Abstract
Since the approval of linezolid in 2000, sporadic reports of resistance have been given and a greater understanding of the underlying mechanisms of resistance has been gained. However, since these developments, an updated status of the in vitro activity of linezolid against gram-positive organisms from the United States has not been reported. The LEADER 2004 surveillance initiative was undertaken to obtain current and representative data on the activity of linezolid against key species, including isolates with significant resistance phenotypes. Organisms were isolated during 2004 and included 2,872 Staphylococcus aureus, 496 coagulase-negative staphylococcus (CNS), 428 Enterococcus faecalis, 196 Enterococcus faecium, and 422 Streptococcus pneumoniae isolates. All S. aureus isolates (54.2% oxacillin resistant) were susceptible to linezolid (MIC90 = 2 μg/ml); MIC distributions were consistent, regardless of oxacillin or multidrug resistance status. For CNS, one nonsusceptible isolate was encountered (Staphylococcus epidermidis; MIC = 32 μg/ml), but overall, the MIC90 (1 μg/ml) was lower than that obtained with S. aureus. For E. faecalis and E. faecium, 99.5% and 96.4% of isolates, respectively, were linezolid susceptible. Both species had an MIC90 of 2 μg/ml, and MIC distributions did not vary with the vancomycin susceptibility status of the populations analyzed. Linezolid nonsusceptibility was not encountered among the S. pneumoniae isolates. These findings indicate that linezolid nonsusceptibility has remained rare among staphylococci and uncommon and sporadic among enterococci. Nonetheless, careful and ongoing monitoring of the in vitro effectiveness of linezolid will be needed so that any changes to the current status may be detected as soon as possible.
Linezolid is the first in the class oxazolidinone that was approved for clinical use in 2000 for the treatment of nosocomial and community-acquired pneumonia, uncomplicated and complicated skin and skin structure infections, and infections caused by vancomycin-resistant Enterococcus faecium (11, 18, 29). The unique mode of action of linezolid involves binding of the agent to the ribosomal 50S subunit in domain V of the 23S rRNA. As a result, the 50S subunit is prevented from interacting with the 30S subunit for the formation of the 70S initiation complex. The unique inhibition of protein synthesis initiation by linezolid confers potent antibacterial properties. This mechanism of action is refractory to cross-resistance from the presence of resistance mechanisms that impact other agents that target ribosome-mediated protein synthesis (e.g., macrolides, lincosamides, streptogramins, and chloramphenicol) (7, 11, 18).
At the time of approval, linezolid demonstrated potent in vitro activity against several gram-positive organisms, including staphylococci, enterococci, and streptococci, even those that were resistant to one or more other classes of antimicrobial agents (1, 8, 11, 20, 29). Subsequent to the approval and use of linezolid over the past 4 years (more than 1 million patients treated [data on file at Pfizer Inc., New York, N.Y.]), there have been sporadic reports of resistance among staphylococci and enterococci, and our understanding of the underlying resistance mechanisms has advanced (15). Despite these reports and advancements, there has not been an extensive, updated analysis of the activity of linezolid against key target organisms in the United States.
LEADER 2004 was a national surveillance initiative specifically designed to analyze linezolid activity in the context of the activities of other gram-positive agents on a broad and representative basis with regard to geographic balance, patient profile, bacterial species, and resistance phenotypes. An important aspect in the analysis of the in vitro activity of linezolid took into consideration what is currently known about resistance development. Briefly, both staphylococci and enterococci have multiple copies of the gene that encodes domain V of the 23S rRNA, the location of the target for linezolid. In both organism groups, a gene dosage effect has been described whereby linezolid MICs increase with the number of gene copies that have mutations (13, 15, 16, 25). Due to this effect, this linezolid surveillance analysis involved not only monitoring strains for absolute nonsusceptible phenotypes (i.e., MIC of ≥8 μg/ml for staphylococci, MIC of ≥4 μg/ml for enterococci and streptococci) (2) but also monitoring MIC distributions for upward shifts that could indicate an increasing number of strains housing mutations in the domain V gene copies prior to absolute resistance development.
MATERIALS AND METHODS
LEADER 2004 involved the prospective collection of gram-positive clinical isolates from 50 hospital laboratories distributed across 33 states and Washington, D.C. Participating institutions were selected to ensure that a representative geographic distribution of strains from all nine U.S. Bureau of Census regions was attained. Diversity with regard to the types of participating institutions was also achieved as community hospitals (n = 21), teaching and university medical centers (n = 24), reference laboratories (n = 3), a children's hospital, and a Veterans Administration hospital were enrolled in the network. From each institution, single patient isolates were requested along with certain demographic (patient age, gender, location) and clinical (source of specimen) information; however, this attendant information was not mandatory for a strain to be included in the study.
