Abstract
WR227825 is an antimalarial pyrroloquinazolinediamine derivative with a high potency but a low therapeutic index. A series of carbamate, carboxamide, succinimide, and alkylamine derivatives of WR227825 were prepared to search for compounds with an improved therapeutic index. The new acetamides and imide showed potent cell growth inhibition against four clones of Plasmodium falciparum (D-6, RCS, W-2, and TM91C235), with a 50% inhibitory concentration of ∼0.01 ng/ml, and were highly active against Plasmodium berghei, with 100% cure at doses from <0.1 mg/kg of body weight to 220 mg/kg. The carbamates and alkyl derivatives, however, showed weak activity against Plasmodium falciparum cell growth but were highly efficacious in tests against P. berghei by the Thompson test. The best compounds, bis-ethylcarbamate (compound 2a) and tetra-acetamide (3a) derivatives, further demonstrated high potency against the sporozoite Plasmodium yoelii in mice and P. falciparum and Plasmodium vivax in aotus monkeys. Against the AMRU-1 strain of P. vivax, which has four dihydrofolate reductase mutations and is highly resistant to antifolates, tetra-acetamide 3a cured the monkeys at doses of 1 and 3 mg/kg. Compound 2a cured only one out of two monkeys at 3 mg/kg. The results indicated that the new derivatives 2a and 3a not only have retained/improved the antimalarial efficacy of the parent compound WR227825 but also were less toxic to the animals used in the tests.
Malaria is one of the most common diseases in countries of Africa, Southeast Asia, and South America (12, 27, 28, 31, 32). The increasing prevalence of multiple-drug-resistant strains of Plasmodium falciparum in most areas where malaria is endemic has significantly reduced the efficacy of current antimalarial drugs for the prophylaxis and treatment of this disease (17, 19). Furthermore, the usefulness of many newer antimalarial drugs was impaired by their side effects. Lethal hemolysis side effects were observed in glucose-6-phosphate dehydrogenase-deficient recipients of 8-aminoquinoline drugs (2, 3), namely, primaquine and tafenoquine. Central nervous system toxicity was a problematic side effect in the patients treated with mefloquine (22, 24). Therefore, there is an eminent need for new and safe antimalarial drugs to combat this disease in areas of malaria endemicity.
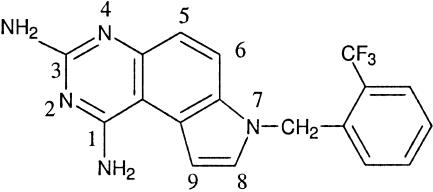
Pyrroloquinazolinediamine (PQZ) derivatives were reported to possess anticancer, antimicrobial, and antimalarial activities (13). Among the PQZ derivatives, WR227825 (compound 1) is one of the most potent antimalarial agents ever reported (26). This compound not only displayed high in vitro efficacy against P. falciparum, with a 50% inhibitory concentration (IC50) of ∼0.01 ng/ml, but was also highly active against Plasmodium berghei in a rodent model, with a 100% curative oral dose of <1 to 20 mg/kg of body weight. However, WR227825 also exhibited high host toxicity, with a subcutaneous 50% lethal dose in mice at less than 20 mg/kg, and produced deaths in aotus monkeys at doses less than 2 mg/kg (33). The PQZ was demonstrated to be an antifolate (15). The low therapeutic index of compound 1 has severely limited its value as an antimalarial agent. Nevertheless, the high efficacy and low therapeutic index of WR227825 make it a challenging lead compound for optimizing the fabrication of new derivatives with improvement in their therapeutic index or pharmacological profiles. In addition to its high host toxicity, the lead compound 1 is sparingly soluble in common organic solvents and water, a highly undesirable physical property for large-scale synthesis and purification (Fig. 1).
FIG. 1.
Chemical structure of the lead compound WR227825.
To overcome the toxicity and solubility problems of the lead molecule, a series of WR227825 derivatives were prepared in this study, wherein the amino groups at positions 1 and 3 were substituted to render the new carbamate, carboxamide, succinimide, or alkyamine derivatives. The antimalarial efficacy of all new derivatives was first assessed in vitro against four clones of P. falciparum, D-6, RCS, W-2, and TM91C235, followed by the Thompson test against P. berghei in mice. Based on the preliminary test results, two of the most promising compounds, 2a and 3a, were further tested in aotus monkeys against P. falciparum and P. vivax, the latter of which is highly resistant to antifolates. Chemical synthesis and antimalarial activities of these new derivatives are discussed in this report.
MATERIALS AND METHODS
Materials.
