Abstract
This study demonstrates for the first time the in vitro transfer of the erythromycin resistance gene erm(B) between two obligate anaerobes, the human spore-forming pathogen Clostridium difficile and the rumen commensal Butyrivibrio fibrisolvens, suggesting that this event might occur also in the natural environment.
Antibiotic resistance genes commonly reside on transmissible plasmids or on other mobile genetic elements which allow the horizontal transfer of these genes between strains, species, and even genera of the normal microflora as well as between pathogens of human and animal origin (6, 18, 24). It is known that antibiotics used in human and veterinary medicine may increase the selective pressure on bacterial populations, which could result in an increase of resistant bacteria (4, 12). The human and animal gastrointestinal tract is a good environment for horizontal gene transfer events (22).
rRNA methylation is the most frequent mechanism of resistance to macrolide-lincosamide-streptogramin B antibiotics in gram-positive bacteria (21). The most widely distributed erm gene class is erm(B), which is present in a large range of hosts, including Clostridium difficile (20, 21). C. difficile is a spore-forming gram-positive bacillus, an opportunistic pathogen that is responsible for many cases of antibiotic-associated diarrhea in humans and animals, and it has been recognized as one of the major causes of nosocomial diarrheic diseases (3, 26). C. difficile strains are frequently resistant to macrolides, and this resistance seems to be associated with strains more prone to cause epidemics (13). C. difficile ErmB resistance determinants can show different genetic arrangements and alleles (8, 27, 28).
Butyrivibrio fibrisolvens is one of the most abundant bacteria isolated from the rumen and has also been identified in the human gastrointestinal tract (29). It is a small, motile, curved rod with tapered ends that analysis of both cell wall structure and 16S rRNA gene sequences indicate it is gram positive (5), although it is currently classified as gram negative. Previous studies demonstrated that some tetracycline resistance determinants could be transferred, under laboratory conditions, from different rumen and human microorganisms to B. fibrisolvens (11, 15).
In this study, we examined the possibility of the erm(B) gene transfer between C. difficile clinical isolates 630, C191, F17, and CD51 harboring different ErmB determinants and B. fibrisolvens strains.
Relevant characteristics of the bacterial strains used in this study are listed in Table 1. The media used in mating experiments were the M2GSC (16) and the RGM (10) broths. Conjugation assays were performed as previously described (23), with the following specific modifications. Donor and recipient bacteria were grown to mid-exponential phase (optimal optical densities at 600 nm [OD600] of 0.3 and 0.4 for C. difficile and B. fibrisolvens, respectively), mixed in a final ratio of 1:1, spread on a sterile nitrocellulose 0.45-μm-pore-size filter on a blood agar (BA) plate (supplemented with 0.1% hemin and 0.1% vitamin K), and incubated for 18 h in an anaerobic cabinet at 35°C. All media were supplemented with 10 μg/ml of tetracycline, 20 μg/ml of erythromycin, and 50 μg/ml of rifampin, as appropriate for each strain. Cycloserine-cefoxitin-fructose-agar (CCFA) (Oxoid, Limited Basingstoke, Hampshire, England), containing 5% egg yolk, was used to discriminate between B. fibrisolvens and C. difficile strains, when necessary.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain | Resistance phenotypea | Source or reference |
|---|---|---|
| C. difficile | ||
| CD51 | Emr, Tcs, Rifs | This study |
| 630 | Emr, Tcr, Rifs | 30 |
| C191 | Emr, Tcr, Rifs | 27 |
| F17 | Emr, Tcr, Rifs | 27 |
| CD37 | Ems, Tcs, Rifr | 25 |
| B. fibrisolvens | ||
| 2221R | Ems, Tcs, Rifr | 1 |
| 1.230 | Ems, Tcr, Rifs | 23 |
| B. fibrisolvens transconjugants | ||
| RE1 | Emr, Tcs, Rifr | This study (CD51 × 2221R) |
| bTE1 | Emr, Tcr, Rifs | This study (RE1 × 1.230) |
Emr, erythromycin resistant; Ems, erythromycin sensitive; Tcr, tetracycline resistant; Tcs, tetracycline sensitive; Rifr, rifampicin resistant; Rifs, rifampicin sensitive.