All isolates were transported to a central laboratory (Focus Bio-Inova, Herndon, Virginia), where they were subcultured onto 5% sheep blood agar twice prior to inoculum preparation for antimicrobial susceptibility testing. Antimicrobial susceptibility testing was performed using broth microdilution (TREK Diagnostics, Cleveland, Ohio) in accordance with CLSI guidelines for staphylococci, enterococci, and Streptococcus pneumoniae (21). After appropriate incubation duration, MICs were read and categorical interpretations of susceptible, intermediate, or resistant were applied using the 2005 CLSI criteria (2). Throughout the study, S. pneumoniae strain ATCC 49619, Staphylococcus aureus strain ATCC 29213, and Enterococcus faecalis strain ATCC 29212 were used as the quality control strains.
For multidrug resistance analysis, staphylococci resistant to three or more agents, including oxacillin, erythromycin, clindamycin, chloramphenicol, tetracycline, trimethoprim-sulfamethoxazole, quinupristin-dalfopristin, rifampin, levofloxacin, vancomycin, and linezolid were classified as multidrug resistant (MDR).
S. pneumoniae multidrug resistance was defined as resistance to three or more agents among penicillin, cefuroxime, amoxicillin-clavulanate, ceftriaxone, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, levofloxacin, vancomycin, and linezolid.
In addition, linezolid MIC distributions were analyzed according to key resistance phenotypes among staphylococci, enterococci, and S. pneumoniae to detect any potential increases in MICs that may be associated with different resistant populations.
RESULTS
A geographic, demographic, and clinical diversity of strains was obtained for analysis. In total, 2,105 isolates were from inpatients, 1,373 isolates were from outpatients, and 612 isolates were from intensive-care unit (ICU) patients, and for 324 isolates, the patient location was not provided. The clinical sources from which isolates were obtained included skin and skin structure specimens (1,602 isolates), blood (1,597 isolates), and respiratory tract specimens (888 isolates), and for some isolates, the specimen source was not provided (327 isolates). Three hundred fifty-three isolates were from pediatric patients aged less than 18 years, 2,321 isolates were from adult patients aged 18 to 64 years, and 1,302 isolates were from elderly patients aged greater than 64 years; for 438 isolates, patient age was not available.
Antimicrobial profiles obtained with each of the organism groups are shown in Table 1. For S. aureus, more than half (54.2%) of the isolates were resistant to oxacillin and the MIC90 (>2 μg/ml) was greater than the CLSI susceptible breakpoint (2). Along with oxacillin resistance, resistance to erythromycin, clindamycin, and the fluoroquinolones (i.e., levofloxacin) was also common, ranging from 29.6% for clindamycin to 66.9% for erythromycin. Resistance to rifampin and trimethoprim-sulfamethoxazole was <3%, and nonsusceptibility to quinupristin-dalfopristin was rare (<0.2% of isolates). Nonsusceptibility to either vancomycin or linezolid was not encountered among any of the 2,872 S. aureus isolates tested.
TABLE 1.
Antimicrobial profiles among key organism groups
| Organism (total no. of isolates) | Agent | MIC (μg/ml)
|
No. (%) of isolatesa
|
|||||
|---|---|---|---|---|---|---|---|---|
| Range | Mode | 50% | 90% | S | I | R | ||
| S. aureus (2,872) | Oxacillin | ≤0.06->2 | >2 | >2 | >2 | 1,316 (45.8) | —b | 1,556 (54.2) |
| Erythromycin | ≤0.12->8 | >8 | >8 | >8 | 885 (30.8) | 66 (2.3) | 1,921 (66.9) | |
| Clindamycin | ≤0.5->4 | ≤0.5 | ≤0.5 | >4 | 1,986 (69.2) | 35 (1.2) | 851 (29.6) | |
| Levofloxacin | ≤0.03->8 | 0.12 | 0.25 | >8 | 1,596 (55.6) | 279 (9.7) | 997 (34.7) | |
| Quinupristin-dalfopristin | ≤0.06-8 | 0.25 | 0.25 | 1 | 2,868 (99.9) | 2 (<0.1) | 2 (<0.1) | |
| Rifampin | ≤0.002->4 | 0.008 | 0.008 | 0.015 | 2,818 (98.1) | 10 (0.3) | 44 (1.5) | |
| Trimethoprim-sulfamethoxazole | ≤0.25->4 | ≤0.25 | ≤0.25 | ≤0.25 | 2,790 (97.1) | — | 82 (2.9) | |
