Abstract
Mycoplasma genitalium is an important pathogen in male nongonococcal urethritis (NGU). Isolation of M. genitalium from clinical specimens by axenic culture is very difficult and time-consuming, and very few strains are available for antibiotic susceptibility testing. Primary isolation of M. genitalium by coculture with Vero cells improves the isolation rate significantly. However, some strains cannot be adapted to axenic culture. In this study, we determined the antibiotic susceptibility of M. genitalium strains grown in Vero cell culture with dilutions of antibiotics. Growth of M. genitalium was monitored by a quantitative PCR assay detecting a single-copy region of the mgpB adhesin gene. Growth inhibition in the presence of antibiotics was expressed as a percentage of the DNA load of controls grown in the absence of antibiotics. Eighteen strains were examined, including 6 new strains isolated from urethral swab specimens and 4 new strains isolated from urine specimens collected from Japanese men. Eight strains adapted to axenic culture were also tested by the conventional broth dilution method. The two methods had an acceptable correlation. Azithromycin was the most active drug against M. genitalium. Among the fluoroquinolones, moxifloxacin had the highest activity, with MICs ranging from 0.03 to 0.5 mg/liter, whereas ciprofloxacin and levofloxacin were considerably less active, with MICs ranging from 0.5 to 16 mg/liter and 0.25 to 4 mg/liter, respectively. MICs for tetracycline ranged from 0.125 to 4 mg/liter. This new method could increase the number of M. genitalium strains available for antibiotic susceptibility testing and significantly shorten the time from sampling to MIC results.
Mycoplasma genitalium was first isolated after prolonged incubation from 2 of 13 men with urethritis (36). Despite repeated attempts, new isolates of M. genitalium were not obtained from urethral specimens by conventional culture techniques (31, 33), but isolation of M. genitalium in cultures of throat and synovial fluid specimens has been reported, although these isolates were mixed with Mycoplasma pneumoniae (1, 35). Aside from these reports, a technique for isolation of M. genitalium from urethral specimens using Vero cells to initially cultivate M. genitalium and subsequent adaptation to conventional broth has been developed (19) and has been used with some success in other laboratories (34). Using this new technique, however, it took more than 50 days to adapt the M. genitalium strains to cell-free medium, i.e., axenic culture. Even today, other urogenital isolates are extremely rare and have been difficult to obtain (23).
M. genitalium is gaining increasing attention as one of the important pathogens in men with nongonococcal urethritis (NGU). While Chlamydia trachomatis can be found in 30 to 40% of NGU patients, M. genitalium has been detected in 15 to 30% (3, 11, 15, 20). Several studies have shown a significant association between the presence of M. genitalium DNA and symptoms and/or signs of urethritis (reviewed in reference 17).
Patients with NGU are usually treated empirically with the same antibiotics regardless of the detection of C. trachomatis. Depending on local recommendations, tetracyclines, macrolides, or quinolones are used. However, several open studies have shown that M. genitalium may persist after treatment with tetracyclines or quinolones (9, 21) and that failure of eradication was associated with recurrence (25). Although resistant strains of C. trachomatis have been characterized in vitro (26), very few reports linking such resistance to clinical treatment failure have been reported (32). However, we do not have much information about the antimicrobial susceptibility of M. genitalium. Pure cultures of M. genitalium strains grown in axenic culture are needed for the conventional method for antibiotic susceptibility testing. Consequently, the number of strains available for antibiotic susceptibility testing has been very limited and the trends of antibiotic susceptibilities among current strains are not known. Using the Vero cell culture method, we isolated M. genitalium from male urethral swabs or urine within 3 to 5 weeks after initiation of the cultivation compared to at least 6 months for adaptation to axenic culture. Recently, six strains from urethral swabs and four strains from urine have been propagated in the Vero cell culture and a new method for antibiotic susceptibility testing of M. genitalium growing only in Vero cell culture was developed and validated.
(Part of this work was presented at the 13th Meeting of the Scandinavian Society for Genitourinary Medicine, Elsinore, Denmark, 22 to 24 September 2004.)
MATERIALS AND METHODS
Culture of Vero cells.
Vero cells were maintained in Eagle's minimal essential medium (MEM) supplemented with 2% Ultroser G serum substitute (Ciphergen, Cergy-Saint-Christophe, France) and 3% sodium bicarbonate, without any antibiotics. Vero cells were propagated in 15-ml or 50-ml plastic culture flasks (NUNC, Roskilde, Denmark) at 37°C.
M. genitalium strains and culture.
M. genitalium strain G37T, an early passage of the M30 strain (18), and M. genitalium strains isolated in our laboratory, designated M2282, M2300, M2321, M2341, M6090, and M6151, were grown in modified Friis's mycoplasma broth medium containing horse serum (19).
M. genitalium strains growing in broth medium were divided in small portions and stored at −80°C for the conventional antibiotic susceptibility test. In addition, these strains were regrown in Vero cell culture in 15-ml flasks before being divided into small portions and stored at −80°C for the new antibiotic susceptibility test.