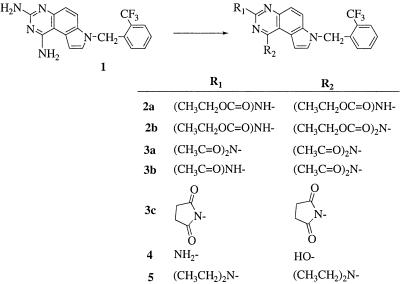
The lead compound WR227825 was prepared by ChemPacific (Baltimore, MD) according to the published procedure (13). New carbamate (compounds 2a and 2b), acetamide (3a and 3b), succinimide (3c), and alkyl (compound 5) derivatives (Fig. 2) were prepared by treating WR227825 (compound 1) with ethyl chloroformate, acetic anhydride, succinic anhydride, and ethyl bromide, respectively. The hydroxy analog 4 was prepared by acid hydrolysis of compound 1 in 6 N hydrochloric acid solution. Detailed information on chemical synthesis and physical properties of the new derivatives can be found in the supplemental material.
FIG. 2.
Chemical structures of pyrroloquinazolinediamine derivatives.
Assessment of antimalarial activities. (i) In vitro antimalarial studies.
The in vitro assays were conducted by using a modification of the semiautomated microdilution technique of Desjardins et al. (7) and Chulay et al. (4). Four P. falciparum malaria parasite clones from the CDC (Indochina III, W-2; Sierra Leone I, D-6), Southeast Asia (TM91C235), and Brazil (RCS) were utilized in susceptibility testing. They were derived by direct visualization and micromanipulation from patient isolates (20). The W-2 clone is susceptible to mefloquine but resistant to chloroquine, sulfadoxine, pyrimethamine, and quinine, whereas the D-6 clone is naturally resistant to mefloquine but susceptible to chloroquine, sulfadoxine, pyrimethamine, and quinine. TM91C235 and RCS are multiple-drug resistance P. falciparum isolates from Southeast Asia and Brazil, respectively. Test compounds were initially dissolved in dimethyl sulfoxide and diluted 400-fold in RPMI 1640 culture medium supplemented with 25 mM HEPES, 32 mM NaHCO3, and 10% Albumax I (Gibco BRL, Grand Island, NY). These solutions were subsequently serially diluted twofold with a Biomek 1000 apparatus (Beckman, Fullerton, CA) over 11 different concentrations. The parasites were exposed to serial dilutions of each compound for 48 h and incubated at 37°C with 5% O2, 5% CO2, and 90% N2 prior to the addition of [3H]hypoxanthine. After a further incubation of 18 h, parasite DNA was harvested from each microtiter well using a Packard Filtermate 196 harvester (Meriden, CT) onto glass filters. The uptake of [3H]hypoxanthine was measured with a Packard TopCount scintillation counter. Concentration-response data were analyzed by a nonlinear regression logistic dose-response model, and the IC50 values for each compound were calculated. The results are shown in Table 1.
TABLE 1.
Antimalarial activity against Plasmodium falciparum cell lines
| Compound no. | IC50 (ng/ml) for parasite clonea:
|
|||
|---|---|---|---|---|
| D-6 | W-2 | RCS | TM91C235 | |
| 1 | 0.0191 | 0.4453 | 0.3569 | 1.2178 |
| 2a | 7.9727 | 127.80 | >250 | >250 |
| 2b | >250 | >250 | >250 | >250 |
| 3a | 0.0236 | 0.0752 | 0.0236 | 0.0845 |
| 3b | 0.0101 | 0.0183 | 0.0116 | 0.0212 |
| 3c | 0.1284 | 0.1284 | 0.2571 | 0.4021 |
| 4 | 27.2403 | 53.9027 | 83.3969 | |
| 5 | 46.033 | 104.0182 | ||
D-6, CDC Sierra Leone I; W-2, CDC Indochina III; RCS, isolates from Brazil; TM91C235, Southeast Asia isolates.
(ii) In vivo antimalarial studies of P. berghei in mice.
The in vivo efficacies of the new compounds were determined by a modified Thompson test (1). This test measures the survivability of mice and parasite clearance following administration of the drug on days 3 to 5 postinfection. In brief, 5 × 106 P. berghei-infected erythrocytes (KBG-173 strain) were inoculated into the intraperitoneal cavity of male mice that weighed 24 to 30 g. By day 3 postinfection, parasitemia ranged from 1.0 to 3.7%. Each drug, suspended in 0.5% hydroxyethylcellulose-0.1% Tween 80, was administered orally (p.o.) once daily from days 3 to 5 postinfection. The volume of drug suspension given depends on the weight of the mouse and the drug concentration of the suspension. In general, the volume is given at 0.01 ml/gram of body weight.
