Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) infection is a prerequisite for the development of Kaposi's sarcoma (KS). Blocking lytic KSHV replication may hinder KS tumorigenesis. Here, we report potent in vitro anti-KSHV activity of 2′-exo-methanocarbathymidine [North-methanocarbathymidine (N-MCT)], a thymidine analog with a pseudosugar ring locked in the northern conformation, which has previously been shown to block the replication of herpes simplex virus types 1 and 2. N-MCT inhibited KSHV virion production in lytically induced KSHV-infected BCBL-1 cells with a substantially lower 50% inhibitory concentration (IC50) than those of cidofovir (CDV) and ganciclovir (GCV) (IC50, mean ± standard deviation: 0.08 ± 0.03, 0.42 ± 0.07, and 0.96 ± 0.49 μM for N-MCT, CDV, and GCV, respectively). The reduction in KSHV virion production was accompanied by a corresponding decrease in KSHV DNA levels in the N-MCT-treated BCBL-1 cells, indicating that the compound blocked lytic KSHV DNA replication. A time- and dose-dependent accumulation of N-MCT-triphosphate (TP) was demonstrated in lytically induced BCBL-1 cells, while uninfected cells showed virtually no accumulation. The levels of N-MCT-TP were significantly decreased in the presence of 5′-ethynylthymidine, a potent inhibitor of herpesvirus thymidine kinase, resulting in the abrogation of anti-KSHV activity of N-MCT. N-MCT-TP more effectively blocked in vitro DNA synthesis by KSHV DNA polymerase with an IC50 of 6.24 ± 0.08 μM (mean ± standard deviation) compared to CDV-diphosphate (14.70 ±2.47 μM) or GCV-TP (24.59 ± 5.60 μM). Taken together, N-MCT is a highly potent and target-specific anti-KSHV agent which inhibits lytic KSHV DNA synthesis through its triphosphate metabolite produced in KSHV-infected cells expressing a virally encoded thymidine kinase.
Kaposi's sarcoma (KS) is a multifocal malignant tumor of endothelial cell origin characterized by the proliferation of spindle-shaped cells with aberrant neovascularization and a large inflammatory cell infiltrate (14). KS usually manifests as pigmented nodular skin lesions, but can often spread to visceral organs in immunocompromised hosts, including AIDS patients (18, 34) and organ transplant recipients (15, 46, 54). This aggressive and disseminated form of KS was recognized as one of the first AIDS-defining conditions at the beginning of the human immunodeficiency virus (HIV) epidemic in the early 1980s (1, 29). Without effective therapy, visceral KS can be highly fatal unless the underlying causes of immune suppression are successfully treated (13, 21). Cytotoxic chemotherapeutic agents are commonly used in disseminated KS with response rates of up to 80% (22, 59). However, the majority of these agents are associated with serious side effects, and the tumor response to any chemotherapeutic regimen is only transient. There is no definitive cure for KS at the present time.
KS-associated herpesvirus (KSHV, also called human herpesvirus 8) was first discovered in KS lesions obtained from patients with AIDS (9). It was subsequently found in all forms of KS and has strongly been implicated in the pathogenesis of KS (41). KSHV is a gamma-2 herpesvirus (genus Rhadinovirus) closely related to other oncogenic gammaherpesviruses, including herpesvirus saimiri (gamma-2), murine gammaherpesvirus (gamma-2), and Epstein-Barr virus (EBV) (gamma-1) (42). Since its discovery, KSHV has also been linked to a rare form of AIDS-associated effusion-based B-cell lymphoma, termed primary effusion lymphoma or body cavity-based lymphoma (BCBL) (8), and a subset of multicentric Castleman's disease (56).
Although the exact etiologic mechanism of these neoplastic disorders is still unclear, KSHV infection is believed to play a critical role in the tumorigenesis and/or tumor progression. A number of studies have shown that higher levels of KSHV viral load in peripheral blood mononuclear cells and/or serum antibody titers against KSHV proteins correlated with increased risk of KS in HIV-infected (49, 60) and uninfected individuals (16, 44). Higher KSHV viral load in peripheral blood has also been associated with progressive KS in HIV-infected individuals (6, 45). Moreover, several clinical studies, including one prospective study, have found that the risk of KS was significantly reduced in AIDS patients who received ganciclovir (GCV) or foscarnet for cytomegalovirus infection (23, 38, 40). These data suggested that the use of antiherpesvirus agents might have deterred the development of KS, presumably by inhibiting KSHV lytic replication.
To exploit the involvement of KSHV in the tumorigenesis, KSHV-targeted molecular intervention has been proposed to treat KS and other KSHV-induced malignancies, including the use of GCV and foscarnet as antiherpetic DNA synthesis inhibitors (33). In the current study, we identified potent in vitro anti-KSHV activity of 2′-exo-methanocarbathymidine [(North)-methanocarbathymidine (N-MCT)], a thymidine analog with a pseudosugar moiety locked in the northern conformation, which has previously been shown to exert strong antiviral activity against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) (37). Our data demonstrate that N-MCT effectively blocks KSHV DNA synthesis through its triphosphate (TP) metabolite, which is efficiently produced in KSHV-infected cells. N-MCT is 5- to 10-fold more potent than the previously identified inhibitors of KSHV DNA synthesis, cidofovir (CDV) and GCV. Higher potency and target specificity of N-MCT against KSHV may make it a more desirable anti-KS agent.
MATERIALS AND METHODS
Cells and compounds.
BCBL-1, a latently KSHV-infected B-cell line established from a primary effusion lymphoma, was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health, contributed by Michael McGrath and Don Ganem (48). Toledo cells (a human B-cell line) and CEM-SS cells (a human T-cell line) were employed to examine the toxicity of the test compounds. The cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, and 1% penicillin-streptomycin-fungizone mixture (final concentrations: 100 U/ml, 100 μg/ml, and 0.25 μg/ml, respectively) (Cambrex, East Rutherford, NJ) at 37°C in 5% CO2-containing humidified air and split at 1:10 every 3 to 4 days.
N-MCT (Fig. 1A) and its southern counterpart, (South)-methanocarbathymidine (S-MCT), which contains the pseudosugar ring locked in the southern conformation, were synthesized as previously described (37). GCV was purchased from Sigma-Aldrich (St. Louis, MO). CDV and 5′-ethynylthymidine (5′-ET) (43) were kindly provided by M. Hitchcock (Gilead Sciences, Inc. Foster City, CA) and M. Bobek (Roswell Park Cancer Institute, Buffalo, NY), respectively. [Methyl-3H]N-MCT (4.7 Ci/mmol), [5-3H]CDV (28.0 Ci/mmol), and [8-3H]GCV (20.4 Ci/mmol) were obtained from Moravek Biochemicals, Inc. (Brea, CA). N-MCT-TP, CDV-diphosphate (DP), and GCV-TP were synthesized by TriLink BioTechnologies, Inc. (San Diego, CA).