Transfer mating results between C. difficile and B. fibrisolvens are shown in Table 2. A successful transfer of erythromycin resistance to the recipient B. fibrisolvens 2221R (Rifr) was obtained using C. difficile CD51 (Emr) as the donor, whereas all attempts to transfer the erythromycin resistance from strains C. difficile 630, C191, and F17 were unsuccessful, even if erythromycin resistance transfer from C. difficile 630 to another two gram-positive bacteria has been previously demonstrated (9, 17). Erythromycin-resistant transconjugants from the mating between C. difficile CD51 and B. fibrisolvens 2221R were obtained in each experiment at average frequencies of 4.7 × 10−8 per donor and 4.6 × 10−7 per recipient, respectively. Onward transfer of erythromycin resistance determinants from B. fibrisolvens RE1 (Rifr, Emr) to B. fibrisolvens 1.230 (Tcr) was also obtained, with average transfer frequencies of erythromycin resistance of 6.4 × 10−4 per donor and 3.6 × 10−6 per recipient, respectively. In these mating experiments, we also observed the tetracycline resistance transfer from the recipient strain B. fibrisolvens 1.230 to the donor B. fibrisolvens RE1. The transconjugants that had acquired the erythromycin or the tetracycline resistance had a different colony morphology and were also identifiable by API rapid ID32A enzymatic test strips (Biomérieux, Marcy l'Etoile, France) for the production of arginine, glycine, and histidine arylamidase by B. fibrisolvens 2221R and by 16S rRNA sequencing (2). The onward transfer of an erm(B) determinant among B. fibrisolvens strains indicates the possibility for the spread of erythromycin resistance among B. fibrisolvens populations in vivo and the potential role of these bacteria as a reservoir of antibiotic resistance genes.
TABLE 2.
Frequency of erythromycin resistance transfer between C. difficile and B. fibrisolvens strains
| Donor strain | Recipient strain | Avg transfer frequency
|
No. of replicates | Progeny saved for further study | |
|---|---|---|---|---|---|
| Per donor | Per recipient | ||||
| CD51 | 2221R | 4.8 × 10−7 | 4.6 × 10−7 | 3 | RE1-RE9 |
| RE1 | 1.230 | 6.4 × 10−4 | 3.6 × 10−5 | 3 | bTE1-bTE9 |
| CD51 | 1.230 | 4.2 × 10−6 | 3.3 × 10−7 | 3 | TE1-TE9 |
Transfer of erythromycin resistance was also obtained between C. difficile CD51 and B. fibrisolvens 1.230, with average frequencies of 4.2 × 10−6 per donor and 3.6 × 10−7 per recipient, respectively. The transfer of the C. difficile CD51 erm(B) determinant to the two different B. fibrisolvens strains, 2221R and 1.230, indicates that it is not influenced by the rapid autoaggregation shown by B. fibrisolvens 2221R (19, 23).
All B. fibrisolvens transconjugants showed high levels of erythromycin resistance (MIC > 256 μg/ml), similar to the donor strain C. difficile CD51. The stability of this resistance was confirmed by replating selected colonies on supplemented BA plates containing or lacking erythromycin (20 μg/ml).
Erythromycin resistance transfer was confirmed in all transconjugants by PCR, using primers ermB1 (5′-CTCAAAACTTTTTAACGAGTG) and ermB2 (5′-CCTCCCGTTAAATAATAGATA) to amplify a 711-bp fragment of erm(B). PCR conditions consisted of 30 cycles of 30 s at 94°C, 1 min at 50°C, and 1 min at 72°C.