Five mice were used in each dosage group. Blood films were taken on day 6 and biweekly for 31 days. Mice that were blood film negative on day 31 postinfection were considered cured. Compounds were considered active when the survival time of the treated mice was greater than twice that of the control mice (i.e., 12 to 14 days). Mice losing >20% of their body weight were sacrificed. The test results are shown in Table 2.
TABLE 2.
Antimalarial activity against Plasmodium berghei
| Compound no. or drug | Oral dose(s) (mg/kg/day) | No. of mice that died/day of death | No. of mice alive on day 31/total no. of mice |
|---|---|---|---|
| 1 | 40 | 1/15, 1/16 | 3/5 (toxic) |
| 20, 10, 2.5, and 1.25 | None | 5/5 | |
| 2a | 80, 40, 20, 10, and 2.5 | None | 5/5 |
| 1.25 | 1/24, 1/27a | 3/5 | |
| 2b | 80, 40, 20, 10, 2.5, and 1.25 | None | 5/5 |
| 3a | 440 | 2/12, 1/14, 2/15 | 0/5 (toxic) |
| 330 | 1/12, 2/14, 2/16 | 0/5 (toxic) | |
| 220, 120, 40, 10, 2.5, and 0.625 | None | 5/5 | |
| 3b | 220, 120, 40, and 10 | None | 5/5 |
| 2.5 and 0.625 | Death in each groupa | 4/5, 3/5 | |
| 3c | 80 | 2/8, 1/9 | 2/5 |
| 40, 20, and 10 | Death in each groupa | 0/5 | |
| 4 | 320 | 3/16, 1/17 | 1/5 |
| 160, 80, and 40 | None | 5/5 | |
| 20 and 10 | Death in each groupa | 2/5, 1/5 | |
| 5 | 160 | 1/11, 1/12, 1/13, 1/14 | 4/8b |
| 80, 40, 20, and 10 | None | 8/8 | |
| Mefloquine | 256 | 1/7, 1/10 | 3/5 (toxic) |
| 128, 64, and 32 | None | 5/5 | |
| 16 | 1/18, 1/19a | 3/5 | |
| 4 | 1/13, 1/18, 1/19a | 2/5 | |
| 2 | 2/18, 1/21, 1/25a | 1/5 | |
| 1 | 3/10, 1/11, 1/22a | 0/5 |
Death due to malaria.
Separate test (eight mice per dose group).
(iii) Sporozoite induction test.
Mice are inoculated in their intraperitoneal cavities with 250,000 sporozoites of Plasmodium yoelii on day 0. Blood examinations and weights are taken at frequent intervals, at least twice a week. The days of examination relative to day 0 vary to avoid weekend work. This sporozoite induction test (5, 25) is run for a minimum of 31 days. All mice alive on the last day with negative blood films are considered cured. Mice losing >20% of their body weight at any time are sacrificed.
Infected, nontreated negative controls are included in every experiment to validate the infectivity of the sporozoites. While these sporozoites usually produce patent infections, a small number of mice remain blood negative. Caution must be taken when judging a compound as prophylactic when the patency rate in the negative controls is less than 100%.
Positive control groups are included occasionally, and they are treated with either primaquine or tafenoquine.
Routinely, 20,000 red blood cells are examined in a thin blood film before an animal is judged negative. The sensitivity of this technique is 0.01% infected red blood cells. Each compound was ground with a mortar and pestle and suspended in 0.5% hydroxyethylcellulose and 0.1% Tween 80 for compounds to be administered p.o., and those given subcutaneously (s.c.) were suspended in peanut oil. Each compound was prepared at three different dose levels.
Four-week-old male CD-1 mice, purchased from Charles River and weighing about 16 to 17 g, were placed five per cage and allowed to acclimate for 4 days before being treated and then inoculated with sporozoites. They were fed food and water ad lib and maintained at 24°C with 12 h of light and 12 h of darkness. The cages and water bottles were changed biweekly. The mice were weighed on days 0, 3, and 6 and then biweekly when blood films were taken.
Plasmodium yoelii (17XNL) was used to infect mice that would be used to infect the mosquitoes. Compounds were administered once a day for three consecutive days, either p.o. or s.c., to mice on the day before (day −1), 4 h before (day 0), and the day after (day 1) the inoculation of sporozoites into the intraperitoneal cavity with 2.5 × 105 sporozoites of P. yoelii on day 0. Whole-body weights were taken on day 0 and day 6 and then twice a week for 31 days. A blood film was taken on day 6 and then twice a week for 31 days. Mice losing more than about 20% of their body weight were sacrificed. All mice alive on day 31 with no parasites in a blood film were considered cured. A compound was considered active against either the sporozoite or the exoerythrocytic stage if no parasites were found in the blood films taken on day 6 or in subsequent blood films taken weekly for 31 days. A compound was considered to exhibit marginal activity if only low levels of parasites were found (less than about 10%) in blood films taken on day 6 or in any blood films taken biweekly for 31 days. Mice alive on day 31 with no parasites found in any blood films were considered cured.