| Vancomycin | 0.5-4 | 1 | 1 | 1 | 2,872 (100) | 0 (0) | 0 (0) | |
| Linezolid | 0.25-4 | 2 | 2 | 2 | 2,872 (100) | — | — | |
| CNS (496) | Oxacillin | ≤0.06->2 | >2 | >2 | >2 | 114 (23.0) | — | 382 (77.0) |
| Erythromycin | ≤0.12->8 | >8 | >8 | >8 | 140 (28.2) | 8 (1.6) | 348 (70.2) | |
| Clindamycin | ≤0.5->4 | ≤0.5 | ≤0.5 | >4 | 291 (58.7) | 12 (2.4) | 193 (38.9) | |
| Levofloxacin | 0.06->8 | >8 | 4 | >8 | 204 (41.1) | 54 (10.9) | 238 (48.0) | |
| Quinupristin-dalfopristin | ≤0.06-2 | 0.25 | 0.25 | 0.5 | 492 (99.2) | 4 (0.8) | 0 (0.0) | |
| Rifampin | ≤0.002->4 | 0.008 | 0.008 | 0.015 | 475 (95.8) | 3 (0.6) | 18 (3.6) | |
| Trimethoprim-sulfamethoxazole | ≤0.25->4 | ≤0.25 | 0.5 | >4 | 296 (59.7) | — | 200 (40.3) | |
| Vancomycin | ≤0.25-8 | 2 | 2 | 2 | 495 (99.8) | 1 (0.2) | 0 (0) | |
| Linezolid | 0.12-32 | 1 | 1 | 1 | 495 (99.8)c | — | — | |
| E. faecalis (428) | Ampicillin | 0.12-8 | 1 | 1 | 2 | 428 (100) | 0 (0) | 0 (0) |
| Teicoplanin | 0.06->128 | 0.25 | 0.25 | 0.25 | 401 (93.7) | 1 (0.2) | 26 (6.1) | |
| Vancomycin | 0.5->128 | 1 | 2 | 8 | 385 (90.0) | 2 (0.5) | 41 (9.6) | |
| Linezolid | 0.25-64 | 2 | 2 | 2 | 426 (99.5) | 1 (0.2) | 1 (0.2) | |
| E. faecium (196) | Ampicillin | 0.12->128 | >128 | 128 | >128 | 20 (10.2) | 0 (0) | 176 (89.8) |
| Teicoplanin | 0.12->128 | 32 | 32 | 128 | 65 (33.2) | 6 (3.1) | 125 (63.8) | |
| Vancomycin | ≤0.25->128 | >128 | >128 | >128 | 53 (27.0) | 1 (0.5) | 142 (72.4) | |
| Quinupristin-dalfopristin | 0.25-16 | 1 | 1 | 2 | 170 (86.7) | 11 (5.6) | 15 (7.7) | |
| Linezolid | 0.25-32 | 2 | 2 | 2 | 189 (96.4) | 3 (1.5) | 4 (2.0) | |
| S. pneumoniae (422) | Penicillin | ≤0.06->2 | ≤0.06 | ≤0.06 | 2 | 282 (66.8) | 78 (18.5) | 62 (14.7) |
| Amoxicillin-clavulanate | ≤0.015-8 | 0.03 | 0.03 | 2 | 399 (94.5) | 2 (0.5) | 21 (5.0) | |
| Cefuroxime-axetil | ≤0.03->4 | ≤0.03 | 0.06 | 4 | 343 (81.3) | 11 (2.6) | 68 (16.1) | |
| Ceftriaxone | ≤0.015->4 | ≤0.015 | 0.03 | 1 | 412 (97.6) | 7 (1.7) | 3 (0.7) | |
| Erythromycin | ≤0.015->1 | 0.06 | 0.06 | >1 | 302 (71.6) | 6 (1.4) | 114 (27.0) | |
| Clindamycin | ≤0.25->1 | ≤0.25 | ≤0.25 | >1 | 371 (87.9) | 3 (0.7) | 48 (11.4) | |
| Levofloxacin | 0.5->8 | 1 | 1 | 1 | 415 (98.3) | 0 (0) | 7 (1.7) | |
| Trimethoprim-sulfamethoxazole | ≤0.06->2 | 0.12 | 0.25 | >2 | 307 (72.7) | 39 (9.2) | 76 (18.0) | |
| Vancomycin | 0.12-0.5 | 0.5 | 0.5 | 0.5 | 422 (100) | — | — | |
| Linezolid | 0.25-1 | 1 | 1 | 1 | 422 (100) | — | — | |
S, susceptible; I, intermediate; R, resistant. Susceptibilities were determined according to standards established by the Clinical and Laboratory Standards Institute (M100-S15) (2).
—, CLSI interpretative criteria not available.
One isolate was linezolid nonsusceptible (MIC, >4 μg/ml).
Antimicrobial patterns among the coagulase-negative staphylococci (CNS) were similar to those found with S. aureus, with some key exceptions (Table 1). Resistance rates to oxacillin (77.0%), erythromycin (70.2%), clindamycin (38.9%), and levofloxacin (48.0%) were substantially higher than those obtained with S. aureus. Trimethoprim-sulfamethoxazole resistance was 40.3%, compared to 2.9% resistance among S. aureus, and nonsusceptibility to vancomycin (one strain) and linezolid (one strain) was encountered. The vancomycin-intermediate strain (MIC = 8 μg/ml) was an isolate of Staphylococcus haemolyticus obtained from a blood culture taken when the 36-year-old male patient was seen as an outpatient. This isolate was susceptible to linezolid (MIC = 1 μg/ml). The linezolid-nonsusceptible strain (MIC = 32 μg/ml) was Staphylococcus epidermidis isolated from a blood culture of an 82-year-old male ICU patient. Further detailed clinical and therapeutic histories were not available for either of these strains. Overall, the MIC90 for linezolid (1 μg/ml) among CNS remained two doubling dilutions below the current CLSI breakpoint of ≤4 μg/ml, and the vancomycin MIC90 of 2 μg/ml was one doubling dilution below its susceptibility breakpoint of ≤4 μg/ml (2).