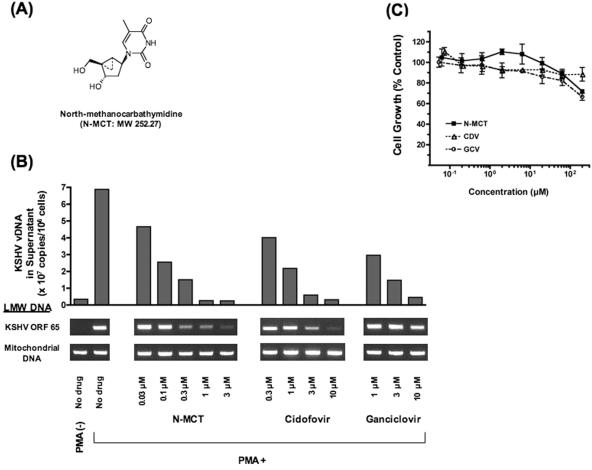
FIG. 1.
(A) Chemical structure of N-MCT. (B) The effects of N-MCT, cidofovir, and ganciclovir on KSHV DNA replication in PMA-stimulated BCBL-1 cells evaluated as KSHV virion-associated DNA copies in the culture supernatants and the amounts of KSHV DNA detected in LMW DNA. The mitochondrial DNA fragment amplified by a primer pair, MTC/F and MTC/R (see text), was included as an internal control for each sample. Shown as a reference is the level of KSHV DNA replication in unstimulated BCBL-1 (PMA −, farthest left lane). The data shown are representative of three independent experiments. (C) Cytotoxic effects of the three compounds examined in PMA-stimulated BCBL-1 cells at the concentrations tested up to 200 μM. The cell growth determined by the XTT method (58) was depicted as a % of the no-drug control (mean ± standard deviation of triplicate wells). The experiment shown is representative of three separate assays. Compounds: (▪) N-MCT; (▵) CDV; (○) GCV.
BCBL-1 culture for evaluation of anti-KSHV activity.
A small fraction (1 to 3%) of BCBL-1 cells in culture are known to spontaneously undergo the lytic cycle and release KSHV virions (30, 48). The antiviral effects of N-MCT and two reference antiherpetic compounds, CDV and GCV, were determined by the relative reduction in the amounts of KSHV DNA and newly released KSHV virion-associated DNA in BCBL-1 cells treated with the test compounds, following lytic induction (48). Briefly, exponentially growing BCBL-1 cells were washed three times with phosphate-buffered saline (PBS) and resuspended in serum-free AIM-V medium with bovine serum albumin (Invitrogen, Carlsbad, CA) at 2 × 105 cells/ml in the absence (unstimulated control) or presence of 20 ng/ml phorbol 12-myristate 13-acetate (PMA, also called 12-O-tetradecanoylphorbol 13-acetate) (Sigma-Aldrich). After 24 h, unstimulated and PMA-stimulated BCBL-1 were harvested, washed once with PBS, and cultured in serum-free AIM-V medium with bovine serum albumin at 2 × 105 cells/ml without PMA in the absence or presence of the test compounds at various concentrations. After 3 days, the cells were counted by the trypan blue dye exclusion method and centrifuged at 1,500 rpm for 5 min. The supernatants were centrifuged at 3,000 rpm for 10 min before being subjected to virion-derived KSHV DNA extraction and quantitation (see below).
The cytotoxicity of the compounds was determined in unstimulated and PMA-stimulated BCBL-1 cells as well as in uninfected Toledo and CEM-SS cells in 96-well microplates, using the XTT {2,3-bis[2-methyoxy-4-nitro-5-sulfophenyl]-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide} assay (58). In selected experiments, the anti-KSHV activity of N-MCT was compared in the presence and absence of 5′-ET, a potent inhibitor of herpesvirus thymidine kinase (TK) (43), in order to investigate whether virally encoded TK played a role in the intracellular production of an active triphosphate metabolite of N-MCT, as has been demonstrated with other nucleoside analogs, such as GCV, in KSHV-infected cells (7).
Measurements of cell- and virion-associated KSHV DNA by PCR.
Low-molecular-weight (LMW) DNA was extracted from the pelleted cells according to Hirt's method (27) and 0.1 μg of LMW DNA was used for KSHV open reading frame 65 (ORF65) PCR by a primer pair, 5′-ACGGTTGTCCAATCGTTGCCTA-3′ and 5′-TCCAACTTTAAGGTGAGAGAC-3′, generating a 529-bp fragment. The ORF65 PCR mixture, containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 0.25 U of Platinum Taq DNA polymerase (Invitrogen), and 200 nM of each primer and template DNA, was subjected to 25 cycles of PCR amplification at 94°C for 60 seconds, 60°C for 60 seconds, and 72°C for 60 seconds, followed by a final extension at 72°C for 5 min. In addition, the mitochondrial DNA primer pair MTC/F (5′-TGGAGCCGGAGCACCCTATGTC-3′) and MTC/R (5′-ATGGGCGGGGGTTGTATTGATG-3′) was used as an internal control for each LMW DNA PCR sample (63). The amplified products were visualized by electrophoresis on a 1.8% agarose gel.
KSHV virions were pelleted from 300 μl of BCBL-1 culture supernatants by a microcentrifugation at 37,000 × g for 2 h at 4°C (53). The pelleted virions were resuspended in 150 μl PBS and treated with 20 units of DNase I (Promega, Madison, WI) at 37°C for 30 min to remove cellular DNA from the samples, followed by incubation with stop solution (20 mM EGTA) at 70°C for 5 min. Virion-associated KSHV DNA (vDNA) was then extracted by QIAamp DNA extraction kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions.
We subjected 1 μl of vDNA eluted in 100 μl of elution buffer to real-time quantitative PCR using a LightCycler instrument (Roche Applied Science, Indianapolis, IN). The 20-μl reaction mixture consisted of the LightCycler FastStart DNA Master SYBR Green I reagent mix (Roche Applied Science), 2.5 mM MgCl2 and 500 nM each of the KSHV ORF26 primer pair (5′-AGCCGAAAGGATTCCACCATT-3′ and 5′-TCCGTGTTGTCTACGTCCAGA-3′). Ten-fold serial dilutions of plasmid pKS330Bam (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health: contributed by Yuan Chang and Patrick Moore), which contains a 330-bp KSHV fragment encoding a portion of the ORF26 gene (9), were included in each assay as external standards to represent 10 to 107 KSHV DNA copies/tube. The number of KSHV vDNA in each supernatant sample was calculated by LightCycler software version 3.5 (Roche Applied Science), adjusted by the cell count, and expressed as copies/106 cells. In selected experiments, 1 μl vDNA per 106 cells was subjected to KSHV ORF65 PCR as described above for 30 cycles.