(iv) Antimalarial activity against P. falciparum in aotus monkey.
Six malaria-naive Aotus lemurinus (male and female; 787 to 978 g) monkeys of karyotypes VIII and IX (14) were housed at the Gorgas Memorial Institute in Panama and cared for and maintained as described by Obaldia (18). They were divided into two groups of two Aotus monkeys each plus two controls and infected with 5 × 106 P. falciparum FVO malaria parasites (1 ml of an appropriate dilution) in RPMI 1640 medium intravenously in the saphenous vein. When their parasitemia reached 5 × 104 parasites/μl, treatment was started. Giemsa-stained thick blood smears were prepared daily with a prick in the ear marginal vein, and parasites were enumerated by the Earle and Perez method (8). Group 1 aotus monkeys received compound 3a at 1 mg/kg once a day for 3 days. Group 2 aotus monkeys received compound 2a at 1 mg/kg once a day for 3 days. The monkeys were weighed and prebled for complete blood count (CBC) and chemistry determination (alanine aminotransferase, blood urea nitrogen [BUN], and creatinine) on day 4 posttreatment (PT). Bleedings for pharmacokinetic determinations were carried out on day 1 PT at time zero, 1 h, 7.5 h, and 24 h PT.
(v) Antimalarial activity against P. vivax in aotus monkey.
Fourteen P. falciparum-cured A. lemurinus monkeys (male and female; 700 to 925 g) were infected intravenously with 5 × 106 parasites of the P. vivax AMRU-1 strain diluted in RPMI 1640 medium obtained from a donor and divided into two larger groups (group I and group II) of six monkeys each (two monkeys per dose level). Group I monkeys and group II monkeys received compounds 3a and 2a, respectively, daily at 0.5, 1.0, and 3 mg/kg/day for a total of 3 days. Two monkeys were left as untreated controls. Treatment started when parasitemia reached 5,000 parasites/μl on day 6 postinoculation. Daily food intake was documented. Daily Giemsa-stained thick blood smears were prepared from a prick in the marginal ear vein, and parasites were enumerated by the Earle and Perez method (8). The animals were weighed at weekly intervals. Blood samples for blood chemistry determination were collected at the following time points: day 0 (CBC and chemistry panel [alanine aminotransferase, creatinine, and BUN]) and day 4 (CBC and chemistry panel). CBC and chemistry panels were done the day prior to the first dose and 24 h after the last dose and at weekly intervals thereafter.
RESULTS
Antimalarial assessment.
A series of carbamate, acetamide, succinimide, and alkyl derivatives of WR227825 and the hydroxy analog 4 were prepared (Fig. 2), and their antimalarial activities were assessed in four clones of P. falciparum cell lines (Table 1) and in a mouse test model against P. berghei (Table 2). The most active compounds, 2a and 3a, were further tested against P. falciparum and P. vivax in aotus monkeys and in the P. yoelii sporozoite-infected mouse model.
Carbamates 2a and 2b showed low in vitro activities against D-6, RCS, W-2, and TM91C235 clones of P. falciparum (Table 1), with an IC50 in the range of 8 to >250 ng/ml, yet retained high in vivo activity against P. berghei (Table 2) in the mouse tests. Both compounds 2a and 2b cured five out of five mice in all dose groups from 2.5 to >80 mg/kg. No significant host toxicity was observed. In the same test, WR227825, the lead compound, showed host toxicity at 40 mg/kg, killing one out of five mice. In contrast to carbamates 2a and 2b, acetamide derivatives 3a and 3b not only exhibited high in vivo antimalarial activity against P. berghei (Table 2) but also were highly potent in vitro against four clones of P. falciparum, with an IC50 of <0.02 ng/ml (Table 1). In the Thompson test, compound 3a produced a 100% cure of mice in all dose groups from 0.625 to 220 mg/kg but exhibited delayed toxicity to all mice at 330 and 440 mg/kg (deaths on days 12 to 16). Compound 3b, though less active than compound 3a, cured more than half (three out of five) of the mice in the low-dose (0.625 mg/kg) groups.
Succinimide 3c showed good cell growth inhibition in all four cell lines, with an IC50 equal to or better than that of the parent drug (compound 1). However, in the Thompson test, compound 3c showed only moderate activity, with no cures in the groups treated with doses of 10 or 40 mg/kg. At a higher dose of 80 mg/kg, two out of five mice showed early death on days 8 and 9, indicating host toxicity at this high-dose level.