Ampicillin resistance was not encountered among the E.faecalis strains, but 10% were vancomycin nonsusceptible (2 intermediate strains and 41 resistant strains) and the vancomycin MIC90 (8 μg/ml) was in the CLSI intermediate range (8 to 16 μg/ml) (2). The E. faecalis MIC90 for linezolid was 2 μg/ml, and 99.5% of isolates were susceptible (Table 1). In contrast to the ampicillin and vancomycin patterns seen with E.faecalis, resistance to both agents among E. faecium was high, 89.8% and 72.4%, respectively, and the MIC90s for both agents were >128 μg/ml. Nonsusceptibility to quinupristin-dalfopristin was common, as 5.6% of isolates were intermediate, 7.7% were resistant, and the MIC90 (2 μg/ml) was in the CLSI intermediate range (2). The E. faecium linezolid MIC90 of 2 μg/ml was the same as that obtained for E. faecalis, and 96.4% of E. faecium isolates were susceptible to linezolid.
Resistance to penicillin, erythromycin, cefuroxime, clindamycin, and trimethoprim-sulfamethoxazole was commonly encountered among the S. pneumoniae isolates and ranged from 11.4% for clindamycin to 27.0% for erythromycin (Table 1). The agents with the highest susceptibility rates included ceftriaxone (97.6%), levofloxacin (98.3%), vancomycin (100%), and linezolid (100%).
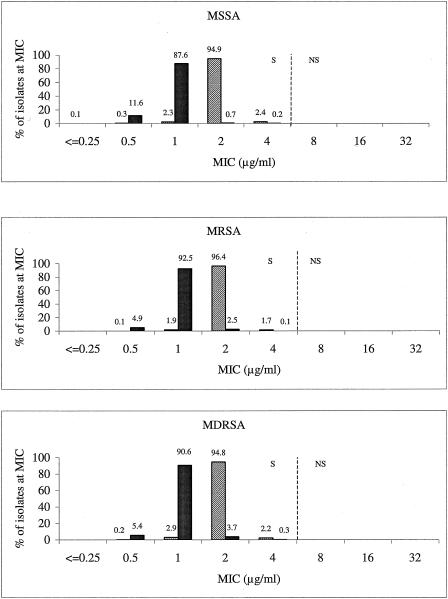
MIC distributions for vancomycin and linezolid according to the S. aureus phenotype populations of methicillin (oxacillin)-susceptible (MSSA), methicillin-resistant (MRSA), and MDR (MDRSA) S. aureus isolates are shown in Fig. 1. For MSSA, MRSA, and MDRSA, linezolid MIC distributions were nearly identical and ranged from 0.5 to 4 μg/ml; one MSSA isolate had an MIC of 0.25 μg/ml. The modal linezolid MIC was 2 μg/ml for all three populations. Relative to each other, none of the three populations demonstrated any upward MIC drift toward the 4-μg/ml susceptible breakpoint. Similarly, the vancomycin MIC distributions did not demonstrate any substantial shift upward across the three populations. However, the percentage of isolates with vancomycin MICs of 2 μg/ml was higher among MDRSA isolates than among the other S. aureus groups (i.e., MSSA, MRSA) (Fig. 1).
FIG. 1.
Vancomycin and linezolid MIC distribution according to different S. aureus phenotype populations (top, MSSA; middle, MRSA; bottom, MDRSA). Vertical dashed lines indicate the current CLSI breakpoints. S, susceptible; NS, nonsusceptible (2).
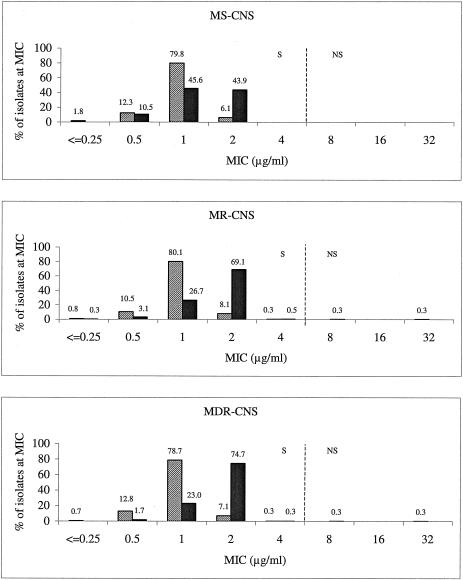
Similar MIC distribution outcomes were noted for the CNS populations (Fig. 2). The linezolid and vancomycin MICs were consistent with the same MIC modes and similar MIC ranges across the methicillin-susceptible (MS-CNS), methicillin-resistant (MR-CNS), and MDR (MDR-CNS) CNS phenotypes. The only exceptions were the single vancomycin-intermediate MDR-CNS isolate and the single linezolid-resistant MDR-CNS isolate that occurred outside the usual MIC range. The CNS linezolid MIC distributions did differ from those of S. aureus in that the mode was 1 μg/ml rather than 2 μg/ml. Also, the linezolid mode for CNS was 1 dilution lower than the vancomycin mode, as opposed to it being 1 dilution higher than the vancomycin mode as seen with S. aureus (Fig. 1).