Evaluation of intracellular phosphorylation of N-MCT.
Exponentially growing BCBL-1 cells or CEM-SS cells were washed three times with PBS and cultured in serum-free AIM-V medium with bovine serum albumin (Invitrogen) at 2 × 105 cells/ml in the absence (unstimulated control) or presence of 20 ng/ml PMA. After 24 h, unstimulated and PMA-stimulated cells were harvested, washed once with PBS, and resuspended in serum-free AIM-V medium with bovine serum albumin at 2 × 105 cells/ml without PMA in the absence or presence of 10 μM N-MCT, CDV, or GCV and 5 μCi/ml of the corresponding radiolabeled compound. Control cultures containing the same concentration of the test compounds but without the radiolabeled formulations were simultaneously set up in an identical manner to assess the cell counts and to evaluate their anti-KSHV activity (see above). In selected experiments, 5′-ET was added at 10 to 50 μM to investigate the changes in the anti-KSHV activity and intracellular phosphorylation profiles of the test compounds. The cells were harvested after 24 or 72 h of incubation.
Upon harvest, the cells were centrifuged at 1,500 rpm for 10 min and washed once with cold PBS. The cell pellets were resuspended in 250 μl of 60% methanol and heated at 95°C for 3 min, followed by a microcentrifugation at 12,000 × g for 10min at 4°C. The clarified supernatant fractions were evaporated under nitrogen, redissolved in 250 μl of water, and subjected to high-pressure liquid chromatography (HPLC) separation of the phosphorylated metabolites as described in detail elsewhere (64). Fractions containing radiolabeled nucleotides were quantitated based on the known specific activity of the parent tritiated nucleoside (64). The phosphorylated metabolites of CDV were identified as CDV-phosphate, CDV-DP (active metabolite), and a phosphate ester adduct of CDV as previously described (28).
In vitro DNA synthesis inhibition assay.
To investigate whether the triphosphate metabolite of N-MCT could directly block KSHV DNA polymerase-mediated DNA synthesis, a rapid microplate-based DNA synthesis assay (50) was carried out in the absence or presence of increasing concentrations of N-MCT-TP, using recombinant KSHV DNA polymerase (rPol) and polymerase processivity factor (rPPF). KSHV rPol and rPPF were expressed and purified from the recombinant baculovirus vector-infected Sf9 cells (12). The DNA synthesis reaction was carried out in a 96-well microplate coated with a 5′-biotinylated 100-mer oligonucleotide template with a 20-mer primer annealed to its 3′-end (primed template, 0.2 pmol/well) with 10 ng each of KSHV rPol and rPPF in a 50-μl reaction mixture, containing 50 mM (NH4)2SO4, 20 mM Tris-HCl (pH 7.5), 3 mM MgCl2, 0.1 mM EDTA, 0.5 mM dithiothreitol (DTT), 2% glycerol, 40 μg/ml bovine serum albumin, 0.625 μM deoxynucleoside triphosphates, and 0.125 μM digoxigenin-11-2′-deoxyuridine-5′-triphosphate (DIG-dUTP) (Roche Applied Science), at 37°C for 60 min in the absence or presence of increasing concentrations of N-MCT-TP, CDV-DP, or GCV-TP. The amounts of newly synthesized DNA which incorporated DIG-dUTP were determined by the DIG detection kit (Roche Applied Science) according to the manufacturer's instructions.
RESULTS
Anti-KSHV activity of N-MCT.
In the BCBL-1 cell-based assay developed for the current study, the number of newly released KSHV virion-associated DNA copies determined by quantitative PCR was consistently 10- to 50-fold increased (median, 16.5-fold) in PMA-induced cells over the uninduced control, with a corresponding increase in the amount of KSHV DNA in the Hirt LMW DNA (Fig. 1B). To determine the biological effects of the test compounds specifically on lytic KSHV DNA replication, the compounds were added to the BCBL-1 culture after the lytic cycle was fully induced by PMA for 24 h. After 3 days, dose-dependent decreases in KSHV vDNA and KSHV DNA in the Hirt DNA were readily observed for N-MCT, CDV, and GCV at the concentrations tested, from 0.03 to 10 μM (Fig. 1B). No significant cytotoxicity was observed with any of the three compounds, although at a much higher concentration (200 μM), mild cytotoxicity was detected with N-MCT or GCV (mean ± standard deviation: 71.9% ± 3.0%, 88.4% ± 11.4% and 65.2% ± 6.0% cell growth for N-MCT, CDV, and GCV, respectively, at 200 μM) (Fig. 1C) (50% cytotoxic concentration >200 μM for all three compounds).
These compounds did not show significant cytotoxicity in unstimulated BCBL-1 cells or in uninfected cell lines, Toledo cells (B-cell line, also EBV negative), and CEM-SS cells (T-cell line), at the concentrations tested up to 200 μM (data not shown). N-MCT exhibited the highest anti-KSHV activity with a 50% inhibitory concentration (IC50) of 0.08 ± 0.03 μM (mean ± standard deviation) (therapeutic index, >2500), compared to 0.42 ± 0.07 (therapeutic index, >476) and 0.96 ± 0.49 (therapeutic index, >208) for CDV and GCV, respectively (Table 1). In contrast, no antiviral activity was observed with S-MCT (data not shown), as has been reported against HSV-1 and HSV-2 (37).
TABLE 1.
Anti-KSHV activity of N-MCT, cidofovir, and ganciclovir in PMA-induced BCBL-1 cells
| Compound | Mean IC50 (μM) ± SDa | Mean IC90 (μM) ± SDa |
|---|---|---|
| N-MCT | 0.08 ± 0.03 | 0.68 ± 0.10 |
| CDV | 0.42 ± 0.07 | 4.01 ± 2.05 |
| GCV | 0.96 ± 0.49 | 7.11 ± 0.28 |
Means from three independent experiments.
Phosphorylation of N-MCT in KSHV-infected and uninfected cells.
The antiviral activity of N-MCT against HSV-1 is mediated through its triphosphate metabolite produced in HSV-1-infected cells (64). To determine whether N-MCT inhibited lytic KSHV DNA replication through a similar mechanism, we examined the intracellular metabolic products of N-MCT in KSHV-infected BCBL-1 cells and uninfected T lymphocyte cells, CEM-SS cells. The latter were used as a reference to compare the intracellular phosphorylation of N-MCT, since it is widely used to screen the anti-HIV activity ofvarious nucleoside compounds, including thymidine analogs (58).