Both 1-hydroxy analog 4 and tetraethyl derivative 5 were more than 1,000-fold less active than the lead compound WR227825 in an in vitro test against the cell growth of P. falciparum isolates (Table 1) yet were highly potent against P. berghei in a mouse test (Table 2). Compound 4 cured five out of five mice in doses from 80 to 160 mg/kg. Deaths due to toxicity were observed at a higher dose level of 320 mg/kg. Tetraethyl derivative 5 produced 100% cure at doses ranging from 10 to 80 mg/kg. Host toxicity became obvious at the higher dose of 160 mg/kg, killing four out of eight mice. Compound 5 was tested in a separate experiment using eight instead of five mice per dose group.
During testing against P. falciparum in aotus monkeys, both compounds 2a and 3a cleared parasitemia by day 3 at an oral dose of 1 mg/kg, and no recrudescence was observed for >100 days. Against P. vivax, which has four dihydrofolate reductase (DHFR) mutations and is highly resistant to antifolates, such as pyrimethamine and cycloguanil, compound 3a cured aotus monkeys at 1 and 3 mg/kg/day p.o for 3 days. Compound 2a was, however, less active at the same doses, with only one out of two monkeys cured at 3 mg/kg and none cured at 0.5 and 1 mg/kg (Table 3).
TABLE 3.
Antimalarial activities against P. falciparum and P. vivax in aotus monkeys
| Compound | Dose (mg/kg) | No. of monkeys cured/total no. of monkeysa
|
|
|---|---|---|---|
| P. vivax | P. falciparum | ||
| 2a | 0.5 | 0/2 | |
| 1 | 0/2 | 2/2 | |
| 3 | 1/2 | ||
| 3a | 0.5 | 0/2 | |
| 1 | 2/2 | 2/2 | |
| 3 | 2/2 | ||
Cured monkeys stayed parasite free for 100 days PT.
Against P. yoelii in sporozoite-challenged mice, compound 3a completely prevented the development of parasitemia at doses ranging from 0.65 to 40 mg/kg for 3 days by oral administration (Table 4). In this test, tafenoquine (21, 29, 30), a new malaria prophylactic drug, was used as a positive control. Compound 3a showed 100% protection of the mice at a dosage as low as 0.625 mg/kg compared to a dosage of 5 mg/kg for tafenoquine in the same test.
TABLE 4.
Antimalarial activity of compound 3a against P. yoelii
| Compound no. or drug | Oral dose (mg/kg) | No. of mice cured/total no. of mice |
|---|---|---|
| 3a | 40 | 5/5 |
| 10 | 5/5 | |
| 2.5 | 5/5 | |
| 0.625 | 5/5 | |
| 0.3125 | 4/5 | |
| 0.15625 | 4/5 | |
| Tafenoquine | 10 | 5/5 |
| 5 | 5/5 | |
| 2.5 | 4/5 | |
| 1.25 | 2/5 |
Preliminary toxicological studies in aotus monkeys were carried out using the same species of monkeys as in the efficacy studies. Two monkeys were used per group. The results indicated that no changes in behavior, weight, or blood chemistry were observed when monkeys were treated with a single oral dose (3 mg/kg) of 2a or 3a. No adverse effects regarding anorexia, vomiting, diarrhea, or behavior changes were observed during a 28-day observation period. No significant changes in body weight were observed. CBC parameters remained within normal ranges. A steady decrease of the hepatic enzyme serum glutamic-pyruvic transaminase from 75.3 to 17.7 units and a decrease of BUN from 24.35 to <10.00 units were observed in one monkey during the observation period. However, the monkey did not present any gastrointestinal or renal abnormal symptoms, although these parameters are indicative of a decrease in hepatic function.