FIG. 2.
Vancomycin and linezolid MIC distribution according to different CNS phenotype populations (top, methicillin-susceptible CNS; middle, methicillin-resistant CNS; bottom, MDR-CNS). Solid bars, vancomycin; hatched bars, linezolid. Vertical dashed lines indicate the current CLSI breakpoints. S, susceptible; NS, nonsusceptible (2).
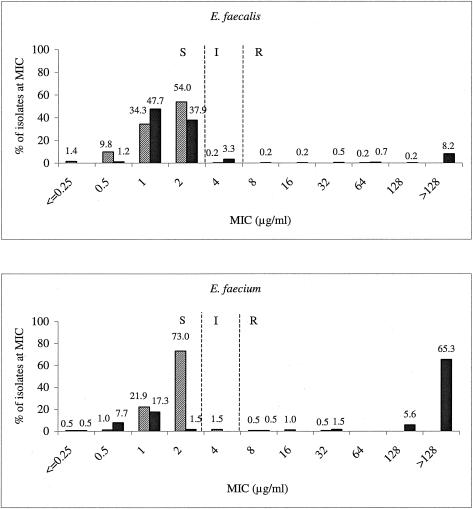
The prevalence of vancomycin nonsusceptibility among both E. faecalis and E. faecium strains resulted in wide vancomycin MIC distributions (Fig. 3), and the extremely high prevalence of vancomycin resistance among E. faecium strains resulted in a mode of >128 μg/ml. Although linezolid-nonsusceptible strains were sporadically encountered with both species (9 of 624 isolates), the MIC distributions remained stable and consistent for both species and showed no evidence of drifting upward with the prevalence of vancomycin resistance. For both E. faecalis and E. faecium, the modal linezolid MIC of 2 μg/ml is at the CLSI susceptible breakpoint of 2 μg/ml (2).
FIG. 3.
Vancomycin and linezolid MIC distribution patterns for E. faecalis (top) and E. faecium (bottom). Solid bars, vancomycin; hatched bars, linezolid. Vertical dashed lines indicate the current CLSI breakpoints. S, susceptible; I, intermediate; R, resistant (2).
Of the 624 enterococcal isolates tested, 9 (1.4%) were nonsusceptible to linezolid. The patient and strain profiles of the nonsusceptible isolates are shown in Table 2. Linezolid MICs ranged from 4 to 64 μg/ml; four isolates had MICs at the intermediate breakpoint (4 μg/ml), one had an MIC on the resistant breakpoint of 8 μg/ml, and the remaining four isolates had MICs of ≥16 μg/ml. The two strains with linezolid MICs of 16 μg/ml were E. faecium blood isolates from the same institution, one from a male inpatient and the other from a female ICU patient.
TABLE 2.
Profiles of linezolid-nonsusceptible enterococcal strains
| Species | Hospital type | Patient location | Gender | Age (yr) | Specimen source | MIC (μg/ml) (interpretative categorya) of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linezolid | Vancomycin | Teicoplanin | Ampicillin | Quinupristin-dalfopristin | Chloramphenicol | ||||||
| Enterococcus faecalis | University | Unknown | Unknown | Unknown | Unknown | 4 (I) | 2 (S) | 0.25 (S) | 2 (S) | 8 (R) | 16 (I) |
| Enterococcus faecalis | University | Inpatient | Male | 53 | Blood | 64 (R) | 1 (S) | 0.25 (S) | 1 (S) | 16 (R) | >32 (R) |
| Enterococcus faecium | University | Unknown | Unknown | Unknown | Unknown | 4 (I) | >128 (R) | 128 (R) | >128 (R) | 1 (S) | 16 (I) |
| Enterococcus faecium | Community | ICU | Male | 78 | Wound | 4 (I) | 1 (S) | 0.5 (S) | 4 (S) | 4 (R) | 8 (S) |
| Enterococcus faecium | Community | Unknown | Unknown | Unknown | Wound | 4 (I) | >128 (R) | 32 (R) | 128 (R) | 1 (S) | 16 (I) |
| Enterococcus faecium | University | ICU | Female | 68 | Wound | 8 (R) | >128 (R) | 128 (R) | 64 (R) | 1 (S) | 16 (I) |
| Enterococcus faecium | University | Inpatient | Male | 30 | Blood | 16 (R) | >128 (R) | 8 (S) | >128 (R) | 1 (S) | 32 (R) |
| Enterococcus faecium | University | ICU | Female | 43 | Blood | 16 (R) | >128 (R) | 32 (R) | >128 (R) | 1 (S) | 32 (R) |
| Enterococcus faecium | University | Inpatient | Female | 42 | Wound | 32 (R) | >128 (R) | 32 (R) | >128 (R) | 1 (S) | 32 (R) |
S, susceptible; I, intermediate; R, resistant. Susceptibilities were determined according to standards established by the Clinical and Laboratory Standards Institute (M100-S15) (2).