BCBL-1 and CEM-SS cells with or without PMA stimulation were incubated with 10 μM N-MCT and 5 μCi/ml [3H]N-MCT for 6, 24 and 72 h, and the methanolic cell extracts were analyzed by gradient anion-exchange HPLC (64). The HPLC profiles clearly showed the presence of N-MCT-monophosphate (MP) in both cell lines regardless of PMA stimulation as early as 6 h of incubation (Fig. 2A and B). Sharp increases in N-MCT-DP and N-MCT-TP levels were also observed in BCBL-1 cells in 24 h, especially in PMA-stimulated BCBL-1 cells, which contained five- to eightfold higher levels of N-MCT-DP and N-MCT-TP than unstimulated BCBL-1 cells (Fig. 2A). The levels of N-MCT-TP were consistently higher than N-MCT-DP in PMA-induced as well as uninduced BCBL-1 cells (Fig. 2A). In contrast, there were no appreciable accumulations of N-MCT-DP and N-MCT-TP in uninfected CEM-SS cells with or without PMA stimulation (Fig. 2B). These data suggested that the intracellular phosphorylation of N-MCT to its monophosphate form could take place in both KSHV-infected and uninfected cells, but the conversion to the di- and triphosphorylated metabolites was significantly more efficient in KSHV-infected cells, especially during the lytic replication cycle.
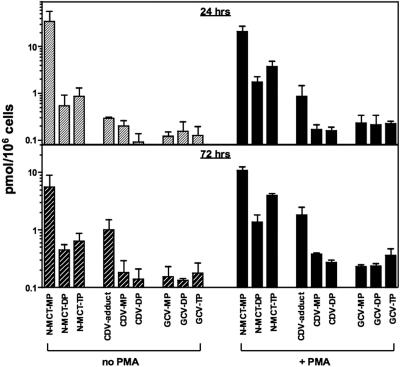
FIG. 2.
Intracellular phosphorylation profiles of N-MCT in KSHV-infected BCBL-1 cells (A) and uninfected CEM-SS cells (B) with (solid lines) and without (dotted lines) PMA stimulation. Shown are the levels of mono-, di-, and triphosphorylated N-MCT metabolites. The data shown are representative of two independent experiments. Compounds: (▪, □) N-MCT-MP; (▴, ▵) N-MCT-DP; (•, ○) N-MCT-TP in PMA-stimulated and unstimulated cells, respectively.
We next compared the levels of phosphorylated metabolites of N-MCT, CDV, and GCV in PMA-induced and uninduced BCBL-1 cells after 24 and 72 h of incubation with 10 μM each of unlabeled and 5 μCi/ml of 3H-labeled compound. As shown in Fig. 3, PMA-stimulated BCBL-1 cells generally contained higher levels of phosphorylated metabolites of all three compounds compared to unstimulated BCBL-1 cells. Notably, the levels of N-MCT-TP were significantly higher than those of CDV-DP and GCV-TP throughout the 72-hour incubation period, especially in PMA-stimulated BCBL-1 cells (Fig. 3).
FIG. 3.
Intracellular phosphorylation profiles of N-MCT, CDV, and GCV in BCBL-1 cells with (+ PMA) and without PMA stimulation (no PMA). Shown are the levels of mono-, di-, and triphosphorylated metabolites of the test compounds analyzed at 24 h (top) and 72 h (bottom) postincubation. Of note, the phosphorylated metabolites of CDV were identified as CDV-monophosphate, CDV-DP (active metabolite), and a phosphate ester adduct of CDV (CDV-adduct) as previously described (28). The data shown are means ± standard deviations of two separate assays.
Herpesvirus TK inhibitor blocks anti-KSHV activity of N-MCT and formation of N-MCT-TP.
KSHV ORF21 has been reported to encode a functionally active TK (7, 26). To further elucidate whether N-MCT-TP formation was directly linked to the anti-KSHV activity of N-MCT, and whether its synthesis was mediated through the virally encoded TK as has been shown in HSV-1-infected cells (64), we evaluated the effects of 5′-ET in PMA-stimulated BCBL-1 cells treated with N-MCT. The thymidine analog 5′-ET has been shown to exert a strong inhibitory activity against HSV-1 TK (43) as well as EBV TK (31), but not against human cellular TK (43).
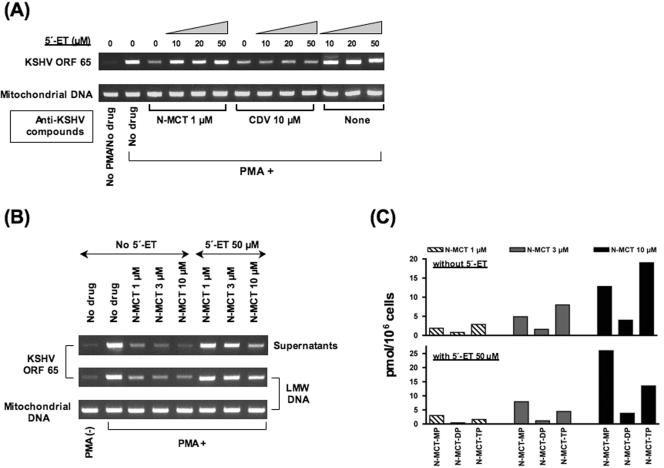
The anti-KSHV activity of N-MCT was first compared in PMA-induced BCBL-1 cells treated with N-MCT alone or in combination with various concentrations of 5′-ET. CDV, which is converted to its active metabolite, CDV-DP, by cellular kinases (11, 28), was used as a reference compound. Compared to cells treated with 1 μM N-MCT alone, marked increases in the level of KSHV DNA in the Hirt DNA were noted in the cells treated with a combination of 1 μM N-MCT and 10, 20, or 50 μM of 5′-ET, with the KSHV DNA level virtually returning to the baseline (no-drug control) at 50 μM of 5′-ET (Fig. 4A). In contrast, the antiviral activity of CDV, tested at 10 μM to achieve an inhibitory effect comparable to that of 1 μM N-MCT, was not affected by the addition of 5′-ET (Fig. 4A).
FIG. 4.
(A) Effects of a potent inhibitor of herpesvirus TK, 5′-ET (43), added at 10, 20, or 50 μM on the anti-KSHV activity of N-MCT (1 μM) and CDV (10 μM) in PMA-stimulated (PMA+) BCBL-1 cells. Shown are the levels of KSHV DNA evaluated by ORF65 PCR and mitochondrial DNA in the Hirt LMW DNA. (B) The effects of 5′-ET (50 μM) on anti-KSHV activity of N-MCT used at 1, 3, or 10 μM in PMA-stimulated BCBL-1. Shown are the amounts of virion-associated (supernatants) and cell-associated KSHV DNA (LMW) as determined by ORF65 PCR along with the levels of control mitochondrial DNA. (C) The levels of phosphorylated metabolites of N-MCT added at 1, 3, or 10 μM in the absence (top) or presence (bottom) of 50 μM 5′-ET in PMA-stimulated BCBL-1 cells.