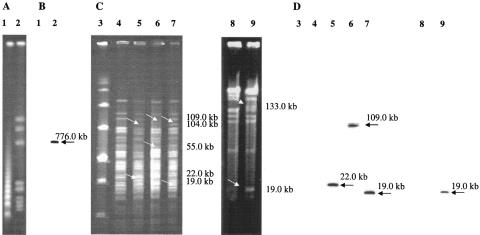
The genomic DNA of C. difficile CD51 and of 27 selected B. fibrisolvens transconjugants (Table 2) were also examined by pulsed-field gel electrophoresis (PFGE) after digestion with SmaI and by hybridization assays. PFGE was performed using a CHEF-Mapper apparatus (Bio-Rad Laboratories) at 6 V/cm for 22 h at 14°C, with an included angle of 120° and linear ramping from 5 to 70 s for C. difficile and from 0.05 to 10 s for B. fibrisolvens. The PCR fragment obtained from the erm(B) amplification was used as probe. When the SmaI-digested C. difficile CD51 genome (Fig. 1A) was hybridized with the erm(B) probe, one hybridizing band at about 770.0 kb was observed (Fig. 1B). The nine B. fibrisolvens 2221R transconjugants showed three different PFGE patterns: the first pattern differed from that of the recipient strain for two additional bands at 22 kb and 104 kb and the loss of one band at 89 kb, the second for two additional bands at 109 kb and 55 kb and the loss of one band at 129 kb, and the third for two additional bands at 19 kb and 104 kb and the loss of one band at 89 kb (Fig. 1C). Three transconjugants showed the first pattern and had an erm(B) hybridizing band at 22 kb, only one transconjugant showed the second pattern and had a hybridizing band at 109 kb, and five transconjugants showed the third pattern and had a band at 19 kb (Fig. 1D). The 18 B. fibrisolvens 1.230 transconjugants showed only one PFGE pattern with two additional bands at 133 kb and 19 kb and the loss of a band at 152 kb (Fig. 1C), regardless of the donor strain (CD51 or RE1). All showed an erm(B) hybridizing band at 19 kb (Fig. 1D). PFGE and hybridization results indicated the acquisition of about 35 kb of chromosomal DNA from the donor in all the examined transconjugants and suggest that the ErmB determinant has a preferred insertion site in the B. fibrisolvens chromosome. Since B. fibrisolvens 1.230 carried tetracycline resistance determinant tet(W) (1), the 18 selected transconjugants derived from this strain were also digested with ApaI, as previously described by Scott et al. (23), to examine this determinant. As already observed, B. fibrisolvens 1.230 showed a tet(W) hybridizing band at 134 kb, whereas the transconjugants showed a band at 120 kb (data not shown). Furthermore, all the transconjugants had an erm(B) hybridizing fragment at 44 kb (data not shown). These results suggest that the insertion site of the ErmB determinant in the B. fibrisolvens 1.230 chromosome is located in proximity of the tet(W) gene, so the insertion of this determinant could introduce a new ApaI restriction site changing the size of tet(W) hybridizing fragments in the B. fibrisolvens 1.230 transconjugants.
FIG. 1.
(A and B) PFGE of C. difficile CD51 genomic DNA digested with SmaI and hybridization assay with the erm(B) probe, respectively. Lane 1, Lambda ladder PFG marker (New England Biolabs, Hitchin, Hertfordshire, United Kingdom); lane 2, C. difficile CD51. (C and D) PFGE of B. fibrisolvens transconjugant genomic DNAs digested with SmaI and hybridization assay with the erm(B) probe, respectively. Lane 3, low-range PFG marker (New England Biolabs, Hitchin, Hertfordshire, United Kingdom); lane 4, B. fibrisolvens 2221R; lanes 5 to 7, B. fibrisolvens 2221R transconjugants; lane 8, B. fibrisolvens 1.230; lane 9, B. fibrisolvens 1.230 transconjugant. The acquired and the hybridizing DNA fragments in B. fibrisolvens transconjugant chromosomes are indicated with arrows, and the relative sizes in kilobase pairs are reported at the side of the figure.
No transfer of erythromycin resistance was obtained when B. fibrisolvens transconjugants TE1 and RE1 (Table 2) were used as donors and C. difficile CD37 (Rifr, Tcs, Ems) as recipient. The basis of this result is not clear, but the mechanism of the ErmB element transposition, its intermediate form, and the structure of the B. fibrisolvens donor strain and that of the C. difficile recipient strain cell wall could be implicated.
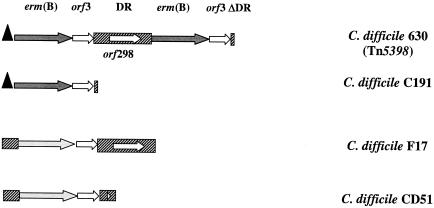
The C. difficile CD51 ErmB determinant showed a new genetic arrangement, named E5 (28), that was also confirmed in all B. fibrisolvens transconjugants (data not shown). The best known element carrying an erm(B) determinant in C. difficile is Tn5398, which has been found in C. difficile 630 (Fig. 2) (8). PCR mapping based on the nucleotide sequence of this element indicated that C. difficile C191, F17, and CD51 ErmB determinants are not carried by Tn5398-like elements (28). The sequence analyses of the C. difficile 630, C191, and F17 ErmB determinants were performed in previous studies (7, 27), and their genetic context is shown in Fig. 2. To compare the ErmB determinants of the different C. difficile isolates used as donor strains, in this study we also completed the characterization of the C. difficile CD51 ErmB determinant. This isolate harbors one copy of the erm(B) gene, visualized as a hybridizing band at 1.5 kb when the genomic DNA digested with Sau3A (7, 27) is hybridized with the erm(B) probe (data not shown). Using the primers designed by Farrow et al. (8), we were able to amplify and sequence a DNA fragment of 1,391 base pairs in length containing the C. difficile CD51 ErmB determinant (EMBL database accession number AJ968665). Sequence analysis demonstrated that this region is 100% identical to that of the ErmB determinant of Arcanobacterium pyogenes OX-7 (between base 1640 and base 3023; GenBank accession no. AY334073) (14). The C. difficile CD51 erm(B) allele, identical to that of C. difficile F17, is not preceded by a leader peptide sequence and is followed by orf3 and by a partial direct repeat sequence due to a deletion of 947 bp (Fig. 2). Further experiments will be performed to complete a C. difficile CD51 erythromycin resistance element characterization and to investigate its mechanism of transfer.