Ten new M. genitalium strains were grown in the Vero cell culture system to high titers. Five were urethral swab specimens from Swedish male attendees of sexually transmitted disease clinics (R5G, R6G, R65G, R66G, and R67G), one (M6312) was from a French male patient with recurrent urethritis from whom strains M6090 and M6151 (18) were previously isolated, and four were from urine specimens from Japanese male patients with NGU (R53G, R69G, R70G, and R74G). When the strains reached titers of >500,000 genome equivalents (geq) as determined by TaqMan 5′ nuclease real-time PCR, they were frozen at −80°C as described above.
Growth curve of M. genitalium in cell culture tubes.
In order to establish the growth pattern of M. genitalium strains in Vero cell culture, the M. genitalium DNA loads were determined in the supernatant and in the cell-containing medium. Vero cells were adjusted to 2.5 × 104/ml in Eagle's MEM supplemented with 2% Ultroser G. M. genitalium strains were cultured with 2 ml of the Vero cell suspensions in Nunclon Delta flat-bottom, screw-cap culture tubes (Nunc, Roskilde, Denmark) at 37°C. At 1, 2, and 3 weeks of incubation, 100 μl of the supernatant was transferred from a culture tube to 300 μl of 20% Chelex 100 slurry (Bio-Rad Laboratories, Hercules, CA). In addition, Vero cells were scraped from the bottom of the same tubes and 100 μl of medium containing Vero cells was transferred to 300 μl of 20% Chelex 100 slurry. Each week, the supernatant and cell-containing medium was harvested from five tubes and the M. genitalium DNA load in each sample was determined by a TaqMan 5′ nuclease real-time PCR as previously described (18). In brief, a 78-bp fragment of the mgpB adhesin gene was amplified and detected with a 6-carboxyfluorescein-labeled minor groove binder (MGB) probe.
Antibiotics for susceptibility testing.
The antibiotics used for susceptibility testing were doxycycline hydrochloride (DOX), tetracycline hydrochloride (TET), erythromycin (ERY), clarithromycin (CLR), azithromycin (AZM), ciprofloxacin hydrochloride (CIP), levofloxacin (LVX), and moxifloxacin hydrochloride (MXF). DOX and ERY were bought from Sigma-Aldrich Denmark, Vallensbaek Strand, Denmark, and TET was bought from Fluka, Buchs, Switzerland. CLR was provided from Abbott Laboratories, North Chicago, Ill. AZM was supplied by Groton Laboratories, Pfizer Inc., Groton, CT. CIP and MXF were supplied by Bayer Health Care, Lyngby, Denmark, and LVX was supplied by Daiichi Pharmacology Company, Tokyo, Japan.
Cell-culture-based antibiotic susceptibility test.
Vero cells grown in 50-ml flasks were trypsinized and resuspended in Eagle's MEM with 2% Ultroser G and adjusted to 1.7 × 104/ml. A 2.2-ml portion of the Vero cell suspension was dispensed in the wells of Multiwell 24-well tissue culture plates (Becton Dickinson, France). Each antibiotic was diluted with Eagle's MEM with 2% Ultroser G in twofold steps, and 0.2 ml of the dilution was added to each well. Triplicate control wells received 0.2 ml of the same medium without antibiotics. After thawing, M. genitalium strains cultured on Vero cells were diluted in cell culture medium and adjusted to contain from 1,000 to 3,000 geq of M. genitalium per 0.1 ml and 0.1 ml of the dilution was used as inoculum, resulting in a total volume of 2.5 ml medium in each well. The plates were covered with sterile sealing tape (Nunc, Roskilde, Denmark) to prevent evaporation of the medium and incubated in an atmosphere with 5% CO2 at 37°C. At 1, 2, and 3 weeks after the inoculation, 100 μl of the supernatant was harvested from each well and added to 300 μl of 20% Chelex 100 slurry, and the M. genitalium DNA load was determined by the TaqMan PCR assay. Inhibition rates of the antibiotics were calculated by the formula inhibition rate (%) = [(average of DNA loads in control wells − DNA load in test well)/(average of DNA loads in control wells)] × 100.
The MIC was defined as the lowest concentration of antibiotic causing 99% inhibition, and the minimum bactericidal concentration (MBC) was defined as the lowest concentration causing 99.9% inhibition.
The initial test ranges of drug concentrations were determined by previous antibiotic susceptibility studies of M. genitalium (2, 9, 20, 26). If the MIC or MBC was not within the initial test range, the strain was reexamined after expansion of the range.
Conventional antibiotic susceptibility tests.
The conventional antibiotic susceptibility test was performed by the broth dilution method, essentially as described by Hannan (14). Briefly, antibiotics were twofold diluted with SP-4 mycoplasma medium and M. genitalium strains which could grow in axenic culture were diluted in SP-4 medium to contain 104 color-changing units/0.1 ml. A 0.1-ml volume of the dilution of the antibiotic was mixed with 0.1 ml of the diluted M. genitalium strains in a Nunclone Delta 96-well microtiter plate (96 MicroWell plates; Nunc, Roskilde, Denmark). The microtiter plates were sealed and incubated at 37°C. The plates were inspected at regular intervals for change of color. The initial MIC was read when the change of color in the broth was first observed in the control wells (usually about 10 to 14 days). The final MIC was read at the time when no further color change in the wells containing antibiotics was observed after approximately 4 weeks.