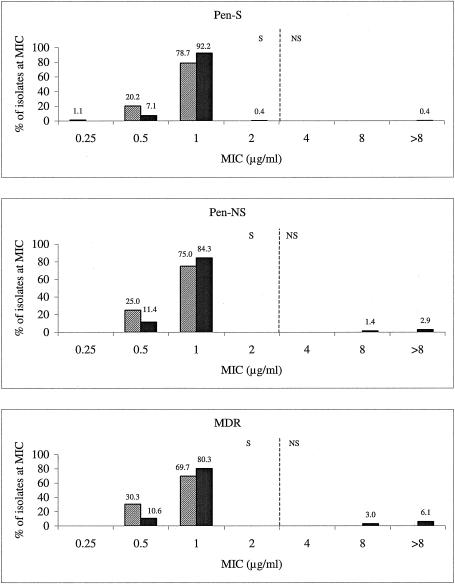
For penicillin-susceptible, penicillin-nonsusceptible, and MDRS. pneumoniae isolates, linezolid and levofloxacin shared the same modal MIC of 1 μg/ml (Fig. 4), which also was the MIC90 for both agents (Table 1). While levofloxacin-resistant strains were sporadically encountered, neither the levofloxacin nor the linezolid MIC distributions exhibited any upward drift, regardless of the populations examined. However, levofloxacin-resistant strains were more common among the MDR population than among the penicillin-nonsusceptible and penicillin-susceptible populations. Overall, the modal MIC and MIC90 of linezolid were 1 μg/ml, which was one doubling dilution below the CLSI susceptible breakpoint of ≤2 μg/ml for S. pneumoniae (2).
FIG. 4.
Levofloxacin and linezolid MIC distributions according to different S. pneumoniae phenotype populations. Solid bars, levofloxacin; hatched bars, linezolid; Pen-S, penicillin susceptible; Pen-NS, penicillin intermediate and resistant. Vertical dashed lines indicate the current CLSI breakpoints. S, susceptible; NS, nonsusceptible (2).
DISCUSSION
To achieve a current and robust representative sample of gram-positive isolates for analyzing current linezolid activity, the LEADER 2004 surveillance initiative collected organisms from diverse geographic and demographic environments as well as from across a variety of clinical specimens. The high resistance rates for important antimicrobial classes found in this study among S. aureus, coagulase-negative staphylococci, E. faecium, E. faecalis, and S. pneumoniae were consistent with the rates reported in other surveillance initiatives (3, 9, 22, 26). Certain key patterns are worth highlighting. Less than half (45.8%) of the S. aureus isolates were oxacillin susceptible, and this low susceptibility pattern was shared with other key agents, such as clindamycin and the fluoroquinolones (levofloxacin). Among enterococci, ampicillin and vancomycin resistance rates continued to be most highly associated with E. faecium, at >89 and 72%, respectively; however, vancomycin nonsusceptibility (10%) among E. faecalis was notably higher than the 2 to 3% previously reported (26). Resistance was also prominent among S. pneumoniae isolates as low susceptibility rates (<90%) were noted for several agents, including penicillin, cefuroxime, macrolides (erythromycin), clindamycin, and trimethoprim-sulfamethoxazole. These antimicrobial profiles demonstrated that the problem of resistance continues to be pervasive across several antimicrobial classes for nearly all commonly encountered key gram-positive species. In addition, these patterns substantiated that the analysis of current linezolid activity was based on its activity within a strong representation of challenging resistance phenotypes as well as on geographic and demographic diversity.
Based on what is currently known about the potential for linezolid resistance development, monitoring activity through analysis of surveillance data was done from two perspectives. The direct perspective involved analysis of rates of “absolute” resistance whereby strains have achieved linezolid MICs beyond current CLSI susceptible breakpoints (2). The second perspective involved the monitoring of MIC distributions for changes or upward shifts. The need for this second perspective is based on the gene dosage effect that has been described for both staphylococci and enterococci. By this effect, an MIC for an isolate increases relative to the number of domain V 23S rRNA genes that contain mutations (13, 14, 15, 16, 24, 25). Careful monitoring of MIC distributions for subtle upward shifts could provide important and early indications that the number of mutations is on the rise among target bacterial populations before changes in “absolute” resistance rates are detected. However, a caveat that must be considered in this approach is that resistant strains may be able to revert to full susceptibility (17).
There have been sporadic reports of absolute linezolid resistance (i.e., nonsusceptibility; MICs of >4 μg/ml) among S.aureus strains, usually associated with long-term courses of linezolid therapy in severely debilitated patients with unremovable infected devices (15, 16, 27, 28). However, based on data from strains collected in studies from 1998 to 2000 that did not find nonsusceptible strains and data from this study in which none of the 2,872 S. aureus isolates had an MIC above 4 μg/ml, the linezolid-nonsusceptible phenotype must be considered extremely rare among S. aureus strains (1, 8, 20). In these earlier surveillance studies, the MIC90 of linezolid was 4 μg/ml, while the current data demonstrated an MIC90 of 2 μg/ml. The reasons for this difference are uncertain but at the very least strongly indicated that linezolid MICs have remained stable even under the selective pressure of more than 1 million patients being treated since its launch (data on file at Pfizer Inc., New York, N.Y.). Data in Fig. 1 provide further perspective on the MIC stability of linezolid. Regardless of the resistant populations examined, linezolid MIC distributions remained nearly identical, with no indication of any upward shift that could be indicative of a mounting mutation burden. With vancomycin as a comparator, it is also interesting to note the absence of any substantial upward MIC shift for this agent that might indicate an increasing frequency of S. aureus vancomycin-heteroresistant or vancomycin-intermediate strains (10).