FIG. 2.
Schematic representation of the ErmB determinant genetic arrangement in the different C. difficile isolates used in this study. The representation is based on the nucleotide sequence of the different ErmB determinants (C. difficile 630, GenBank accession number AF109075; C. difficile C191, GenBank accession number AJ294530; C. difficile F17, GenBank accession number AJ294529; C. difficile CD51, EMBL accession number AJ968665). The approximate extent and organization of the determinants are not necessarily to scale. Regions of nucleotide sequence similarity are indicated by the same shading. The arrows indicate the individual open reading frames and their respective direction of transcription. The two erm(B) variants are indicated by the different color of the arrows. The leader peptide sequence is represented by a black triangle. DR, direct repeat.
The data presented in this paper provide the first evidence of the erythromycin resistance transfer between the human pathogen C. difficile and the rumen commensal B. fibrisolvens by conjugation in vitro, supplying additional proof that the resistance gene horizontal transfer among gastrointestinal anaerobic microorganisms could involve bacteria belonging to different ecosystems and normally found in different hosts.
Nucleotide sequence accession number.
The DNA sequence of the C. difficile CD51 ErmB determinant was submitted to the EMBL database under accession no. AJ968665.
Acknowledgments
This work was partially supported by the European Community's Fifth Framework Programme “Quality of Life and Management of Living Resources,” Contract no. QLK2-CT-2002-00843-ARTRADI.
We are indebted to Karen Scott (Rowett Research Institute, Bucksburn, Aberdeen, United Kingdom) for constructive comments and suggestions and for supplying B. fibrisolvens strains. We also thank Peter Mullany (Eastman Dental Institute for Oral Health Care Sciences, University College London, United Kingdom) for C. difficile CD37. We are grateful to Tonino Sofia for editing the manuscript.
REFERENCES
- 1.Barbosa, T. M., K. P. Scott, and H. J. Flint. 1999. Evidence for recent intergeneric transfer of a new tetracycline resistance gene, tet(W), isolated from Butyrivibrio fibrisolvens, and the occurence of tet(O) in ruminal bacterial. Environ. Microbiol. 1:53-64. [DOI] [PubMed] [Google Scholar]
- 2.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., N. Moon, T. W. Chang, N. Taylor, and A. B. Onderdonk. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778-782. [PubMed] [Google Scholar]
- 4.Casewell, M., C. Friis, E. Marco, P. McMullin, and I. Phillips. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52:159-161. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, K.-J., and J. W. Costerton. 1977. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium? J. Bacteriol. 129:1506-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Cruz, F., and J. Davies. 2000. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 8:128-133. [DOI] [PubMed] [Google Scholar]
- 7.Farrow, K. A., D. Lyras, and J. I. Rood. 2000. The macrolide-lincosamide-streptogramin B resistance determinant from Clostridium difficile 630 contains two erm(B) genes. Antimicrob. Agents Chemother. 44:411-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrow, K. A., D. Lyras, and J. I. Rood. 2001. Genomic analysis of the erythromycin resistance element Tn5398 from Clostridium difficile. Microbiology 147:2717-2728. [DOI] [PubMed] [Google Scholar]
- 9.Hachler, H., B. Berger-Bachi, and F. H. Kayser. 1987. Genetic characterization of a Clostridium difficile erythromycin-clindamycin resistance determinant that is transferable to Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hespell, R. B., R. Wolf, and R. J. Bothast. 1987. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl. Environ. Microbiol. 53:2849-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hespell, R. B., and T. R. Whitehead. 1991. Conjugal transfer of Tn916, Tn916AE, and pAMR1 from Enterococcus faecalis to Butyrivibrio fibrisolvens strains. Appl. Environ. Microbiol. 57:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosein, I. K., D. W. Hill, L. E. Jenkins, and J. T. Magee. 2002. Clinical significance of the emergence of bacterial resistance in the hospital environment. J. Appl. Microbiol. 92(Suppl.):90S-97S. [PubMed] [Google Scholar]