The inhibitory effect of 50 μM 5′-ET on the anti-KSHV activity of N-MCT was clearly demonstrated even at higher concentrations of N-MCT tested up to 10 μM. PMA-induced BCBL-1 cells were treated with 1, 3, or 10 μM N-MCT alone or in combination with 50 μM 5′-ET. The amounts of virion-and cell-associated KSHV DNA were significantly higher in the cells treated with both N-MCT and 5′-ET compared to that of the cells treated with N-MCT alone at all three concentrations (Fig. 4B). The intracellular levels of phosphorylated N-MCT metabolites N-MCT-MP, N-MCT-DP, and N-MCT-TP were dose-dependently increased in the cells treated with 1, 3, or 10 μM N-MCT and 5 μCi/ml [3H]N-MCT (Fig. 4C, top panel). In the presence of 50 μM 5′-ET, which significantly diminished the anti-KSHV effect of N-MCT in PMA-induced BCBL-1 cells (Fig. 4B), the levels of N-MCT-DP and N-MCT-TP in the methanolic cell extracts were substantially decreased, while there appeared to be an accumulation of N-MCT-MP (Fig. 4C, bottom). These data suggested that anti-KSHV activity of N-MCT was most likely mediated through its triphosphate metabolite, N-MCT-TP, which was converted from its precursor, N-MCT-MP, through N-MCT-DP more efficiently in KSHV-infected cells expressing the viral TK.
N-MCT-TP inhibits DNA synthesis in vitro.
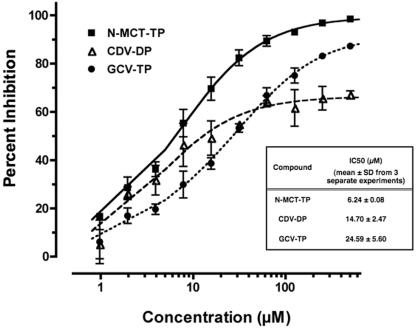
We have previously shown that inhibitors of KSHV Pol-mediated processive DNA synthesis could be screened by a rapid microplate-based in vitro DNA synthesis assay (50). In order to further ascertain that N-MCT-TP was indeed an active metabolite of N-MCT, which blocked lytic KSHV DNA replication, we evaluated the inhibitory effect of N-MCT-TP on processive DNA synthesis in vitro, using baculovirus-expressed recombinant rPol and rPPF (12). The KSHV Pol-specific accessory protein KSHV PPF, which specifically associates with Pol and tethers it onto extending DNA to facilitate processive DNA polymerization (50), was added to the rPol DNA synthesis reaction mixture in order to emulate specific KSHV DNA replication. Active forms of phosphate metabolites of CDV and GCV, CDV-DP and GCV-TP, respectively, were included as a reference.
All three phosphorylated compounds blocked KSHV rPol- and rPPF-mediated DNA synthesis (Fig. 5) with IC50 values (mean ± standard deviation from three independent experiments) of 6.24 ± 0.08 μM, 14.70 ± 2.47 μM, and 24.59 ± 5.60 μM for N-MCT-TP, CDV-DP, and GCV-TP, respectively (Fig. 5, inset). Within the concentrations tested up to 500 μM, N-MCT-TP was the only compound that achieved greater than 90% inhibition (IC90: 76.47 ± 13.95 μM) (Fig. 5). Although CDV-DP inhibited in vitro DNA synthesis more effectively than GCV-TP at lower concentrations, its inhibitory activity appeared to level off around 60 to 70%, whereas GCV-TP dose-dependently blocked DNA synthesis (Fig. 5).
FIG. 5.
Inhibitory activity of N-MCT-TP, CDV-DP, and GCV-TP on in vitro DNA synthesis, mediated by recombinant KSHV polymerase and polymerase processivity factor, depicted as % inhibition (mean ± standard deviation of triplicate wells). Shown in the inset are IC50 values (means ±standard deviations) from three independent experiments for each compound. Compounds: (▪) N-MCT-TP; (▵) CDV-DP; (•) GCV-TP.
DISCUSSION
The unique aspects of intracellular phosphorylation of N-MCT were first discovered in HSV-1-infected cells (64). Unlike acyclovir and GCV, which are selectively monophosphorylated in herpesvirus-infected cells because they are better substrates for virally encoded kinases than for cellular nucleoside kinases (17, 19), N-MCT was found to be efficiently monophosphorylated in HSV-1-infected as well as in uninfected cells, indicating that the compound was a suitable substrate for cellular TK for monophosphorylation (64). However, the successive conversion of N-MCT-MP to N-MCT-DP and N-MCT-TP could only be detected in the HSV-1-infected cells, and the use of an HSV-1 TK inhibitor resulted in the accumulation of N-MCT-MP in the infected cells (64). HSV-1 TK is a multifunctional enzyme with diverse substrate specificity, known to exhibit TK and thymidylate kinase activities (10). Findings by Zalah et al. suggested that N-MCT-MP was not recognizable by cellular thymidylate kinase, and that the rate-limiting step for N-MCT activation was the conversion of N-MCT-MP to N-MCT-DP presumably catalyzed by HSV-1-encoded TK/thymidylate kinase (10), as N-MCT-DP was thought to be readily phosphorylated to N-MCT-TP by cytosolic nucleoside diphosphate kinase (64). The discovery also suggested that N-MCT could be specifically activated (tri-phosphorylated) in cells infected with herpesviruses, which encoded TK/thymidylate kinases capable of recognizing N-MCT-MP as an optimal substrate.
Inhibitory activities of various nucleoside analogs against KSHV replication have previously been evaluated in KSHV-infected cell lines (such as BCBL-1) lytically induced by PMA (30, 39). Of the compounds examined to date, CDV has been identified as one of the most potent anti-KSHV agents, while GCV was found to be moderately active against KSHV (30, 39). In the current study, we found that N-MCT blocked KSHV lytic replication in BCBL-1 cells at a 5- to 10-fold lower IC50 than those of CDV and GCV without notable cytotoxicity (the 50% cytotoxic concentration of N-MCT was >200 μM). As has been shown in HSV-1-infected cells exposed to N-MCT (64), we observed a time- and dose-dependent accumulation of N-MCT-TP almost exclusively in KSHV-infected cells, while both uninfected and infected cell lines contained abundant levels of N-MCT-MP.
Our data suggested that the intracellular conversion of N-MCT-MP to N-MCT-DP was most likely mediated by KSHV ORF21-encoded TK, which has been shown to exhibit thymidylate kinase activity (26). Indeed, in the presence of a potent herpesvirus TK inhibitor, 5′-ET (31, 43), the levels of N-MCT-DP and N-MCT-TP were significantly reduced, resulting in the abrogation of anti-KSHV activity of N-MCT. These findings further supported our notion that KSHV TK catalyzed phosphorylation of N-MCT-MP to N-MCT-DP, which was then converted to N-MCT-TP by cellular nucleoside diphosphate kinase, and that the triphosphate form of N-MCT was directly responsible for the anti-KSHV activity. Interestingly, we found that the intracellular accumulation of N-MCT-TP was significantly greater than those of CDV-DP and GCV-TP, the active metabolites of CDV and GCV, respectively, in BCBL-1 cells treated with each compound at the same concentration. These data may, at least in part, account for the superior anti-KSHV activity of N-MCT identified in our study.