DISCUSSION
The antimalarial efficacies of the new compounds were evaluated against the cell growth of four P. falciparum isolates, D-6, W-2, RCS, and TM91C235, and by the Thompson test against P. berghei. Two of the compounds, 2a and 3a, were tested further in aotus monkey against P. falciparum and P. vivax. The structure-activity relationship studies indicated that carbamylation or alkylation of WR227825 (compounds 2a, 2b, and 5) resulted in a dramatic reduction in cell growth inhibition against clones of P. falciparum from several hundredfold to more than 1,000-fold. Conversion of the 3-amino group to hydroxy analog 4 likewise decreased the in vitro antimalarial activity. In contrast, acetamide and succinimide derivatives (compounds 3a, 3b, and 3c) retained or increased the cell growth inhibitory activity in all four isolates of P. falciparum, D-6, W-2, RCS, and TM91C235. Nevertheless, all five classes of derivatives prepared in this study exhibited strong antimalarial activity in the Thompson test against P. berghei (Table 2). Ethyl carbamates 2a and 2b cured five out of five mice treated with doses from 2.5 mg/kg to 80 mg/kg. Acetamide derivative 3a cured 100% of mice groups treated with doses ranging from 0.625 to 220 mg/kg. Toxicity was found at doses of 330 and 440 mg/kg. Succinimide 3c, although it showed potent in vitro activity, is the least active in the Thompson test against P. berghei among the compounds studied, suggesting a bioavailability problem of oral administration. Further structure-activity relationship studies led to the synthesis of 1-hydroxy-3-amino analog of WR227825, compound 4, an analog of compound 1 with the 1-amino group replaced with a hydroxy group. As expected, compound 4 showed no inhibitory activity against cell growth in an in vitro test, since both amino groups at positions 1 and 3 of the lead molecule WR227825 are essential for the inhibition of DHFR (15). Surprisingly, compound 4 showed potent antimalarial activity in animal studies. It cured 100% of mice infected with P. berghei at an oral dose range of 80 to 180 mg/kg (Table 2). Poor in vitro and good in vivo activity of carbamates 2a and 2b are typical biochemical responses of a prodrug. However, the in vivo antimalarial activity of compound 4 cannot be explained by the prodrug rationale. Since the 1,3-diamino functions in the quinazoline ring of the lead molecule WR227825 are essential for the inhibition of DHFR, which was believed to be the mechanism of action of WR227825, compound 4 may inhibit a target other than DHFR. The identification of active metabolites of compound 4 in rats or monkeys is in progress.
The alkylation of compound 1 gave tetraethylamine (compound 5), which is over 10,000-fold less active than the lead compound 1 against both sensitive and drug-resistant P. falciparum cell lines in vitro (Table 1). However, compound 5 exhibited potent in vivo activity against P. berghei, with curative oral dosages of 10 to 80 mg/kg in the Thompson test (Table 2). Compound 5 is an analog of compound 3a, with the acetyl groups being replaced with chemically stable ethyl substituents. Like the carbamates (2a and 2b), tetraethyl derivative 5 is probably a prodrug of the lead compound 1. However, for compound 5 to act as a prodrug, extensive metabolic dealkylation of compound 5 to compound 1 must take place prior to its antimalarial action.
In contrast to compounds 2a, 2b, 4, and 5, both the acetamides (compounds 3a and 3b) and succinimide 3c showed high antimalarial potency in in vitro and in vivo tests. The results suggest that compounds 3a, 3b, and 3c may possess intrinsic antimalarial activities or that these compounds are unstable chemically, resulting in hydrolysis in the tissue culture to lead compound 1. In the case of hydrolysis, high host toxicity is expected in mice treated with compounds 3a, 3b, and 3c. However, the efficacy and toxicity results support the intrinsic activity contention.
Among the new derivatives, acetamide 3a and carbamate 2a were the most potent compounds, with no obvious signs of toxicity at 220 mg/kg but with toxicity at 330 mg/kg in host animals in Thompson tests against P. berghei, and were, thus, further tested against P. falciparum and P. vivax in aotus monkeys. Against P. falciparum in aotus monkey, both compounds 2a and 3a are equally active and cleared parasitemia by day 3 at an oral dose of 1 mg/kg, and no recrudescence was observed for >100 days. However, compound 3a was more active than compound 2a against a clone of P. vivax which has four DHFR mutations and is highly resistant to antifolates. Compound 3a cured aotus monkeys at both 1 and 3 mg/kg/day p.o for 3 days. However, compound 2a was much less active at the same dose level; only one out of the two monkeys was cured at 3 mg/kg, but no monkeys were cured at 1 mg/kg. If compound 3a also acts as a prodrug of WR227825 as does compound 2a, one would expect both 2a and 3a to be equal in efficacy in the test against P. vivax. This observation plus the fact that acetamides and succinimides are generally very stable chemically and metabolically further suggest that compounds 3a and 3c may possess intrinsic activity and may not simply act as a prodrug of WR227825.
The chemical modifications of WR227825 gave new compounds with not only retained or enhanced efficacy but also much less toxicity than the lead compound. While an oral lethal dose of WR227825 in aotus monkey is less than 2 mg/kg, no changes in behavior, weight, or blood chemistry in aotus monkeys were observed with a single oral dose of compound 2a or 3a at 3 mg/kg. Furthermore, no high-dose-related deaths in mice treated with 80 or 220 mg/kg of compounds 2a and 3a, respectively, were demonstrated by the Thompson test (Table 2).A level of 330 mg/kg of compound 3a was toxic.