- 13.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. Rood, P. De Girolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 14.Jost, B. H., H. T. Trinh, J. G. Songer, and S. J. Billington. 2004. A second tylosin resistance determinant, ErmB, in Arcanobacterium pyogenes. Antimicrob. Agents Chemother. 48:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville, C. M., K. P. Scott, D. K. Mercer, and H. J. Flint. 2001. Novel tetracycline resistance gene, tet(32), in the Clostridium-related human colonic anaerobe K10 and its transmission in vitro to the rumen anaerobe Butyrivibrio fibrisolvens. Antimicrob. Agents Chemother. 45:3246-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki, K., J. C. Martin, R. Marinsek-Logar, and H. J. Flint. 1997. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii B14. Anaerobe 3:373-381. [DOI] [PubMed] [Google Scholar]
- 17.Mullany, P., M. Wilks, I. Lamb, C. Clayton, B. Wren, and S. Tabaqchali. 1990. Genetic analysis of a tetracycline resistance element from Clostridium difficile and its conjugal transfer to and from Bacillus subtilis. J. Gen. Microbiol. 136:1343-1349. [DOI] [PubMed] [Google Scholar]
- 18.Oppegaard, H., T. M. Steinum, and Y. Wasteson. 2001. Horizontal transfer of a multi-drug resistance plasmid between coliform bacteria of human and bovine origin in a farm environment. Appl. Environ. Microbiol. 67:3732-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reniero, J., P. Cocconcelli, V. Bottazzi, and L. Morelli. 1992. High frequency of conjugation in Lactobacillus mediated by an aggregation-promoting factor. J. Gen. Microbiol. 138:763-768. [Google Scholar]
- 20.Roberts, M. C. 1995. Distribution of tetracycline and macrolide-lincosamide-streptogramin B resistance genes in anaerobic bacteria. Clin. Infect. Dis. 20(Suppl. 2):S367-S369. [DOI] [PubMed] [Google Scholar]
- 21.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott, K. P. 2002. The role of conjugative transposons in spreading antibiotic resistance between bacteria that inhabit the gastrointestinal tract. Cell Mol. Life Sci. 59:2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott, K. P., T. M. Barbosa, K. J. Forbes, and H. J. Flint. 1997. High-frequency transfer of a naturally occurring chromosomal tetracycline resistance element in the ruminal anaerobe Butyrivibrio fibrisolvens. Appl. Environ. Microbiol. 63:3405-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoemaker, N. B., G. R. Wang, and A. A. Salyers. 1992. Evidence for natural transfer of a tetracycline resistance gene between bacteria from the human colon and bacteria from the bovine rumen. Appl. Environ. Microbiol. 58:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, C. J., S. M. Markowitz, and F. L. Macrina. 1981. Transferable tetracycline resistance in Clostridium difficile. Antimicrob. Agents Chemother. 19:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer, R. C. 1998. Clinical impact and associated costs of Clostridium difficile-associated disease. J. Antimicrob. Chemother. 41(Suppl. C):5-12. [DOI] [PubMed] [Google Scholar]
- 27.Spigaglia, P., and P. Mastrantonio. 2003. Analysis of macrolide-lincosamide-streptogramin B (MLSB) resistance determinant in strains of Clostridium difficile. Microb. Drug Resist. 8:45-53. [DOI] [PubMed] [Google Scholar]
- 28.Spigaglia, P., V. Carucci, F. Barbanti, and P. Mastrantonio. 2005. ErmB determinants and Tn916-like elements from clinical isolates of Clostridium difficile. Antimicrob. Agents Chemother. 49:2550-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart, C. S., H. J. Flint, and M. P. Bryant. 1999. The rumen bacteria, In P. N. Hobson (ed.), The rumen microbial ecosystem, 2nd ed. Elsevier Applied Science, London, United Kingdom.
- 30.Wüst, J., and U. Hardegger. 1983. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob. Agents Chemother. 23:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]