Compared to HSV-1 TK, which is known to possess a broad range of substrate specificities, KSHV TK has more restricted substrate specificity. It has been reported that KSHV TK preferentially phosphorylated thymidine derivatives, while GCV, a guanine analog, was a poor substrate for the enzyme (26). Although it is still possible that GCV may be phosphorylated by a KSHV ORF36-encoded phosphotransferase as has previously been suggested (7), we found that the intracellular level of GCV-TP was, nonetheless, significantly lower than that of N-MCT-TP in KSHV-infected BCBL-1 cells, corresponding to the lower anti-KSHV efficacy of GCV than of N-MCT. Our data further support future efforts to explore and develop thymidine-based analogs as anti-KSHV agents. In addition to KSHV, N-MCT may also exert antiviral activity against another gammaherpesvirus, EBV, which has been shown to encode a TK similar to KSHV TK, exhibiting thymidylate kinase activity and a substrate preference for thymidine analogs (25). Considering the lack of well-established, effective anti-EBV agents available at the present time, further studies are warranted to explore the inhibitory activity of N-MCT against EBV replication and its possible utility in EBV-induced malignancies.
The intracellular accumulation of monophosphorylated (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU-MP) or (E)-5-(2-iodovinyl)-2′-deoxyuridine (IVDU-MP) has been linked to cytostatic effects in TK-deficient tumor cells expressing HSV TK (3). BVDU-MP and IVDU-MP were suspected to target host thymidylate synthase, thereby hindering cellular DNA synthesis (3). While both KSHV-infected and uninfected cells exposed to 10 μM N-MCT were found to contain abundant levels of N-MCT-MP in our study, there was no significant cytotoxicity noted in either cell group until the test concentration reached 200 μM. Therefore, it is unlikely that N-MCT-MP interferes with host thymidylate synthase in cells exposed to KSHV-inhibitory concentrations of N-MCT. KSHV also encodes a functional thymidylate synthase (20). Although it has yet to be determined whether N-MCT-MP can interfere with virally encoded thymidylate synthase, the role of N-MCT-MP in KSHV inhibition is probably minimal, since the KSHV core lytic DNA replication machinery does not include KSHV thymidylate synthase (51, 61).
Another critical determinant of antiherpetic activity of nucleoside-based agents is the efficiency with which the active metabolites are “misincorporated” into viral DNA. For example, S-MCT has not been associated with significant inhibitory activity against HSV-1 (37) or KSHV, as observed in the current study, despite evidence to suggest that it is an excellent substrate for virally encoded TK (36, 52). This is probably because S-MCT-TP is not a preferred substrate for DNA polymerases compared to N-MCT-TP (36), clearly illustrating the two distinct factors involved in attaining antiviral activity. It has also been shown that herpesvirus polymerases possess an inherent 3′ to 5′ exonuclease activity (35, 57), as with other well-characterized DNA polymerases (5, 24). Therefore, the antiviral potency of nucleoside analogs can be greatly influenced by the sensitivity or insensitivity (resistance) of phosphorylated metabolites to the exonuclease activity of viral polymerases. Furthermore, the processivity factors of HSV-1 and EBV polymerases, UL42 and BMRF1, respectively, have been shown to enhance the exonuclease activity of the viral polymerases, substantially reducing the extent of nucleotide misincorporation into DNA (55, 57).
It is highly plausible that KSHV Pol exhibits a similar exonuclease activity, and in the presence of KSHV PPF, the enzyme can efficiently remove mismatched nucleotides from the DNA chain during processive DNA synthesis. In the current study, N-MCT-TP was shown to block in vitro DNA synthesis mediated by KSHV rPol and rPPF more effectively than CDV-DP and GCV-TP. The data not only indicate that N-MCT-TP is efficiently incorporated into DNA, ultimately terminating the processive DNA synthesis, but also imply that N-MCT-MP may be more resistant to excision than two other reference compounds examined. In contrast to dideoxynucleoside compounds known as immediate DNA chain terminators (2, 32), the active metabolites of N-MCT, CDV, and GCV do not block DNA chain elongation at the site of incorporation (4, 47, 62). Although the mechanisms are unclear, this mode of delayed chain termination may confer relative resistance to excision (4, 62). It will be of great interest to investigate whether the rigid conformation of the pseudosugar moiety of N-MCT plays a critical role in excision resistance.
In summary, we discovered the potent anti-KSHV activity of N-MCT, which is specifically triphosphorylated in KSHV-infected cells undergoing lytic replication and efficiently blocks KSHV DNA replication. The compound may represent a new option for the prevention and treatment of KSHV-induced malignancies.
Acknowledgments
We thank M. Hitchcock and M. Bobek for providing us with cidofovir and 5′-ethynylthymidine, respectively, and Q. Yang and J. Brubaker for technical assistance.