The inhibition of DHFR was reported to be the mechanism of action of 1,3-diaminopyrroloquinazolines (15). Cell growth inhibition by 1,3-diaminopyrroloquinazoline derivatives and the analogous diaminoquinazolines or triazines, however, was not reversed by the addition of folinic acid in the cell culture, indicating that this class of compound may also inhibit enzyme targets other than DHFR (16). Since carboxamides and especially acetamides are chemically and enzymatically stable and 1,3-diamino groups of compound 1 are essential for the inhibition of DHFR (6, 9, 10, 11, 23), the extremely high antimalarial activities of acetamides (3a and 3b) in the cell culture as well as in the Thompson test were an unexpected and surprising discovery. Furthermore, the alkylcarbamate and acetamide derivatives (2a and 2b and 3a and 3b) are all substantially less toxic than the parent compound, 1. The results of toxicological and pharmacokinetic studies in rats further indicate that compounds 2a and 3a are superior to the lead compound 1. The results will be published in separate reports.
Supplementary Material
Acknowledgments
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
This research is supported by funding from the Military Infectious Diseases Research Program, U.S. Army Medical Research and Materiel Command, and the U.S. Department of Defense.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Ager, A. L. 1984. Experimental models: rodent malaria models (in vivo), p. 225-254. In W. Peters and W. H. G. Richards (ed.), Handbook of experimental pharmacology: antimalarial drugs, vol. 68. Springer-Verlag, New York, N.Y. [Google Scholar]
- 2.Carson, P. E., C. I. Flanagan, C. E. Ickes, and A. S. Alving. 1956. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science (Washington, D.C.) 124:484-485. [DOI] [PubMed] [Google Scholar]
- 3.Carson, P. E., R. Hohl, M. V. Nora, G. W. Parkhurst, T. Ahmad, S. Scanlan, and H. Frischer. 1981. Toxicology of the 8-aminoquinolines and generic factors associated with their toxicity in man. Bull. W. H. O. 59:427-437. [PMC free article] [PubMed] [Google Scholar]
- 4.Chulay, J. D., J. D. Haynes, and C. L. Diggs. 1983. Plasmodium falciparum: assessment of in vitro growth by [3H]-hypoxanthine incorporation. Exp. Parasitol. 55:138-146. [DOI] [PubMed] [Google Scholar]
- 5.Davidson, D. E., A. L. Ager, J. L. Brown, F. E. Chapple, R. E. Whitmire, and R. N. Rossan. 1981. Recent developments of tissue schizonticidal antimalarial drugs. Bull. W. H. O. 59:463-479. [PMC free article] [PubMed] [Google Scholar]
- 6.Davoll, J., A. M. Johnson, H. J. Davies, O. D. Bird, J. Clarke, and E. F. Elslager. 1972. Folate antagonists. 2. 2,4-Diamino-6-([aralkyl and {heterocyclic}-methyl]amino)quinazolines, a novel class of antimetabolites of interest in drug-resistant malaria and Chagas' disease. J. Med. Chem. 15:812-826. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earle, W. C., and M. Perez. 1932. Enumeration of parasites in the blood of malarial patients. J. Lab. Clin. Med. 17:1124-1130. [Google Scholar]
- 9.Elslager, E. F., M. P. Hutt, P. Jacob, J. Johnson, B. Temporelli, L. M. Werbel, D. F. Worth, and L. Rane. 1979. Folate antagonists. 15. 2,3-Diamino-6-(2-naphthylsulfonyl)-quinazoline and related 2,4-diamino-6-[(phenyl and naphthyl)-sulfinyl and sulfonyl]quinazolines, a potent new class of antimetabolites with phenomenal antimalarial activity. J. Med. Chem. 22:1247-1257. [DOI] [PubMed] [Google Scholar]
- 10.Elslager, E. F., J. Clarke, L. M. Werbel, D. F. Worth, and J. Davoll. 1972. Folate antagonists. 3. 2,4-Diamino-6-(heterocyclic)-quinazolines, a novel class of antimetabolites with potent antimalarial and antibacterial activity. J. Med. Chem. 15:827-836. [DOI] [PubMed] [Google Scholar]
- 11.Genther, C. S., and C. C. Smith. 1977. Antifolate studies. Activities of 40 potential antimalarial compounds against sensitive and chlorguanide triazine resistant strains of folate-requiring bacteria and Escherichia coli. J. Med. Chem. 20:237-243. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg, A. E., and H. O. Lobel. 1990. Mortality from Plasmodium falciparum malaria in travelers from the United States, 1959-1987. Ann. Intern. Med. 113:326-327. [DOI] [PubMed] [Google Scholar]
- 13.Ledig, K. W. October1978. 7-(Substituted)-7H-pyrrolo[3,2,-f]quinazoline-1,3-diamines. U.S. patent 4,118,561.