Linezolid nonsusceptibility has rarely been encountered among CNS in previous surveillance studies (1, 8, 19, 20), while in the current study, 1 isolate out of 496 (0.2%) had a nonsusceptible phenotype (MIC = 32 μg/ml). The fact that this phenotype is a rare finding was supported by the MIC analysis. The linezolid MIC90 of 1 μg/ml was one doubling dilution lower than that for S. aureus and one doubling dilution lower than that reported in earlier surveillance initiatives (1, 20). Also, the MIC distribution of linezolid among CNS did not vary with resistance to other agents (Fig. 2). Furthermore, in contrast to S. aureus, the linezolid MIC distributions for CNS trended lower than did those for vancomycin. The one vancomycin-intermediate CNS strain encountered (S. haemolyticus; MIC = 8 μg/ml) was susceptible to linezolid. Overall, no upward trend in vancomycin MICs was observed for CNS.
While obvious differences continue to be noted between E.faecalis and E. faecium with regard to ampicillin and vancomycin resistance rates, the levels of linezolid activity have remained comparable and high for these two species. Linezolid susceptibility rates were above 95% for both. While this level of susceptibility is high, it did represent a decrease in susceptibility from that observed in an early surveillance study that examined strains of enterococci collected between 1998 and 2000 in which no resistance was reported (20). In this current study, intermediate and resistant strains were more common among E. faecium than among E. faecalis. Sporadic previous reports of linezolid resistance among enterococci have also involved E. faecium more commonly than E. faecalis, though resistance has been reported for both species (5, 6, 15, 19, 23). However, there is no clear evidence to suggest that E. faecium has certain underlying genetic or physiological characteristics that would predispose this species over E. faecalis to oxazolidinone resistance. Although molecular typing was not done to determine the potential clonality of the two strains from the same institution, this was a possibility, as the nosocomial spread of linezolid- and vancomycin-resistant E. faecium has been previously described (6).
In any case, the results demonstrated that linezolid nonsusceptibility remains a sporadic and uncommon occurrence among enterococci. Furthermore, the MIC distributions appeared stable for both species. Finally, there was no detectable upward MIC shift for either species, which suggested that E.faecium does not appear to have an increased mutation burden relative to E. faecalis (Fig. 3).
Of practical importance, with regard to the linezolid MIC distributions for enterococci, was that the MIC90 is at the current CLSI susceptible breakpoint of ≤2 μg/ml (2). Therefore, any artifacts generated as a result of technical interpretations, susceptibility testing materials, or testing systems that falsely increase the MIC reading by 1 dilution would result in what Livermore has termed “artifactual resistance,” or false nonsusceptibility (11). That this can and does occur has been reported previously (11, 12; D. F. Sahm, D. C. Draghi, R. S. Blosser, P. A. Hogan, and D. J. Sheehan, Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. C-320, 2005). Careful review of any laboratory result indicates that linezolid nonsusceptibility should be carefully scrutinized, especially given that such a phenotype was uncommon.
Linezolid resistance among S. pneumoniae strains has been rarely reported and was not encountered among the 422 isolates tested in this surveillance study (1, 4, 8, 15, 20). The MIC90 of 1 μg/ml was the same as that obtained with isolates tested from 1998 to 2000, and there was no upward shift in MIC distributions, regardless of the populations analyzed (20).
Prior to this current report, an intensive analysis of the in vitro activity of linezolid against key gram-positive pathogens, including those expressing problematic resistance phenotypes for other antimicrobial classes, had not been examined in over 4 years. Since that time, sporadic cases of resistant staphylococci and enterococci have been reported, as have more basic insights into the molecular mechanisms associated with linezolid resistance development. Analysis of the data generated through the LEADER 2004 initiative has demonstrated that staphylococcal nonsusceptibility remains rare and that enterococcal resistance is both uncommon and sporadic. Nonetheless, careful and ongoing monitoring of the in vitro effectiveness of linezolid is needed so that any changes contrary to these projections may be detected as soon as possible. To this end, the MIC profiles established through LEADER 2004 will serve as a useful benchmark to detect any MIC shifts that may occur longitudinally as clinical experience with linezolid continues to grow.
Acknowledgments
This study was funded by Pfizer Inc., Pfizer Global Pharmaceuticals, New York, N.Y.