This work was supported, in part, by federal funds from the National Cancer Institute, National Institutes of Health, under contract number NO1-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
REFERENCES
- 1.Anonymous. 1981. Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. Morb. Mortal. Wkly. Rep. 30:305-308. [PubMed] [Google Scholar]
- 2.Atkinson, M. R., M. P. Deutscher, A. Kornberg, A. F. Russell, and J. G. Moffatt. 1969. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2′,3′-dideoxyribonucleotide. Biochemistry 8:4897-4904. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J., E. De Clercq, A. Verbruggen, D. Ayusawa, K. Shimizu, and T. Seno. 1987. Thymidylate synthase is the principal target enzyme for the cytostatic activity of (E)-5-(2-bromovinyl)-2′-deoxyuridine against murine mammary carcinoma (FM3A) cells transformed with the herpes simplex virus type 1 or type 2 thymidine kinase gene. Mol. Pharmacol. 32:410-416. [PubMed] [Google Scholar]
- 4.Boyer, P. L., J. G. Julias, V. E. Marquez, and S. H. Hughes. 2005. Fixed conformation nucleoside analogs effectively inhibit excision-proficient HIV-1 reverse transcriptases. J. Mol. Biol. 345:441-450. [DOI] [PubMed] [Google Scholar]
- 5.Brutlag, D., and A. Kornberg. 1972. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3′ leads to 5′ exonuclease activity in deoxyribonucleic acid polymerases. J. Biol. Chem. 247:241-248. [PubMed] [Google Scholar]
- 6.Campbell, T. B., M. Borok, L. Gwanzura, S. MaWhinney, I. E. White, B. Ndemera, I. Gudza, L. Fitzpatrick, and R. T. Schooley. 2000. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS 14:2109-2116. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, J. S., F. Hamzeh, S. Moore, J. Nicholas, and R. F. Ambinder. 1999. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J. Virol. 73:4786-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 10.Chen, M. S., J. Walker, and W. H. Prusoff. 1979. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J. Biol. Chem. 254:10747-10753. [PubMed] [Google Scholar]
- 11.Cihlar, T., and M. S. Chen. 1996. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 50:1502-1510. [PubMed] [Google Scholar]
- 12.Dorjsuren, D., Y. Badralmaa, J. Mikovits, A. Li, R. Fisher, R. Ricciardi, R. Shoemaker, and S. Sei. 2003. Expression and purification of recombinant Kaposi's sarcoma-associated herpesvirus DNA polymerase using a Baculovirus vector system. Protein Expr. Purif. 29:42-50. [DOI] [PubMed] [Google Scholar]
- 13.Dupont, C., E. Vasseur, A. Beauchet, P. Aegerter, H. Berthe, P. de Truchis, D. Zucman, E. Rouveix, and P. Saiag. 2000. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodeficience humaine. AIDS 14:987-993. [DOI] [PubMed] [Google Scholar]
- 14.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 37:1251-1269. [DOI] [PubMed] [Google Scholar]
- 15.Farge, D. 1993. Kaposi's sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur. J. Med. 2:339-343. [PubMed] [Google Scholar]
- 16.Farge, D., C. Lebbe, Z. Marjanovic, P. Tuppin, C. Mouquet, M. N. Peraldi, P. Lang, C. Hiesse, C. Antoine, C. Legendre, J. Bedrossian, M. F. Gagnadoux, C. Loirat, C. Pellet, J. Sheldon, J. L. Golmard, F. Agbalika, and T. F. Schulz. 1999. Human herpes virus-8 and other risk factors for Kaposi's sarcoma in kidney transplant recipients. Groupe Cooperatif de Transplantation d' Ile de France (GCIF). Transplantation 67:1236-1242. [DOI] [PubMed] [Google Scholar]
- 17.Field, A. K., M. E. Davies, C. DeWitt, H. C. Perry, R. Liou, J. Germershausen, J. D. Karkas, W. T. Ashton, D. B. Johnston, and R. L. Tolman. 1983. 9-([2-Hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc. Natl. Acad. Sci. USA 80:4139-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman-Kien, A. E., and B. R. Saltzman. 1990. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi's sarcoma. J. Am. Acad. Dermatol. 22:1237-1250. [DOI] [PubMed] [Google Scholar]
- 19.Fyfe, J. A., P. M. Keller, P. A. Furman, R. L. Miller, and G. B. Elion. 1978. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J. Biol. Chem. 253:8721-8727. [PubMed] [Google Scholar]
- 20.Gaspar, G., E. De Clercq, and J. Neyts. 2002. Human herpesvirus 8 gene encodes a functional thymidylate synthase. J. Virol. 76:10530-10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill, J., D. Bourboulia, J. Wilkinson, P. Hayes, A. Cope, A. G. Marcelin, V. Calvez, F. Gotch, C. Boshoff, and B. Gazzard. 2002. Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma-associated herpesvirus infection in patients with and without Kaposi sarcoma. J. Acquir. Immune Defic. Syndr. 31:384-390. [DOI] [PubMed] [Google Scholar]
- 22.Gill, P. S., M. Rarick, J. A. McCutchan, L. Slater, B. Parker, E. Muchmore, M. Bernstein-Singer, B. Akil, B. M. Espina, M. Krailo, et al. 1991. Systemic treatment of AIDS-related Kaposi's sarcoma: results of a randomized trial. Am. J. Med. 90:427-433. [PubMed] [Google Scholar]
- 23.Glesby, M. J., D. R. Hoover, S. Weng, N. M. Graham, J. P. Phair, R. Detels, M. Ho, and A. J. Saah. 1996. Use of antiherpes drugs and the risk of Kaposi's sarcoma: data from the Multicenter AIDS Cohort Study. J Infect. Dis. 173:1477-1480. [DOI] [PubMed] [Google Scholar]
- 24.Goscin, L. P., and J. J. Byrnes. 1982. DNA polymerase delta: one polypeptide, two activities. Biochemistry 21:2513-2518. [DOI] [PubMed] [Google Scholar]
- 25.Gustafson, E. A., A. C. Chillemi, D. R. Sage, and J. D. Fingeroth. 1998. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 42:2923-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafson, E. A., R. F. Schinazi, and J. D. Fingeroth. 2000. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J. Virol. 74:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 28.Ho, H. T., K. L. Woods, J. J. Bronson, H. De Boeck, J. C. Martin, and M. J. Hitchcock. 1992. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. Mol. Pharmacol. 41:197-202. [PubMed] [Google Scholar]
- 29.Hymes, K. B., T. Cheung, J. B. Greene, N. S. Prose, A. Marcus, H. Ballard, D. C. William, and L. J. Laubenstein. 1981. Kaposi's sarcoma in homosexual men-a report of eight cases. Lancet ii:598-600. [DOI] [PubMed] [Google Scholar]
- 30.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kira, T., S. P. Grill, G. E. Dutschman, J. S. Lin, F. Qu, Y. Choi, C. K. Chu, and Y. C. Cheng. 2000. Anti-Epstein-Barr virus (EBV) activity of β-L-5-iododioxolane uracil is dependent on EBV thymidine kinase. Antimicrob. Agents Chemother. 44:3278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopf, K. W., E. R. Kaufman, and C. Crumpacker. 1981. Physical mapping of drug resistance mutations defines an active center of the herpes simplex virus DNA polymerase enzyme. J. Virol. 39:746-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krown, S. E. 2003. Therapy of AIDS-associated Kaposi's sarcoma: targeting pathogenetic mechanisms. Hematol. Oncol. Clin. N. Am. 17:763-783. [DOI] [PubMed] [Google Scholar]