- 14.Ma, N. S., R. N. Rossan, S. T. Kelly, J. S. Harper, M. T. Bedard, and T. C. Jones. 1978. Banding patterns of the chromosomes of two new karyotypes of the owl monkey, Aotus, captured in Panama. J. Med. Primatol. 7:146-155. [DOI] [PubMed] [Google Scholar]
- 15.McCormack, J. J., A. A. Barbara, K. W. Ledig, and B. L. Hillcoat. 1979. Inhibition of dihydrofolate reductases by derivatives of 2,4-diaminopyrroloquinazoline. Biochem. Pharmacol. 28:3227-3229. [DOI] [PubMed] [Google Scholar]
- 16.Milhous, W. K., N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1985. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob. Agents Chemother. 27:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosten, F., F. Ter Kuile, T. Chongsuphajaisiddhi, C. Luxemburger, H. K. Webster, M. Edstein, L. Phaipun, and N. J. White. 1982. Mefloquine-resistant Falciparum malaria on the Thai-Burmes border. Lancet 337:1140-1143. [DOI] [PubMed] [Google Scholar]
- 18.Obaldia, N., III. 1991. Detection of Klebsiella pneumoniae antibodies in Aotus l. lemurinus (Panamanian owl monkey) using an enzyme linked immunosorbent assay (ELISA) test. Lab. Anim. 25:133-141. [DOI] [PubMed] [Google Scholar]
- 19.Oduola, A. M., W. K. Milhous, L. A. Salako, O. Walker, and R. E. Desjardins. 1987. Reduced in vitro susceptibility to mefloquine in West African isolates of Plasmodium falciparum. Lancet ii:1304-1305. [DOI] [PubMed] [Google Scholar]
- 20.Oduola, A. M., N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1988. Plasmodium falciparum: cloning by single-erythrocyte micromanipulation and heterogeneity in vitro. Exp. Parasitol. 66:86-95. [DOI] [PubMed] [Google Scholar]
- 21.Peters, W. 1999. The evolution of tafenoquine—antimalarial for a new millennium? J. R. Soc. Med. 92:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips-Howard, P. A., and F. O. ter Kuile. 1995. CNS adverse events associated with antimalarial agents: fact or fiction? Drug Saf. 12:370-383. [DOI] [PubMed] [Google Scholar]
- 23.Rosowsky, A., P. C. Huang, N. Papathanasopoulos, and E. J. Modest. 1974. Quinazolines. 12. 1,3-Diaminobenzo(f)quinazolines containing long-chain alkyl or chloro-substituents on the central ring. Synthesis and biological evaluation as candidate antifolate and antimalarial agents. J. Med. Chem. 17:1217-1222. [DOI] [PubMed] [Google Scholar]
- 24.Schlagenhauf, P. 1999. Mefloquine for malaria chemoprophylaxis 1991-1998: a review. J. Travel Med. 6:122-133. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney, T. 1991. A survey of compounds from the antimalarial drug development program of the U.S. Army Medical Research and Development Command, vol. 1. Walter Reed Army Institute of Research, Silver Spring, Md.
- 26.Sweeney, T. R. 1991. Thomas R. Sweeney (TRS) malaria compendium, p. 817, Table 100. WRAIR, Silver Spring, Md.
- 27.Trigg, P. I., and A. V. Kondrachine. 1998. The current global malaria situation, p. 11-22. In I. W. Sherman (ed.), Malaria parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 28.Wallace, M. R., T. W. Sharp, B. Smoak, C. Iriye, P. Rozmajzl, S. A. Thornton, R. Batchelor, A. J. Magill, H. O. Lobel, C. F. Longer, and J. P. Burans. 1996. Malaria among United States troops in Somalia. Am. J. Med. 100:49-55. [DOI] [PubMed] [Google Scholar]
- 29.Walsh, D. S., P. Wilairatana, D. B. Tang, D. G. Heppner, Jr., T. G. Brewer, S. Krudsood, U. Silachamroon, W. Phumratanaprapin, D. Siriyanonda, and S. Looareesuwan. 2004. Randomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapse. Clin. Infect. Dis. 39:1095-1103. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, D. S., C. Eamsila, T. Sasiprapha, S. Sangkharomya, P. Khaewsathien, P. Supakalin, D. B. Tang, P. Jarasrumgsichol, C. Cherdchu, M. D. Edstein, K. H. Rieckmann, and T. Brewer. 2004. Efficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant P. falciparum malaria. J. Infect. Dis. 190:1456-1463. [DOI] [PubMed] [Google Scholar]
- 31.White, N. J. 1998. Drug resistance in malaria. Br. Med. Bull. 54:703-715. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 1997. World malaria situation in 1994. Part I. Population at risk. Wkly. Epidemiol. Rec. 72:269-274. [PubMed] [Google Scholar]
- 33.WRAIR. Unpublished data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.