REFERENCES
- 1.Ballow, C. H., R. N. Jones, D. J. Biedenbach, and the North American ZAPS Research Group. 2002. A multicenter evaluation of linezolid antimicrobial activity in North America. Diagn. Microbiol. Infect. Dis. 43:75-83. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement, vol. 25, no. 1. M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 3.Diekema, D. J., B. J. BootsMiller, T. E. Vaughn, R. F. Woolson, J. W. Yankey, E. J. Ernst, S. D. Flach, M. M. Ward, C. L. J. Franciscus, M. A. Pfaller, and B. N. Doebbeling. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin. Infect. Dis. 38:78-85. [DOI] [PubMed] [Google Scholar]
- 4.Farrell, D. J., I. Morrissey, S. Bakker, S. Buckridge, and D. Felmingham. 2004. In vitro activities of telithromycin, linezolid, and quinupristin-dalfopristin against Streptococcus pneumoniae with macrolide resistance due to ribosomal mutations. Antimicrob. Agents Chemother. 48:3169-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 6.Herrero, I. A., N. C. Issa, and R. Patel. 2002. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. N. Engl. J. Med. 346:867-869. [DOI] [PubMed] [Google Scholar]
- 7.Hershberger, E., S. Donabedian, K. Konstantinou, and M. J. Zervos. 2004. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin. Infect. Dis. 38:92-98. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., C. H. Ballow, D. J. Biedenbach, and the ZAPS Study Group Medical Centers. 2001. Multi-laboratory assessment of the linezolid spectrum of activity using the Kirby-Bauer disk diffusion method: report of the Zyvox Antimicrobial Potency Study (ZAPS) in the United States. Diagn. Microbiol. Infect. Dis. 40:59-66. [DOI] [PubMed] [Google Scholar]
- 9.Karlowsky, J. A., C. Thornsberry, M. E. Jones, A. T. Evangelista, I. A. Critchley, and D. F. Sahm. 2003. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin. Infect. Dis. 26:963-970. [DOI] [PubMed] [Google Scholar]
- 10.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 47:3040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore, D. M. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 51(Suppl. 2):ii9-ii16. [DOI] [PubMed] [Google Scholar]
- 12.Livermore, D. M., S. Mushtaq, and M. Warner. 2001. Susceptibility testing with linezolid by different methods, in relation to published ‘general breakpoints’. J. Antimicrob. Chemother. 48:452-454. [DOI] [PubMed] [Google Scholar]
- 13.Lobritz, M., R. Hutton-Thomas, S. Marshall, and L. B. Rice. 2003. Recombination proficiency influences frequency and locus of mutational resistance to linezolid in Enterococcus faecalis. Antimicrob. Agents Chemother. 47:3318-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall, S. H., C. J. Donskey, R. Hutton-Thomas, R. A. Salata, and L. B. Rice. 2002. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents. Chemother. 46:3334-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meka, V. G., and H. S. Gold. 2004. Antimicrobial resistance to linezolid. Clin. Infect. Dis. 39:1010-1015. [DOI] [PubMed] [Google Scholar]
- 16.Meka, V. G., S. K. Pillai, G. Sakoulas, C. Wennersten, L. Venkataraman, P. C. DeGirolami, G. M. Eliopoulos, R. C. Moellering, Jr., and H. S. Gold. 2004. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J. Infect. Dis. 190:311-317. [DOI] [PubMed] [Google Scholar]
- 17.Meka, V. G., H. S. Gold, A. Cooke, L. Venkataraman, G. M. Eliopoulos, R. C. Moellering, Jr., and S. G. Jenkins. 2004. Reversion to susceptibility in a linezolid-resistant clinical isolate of Staphylococcus aureus. J. Antimicrob. Chemother. 54:818-820. [DOI] [PubMed] [Google Scholar]
- 18.Moellering, R. C., Jr. 2003. Linezolid: the first oxazolidinone antimicrobial. Ann. Intern. Med. 138:135-142. [DOI] [PubMed] [Google Scholar]
- 19.Mutnick, A. H., V. Enne, and R. N. Jones. 2003. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 37:769-774. [DOI] [PubMed] [Google Scholar]
- 20.Mutnick, A. H., D. J. Biedenbach, J. D. Turnidge, and R. N. Jones. 2002. Spectrum and potency evaluation of a new oxazolidinone, linezolid: report from the SENTRY Antimicrobial Surveillance Program, 1998-2000. Diagn. Microbiol. Infect. Dis. 43:65-73. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed., vol. 23, no. 2. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.NNIS System. 2003. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498. [DOI] [PubMed] [Google Scholar]
- 23.Pai, M. P., K. A. Rodvold, P. C. Schreckenberger, R. D. Gonzales, J. M. Petrolatti, and J. P. Quinn. 2002. Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 35:1269-1272. [DOI] [PubMed] [Google Scholar]
- 24.Pillai, S. K., G. Sakoulas, C. Wennersten, G. M. Eliopoulos, R. C. Moellering, M. J. Ferraro, and H. S. Gold. 2002. Linezolid resistance in Staphylococcus aureus: characterization and stability of resistant phenotype. J. Infect. Dis. 186:1603-1607. [DOI] [PubMed] [Google Scholar]
- 25.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database—USA. Clin. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 27.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, Jr., and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 29.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]