- 34.Lemlich, G., L. Schwam, and M. Lebwohl. 1987. Kaposi's sarcoma and acquired immunodeficiency syndrome. Postmortem findings in twenty-four cases. J. Am. Acad. Dermatol. 16:319-325. [DOI] [PubMed] [Google Scholar]
- 35.Marcy, A. I., P. D. Olivo, M. D. Challberg, and D. M. Coen. 1990. Enzymatic activities of overexpressed herpes simplex virus DNA polymerase purified from recombinant baculovirus-infected insect cells. Nucleic Acids Res. 18:1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquez, V. E., T. Ben-Kasus, J. J. Barchi, Jr., K. M. Green, M. C. Nicklaus, and R. Agbaria. 2004. Experimental and structural evidence that herpes 1 kinase and cellular DNA polymerase(s) discriminate on the basis of sugar pucker. J. Am. Chem. Soc. 126:543-549. [DOI] [PubMed] [Google Scholar]
- 37.Marquez, V. E., M. A. Siddiqui, A. Ezzitouni, P. Russ, J. Wang, R. W. Wagner, and M. D. Matteucci. 1996. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem. 39:3739-3747. [DOI] [PubMed] [Google Scholar]
- 38.Martin, D. F., B. D. Kuppermann, R. A. Wolitz, A. G. Palestine, H. Li, and C. A. Robinson. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 340:1063-1070. [DOI] [PubMed] [Google Scholar]
- 39.Medveczky, M. M., E. Horvath, T. Lund, and P. G. Medveczky. 1997. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 11:1327-1332. [DOI] [PubMed] [Google Scholar]
- 40.Mocroft, A., M. Youle, B. Gazzard, J. Morcinek, R. Halai, and A. N. Phillips. 1996. Anti-herpesvirus treatment and risk of Kaposi's sarcoma in HIV infection. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. AIDS 10:1101-1105. [PubMed] [Google Scholar]
- 41.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 42.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nutter, L. M., S. P. Grill, G. E. Dutschman, R. A. Sharma, M. Bobek, and Y. C. Cheng. 1987. Demonstration of viral thymidine kinase inhibitor and its effect on deoxynucleotide metabolism in cells infected with herpes simplex virus. Antimicrob. Agents Chemother. 31:368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellet, C., S. Chevret, C. Frances, S. Euvrard, M. Hurault, C. Legendre, S. Dalac, D. Farge, C. Antoine, C. Hiesse, M. N. Peraldi, P. Lang, D. Samuel, Y. Calmus, F. Agbalika, P. Morel, F. Calvo, and C. Lebbe. 2002. Prognostic value of quantitative Kaposi sarcoma-associated herpesvirus load in posttransplantation Kaposi sarcoma. J. Infect. Dis. 186:110-113. [DOI] [PubMed] [Google Scholar]
- 45.Quinlivan, E. B., C. Zhang, P. W. Stewart, C. Komoltri, M. G. Davis, and R. S. Wehbie. 2002. Elevated virus loads of Kaposi's sarcoma-associated human herpesvirus 8 predict Kaposi's sarcoma disease progression, but elevated levels of human immunodeficiency virus type 1 do not. J. Infect. Dis. 185:1736-1744. [DOI] [PubMed] [Google Scholar]
- 46.Qunibi, W., M. Akhtar, K. Sheth, H. E. Ginn, O. Al-Furayh, E. B. DeVol, and S. Taher. 1988. Kaposi's sarcoma: the most common tumor after renal transplantation in Saudi Arabia. Am. J. Med. 84:225-232. [DOI] [PubMed] [Google Scholar]
- 47.Reid, R., E. C. Mar, E. S. Huang, and M. D. Topal. 1988. Insertion and extension of acyclic, dideoxy, and ara nucleotides by herpesviridae, human alpha and human beta polymerases. A unique inhibition mechanism for 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J. Biol. Chem. 263:3898-3904. [PubMed] [Google Scholar]
- 48.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 49.Renwick, N., T. Halaby, G. J. Weverling, N. H. Dukers, G. R. Simpson, R. A. Coutinho, J. M. Lange, T. F. Schulz, and J. Goudsmit. 1998. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. AIDS 12:2481-2488. [DOI] [PubMed] [Google Scholar]
- 50.Ricciardi, R. P., K. Lin, X. Chen, D. Dorjsuren, R. Shoemaker, and S. Sei. 2004. Rapid screening of chemical inhibitors that block processive DNA synthesis of herpesviruses: potential application to high-throughput screening. Methods Mol. Biol. 292:481-492. [DOI] [PubMed] [Google Scholar]
- 51.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schelling, P., M. T. Claus, R. Johner, V. E. Marquez, G. E. Schulz, and L. Scapozza. 2004. Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J. Biol. Chem. 279:32832-32838. [DOI] [PubMed] [Google Scholar]
- 53.Sei, S., Q. E. Yang, D. O'Neill, K. Yoshimura, K. Nagashima, and H. Mitsuya. 2000. Identification of a key target sequence to block human immunodeficiency virus type 1 replication within the gag-pol transframe domain. J. Virol. 74:4621-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepherd, F. A., E. Maher, C. Cardella, E. Cole, P. Greig, J. A. Wade, and G. Levy. 1997. Treatment of Kaposi's sarcoma after solid organ transplantation. J. Clin. Oncol. 15:2371-2377. [DOI] [PubMed] [Google Scholar]
- 55.Song, L., M. Chaudhuri, C. W. Knopf, and D. S. Parris. 2004. Contribution of the 3′- to 5′-exonuclease activity of herpes simplex virus type 1 DNA polymerase to the fidelity of DNA synthesis. J. Biol. Chem. 279:18535-18543. [DOI] [PubMed] [Google Scholar]
- 56.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 57.Tsurumi, T., T. Daikoku, and Y. Nishiyama. 1994. Further characterization of the interaction between the Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit with regard to the 3′-to-5′ exonucleolytic activity and stability of initiation complex at primer terminus. J. Virol. 68:3354-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weislow, O. S., R. Kiser, D. L. Fine, J. Bader, R. H. Shoemaker, and M. R. Boyd. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 81:577-586. [DOI] [PubMed] [Google Scholar]
- 59.Welles, L., M. W. Saville, J. Lietzau, J. M. Pluda, K. M. Wyvill, I. Feuerstein, W. D. Figg, R. Lush, J. Odom, W. H. Wilson, M. T. Fajardo, R. W. Humphrey, E. Feigal, D. Tuck, S. M. Steinberg, S. Broder, and R. Yarchoan. 1998. Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi's sarcoma. J. Clin. Oncol. 16:1112-1121. [DOI] [PubMed] [Google Scholar]
- 60.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, A. S. Denton, et al. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]
- 61.Wu, F. Y., J. H. Ahn, D. J. Alcendor, W. J. Jang, J. Xiao, S. D. Hayward, and G. S. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong, X., J. L. Smith, and M. S. Chen. 1997. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 41:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, Q. E., A. G. Stephen, J. W. Adelsberger, P. E. Roberts, W. Zhu, M. J. Currens, Y. Feng, B. J. Crise, R. J. Gorelick, A. R. Rein, R. J. Fisher, R. H. Shoemaker, and S. Sei. 2005. Discovery of small-molecule human immunodeficiency virus type 1 entry inhibitors that target the gp120-binding domain of CD4. J. Virol. 79:6122-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zalah, L., M. Huleihel, E. Manor, A. Konson, H. Ford, Jr., V. E. Marquez, D. G. Johns, and R. Agbaria. 2002. Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells. Antiviral Res. 55:63-75. [DOI] [PubMed] [Google Scholar]