Statistical methods.
Differences between MIC determinations by the cell culture and the conventional method were compared using the Wilcoxon's signed-rank test.
RESULTS
Growth curves of M. genitalium strains in cell culture tubes.
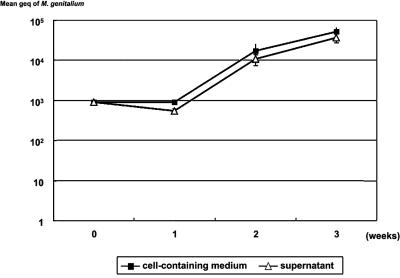
The growth curve of M. genitalium strain R6G in a Vero cell culture grown in flat-bottom culture tubes is shown in Fig. 1. R6G did not grow after 1 week of incubation, but the DNA load increased after 2 weeks. There was no difference in the M.genitalium DNA load between the supernatant and in the cell-containing medium of the Vero cell culture. Therefore, we considered monitoring of the DNA load in the supernatant indicative of the total growth in the Vero cell culture.
FIG. 1.
Growth of M. genitalium strain R6G in Vero cell culture. M. genitalium strain R6G was grown with Vero cells in air with 5% CO2. The M. genitalium DNA load was determined at 1, 2, and 3 weeks of incubation from the supernatant and from a mixture of supernatant and Vero cells scraped from the bottom of the same tubes.
Growth of M. genitalium strains in tissue culture plates without antibiotics.
The change of M. genitalium DNA loads was monitored by harvesting the supernatant from the control wells of a 24-well tissue culture plate. The DNA loads of R6G slightly decreased at 1 week after the inoculation and started to increase after 2 weeks of incubation. R5G, R53G, R65G, R69G, R70G, R74G, M6312, M30, M2321, M2300, M6090, and M6151 had the same growth pattern as R6G. G37T, M2341, and M2282 had more rapid growth patterns. At 1 week after incubation, DNA loads of these strains slightly increased and continued to increase at 3 weeks. R66G and R67G had a slow growth pattern. The DNA loads of these strains did not increase at 2 weeks after inoculation. At 3 weeks, DNA loads increased dramatically, but at 4 weeks, all Vero cells were detached from the bottom of the wells.
Inhibition of growth of M. genitalium on Vero cell culture with antibiotics.
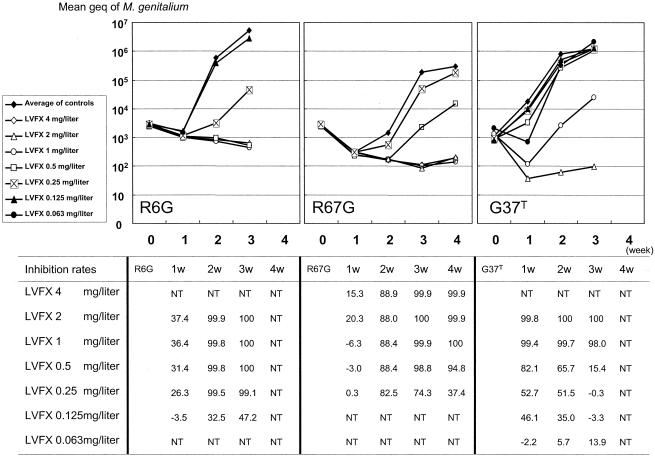
The inhibition rate of LVX is shown in Fig. 2 for three strains which had different growth patterns in the Vero cell culture. For R6G, MIC and MBC were determined to be 0.25 mg/liter and 2 mg/liter, respectively, at 2 weeks after the inoculation. At 3 weeks, MIC and MBC were 0.25 mg/liter and 0.5 mg/liter, respectively. G37T had a rapid growth pattern. However, MBC could not be determined at 1 week after inoculation of G37T. MIC and MBC were 1 mg/liter and 2 mg/liter at 2 weeks, respectively. MIC and MBC were 2 mg/liter at 3 weeks. R67G grew slowly, and MIC and MBC could not be determined at 1 and 2 weeks. The MIC and MBC were 1 mg/liter at both 3 and 4 weeks after the incubation. Based on these experiences with strains with different growth patterns, it was decided to determine the MIC and MBC at 3 weeks of incubation. MICs for all strains determined at this time point are shown in Table 1.
FIG. 2.
Inhibition of growth of M. genitalium by levofloxacin. M. genitalium strains grown in Vero cell suspensions with various concentrations of levofloxacin. Three strains with different growth rates in Vero cell culture are shown. At 1, 2, 3, and 4 weeks of incubation, the M. genitalium DNA load in the supernatant was determined. The inhibition rate was calculated by the formula inhibition rate (%) = [(average of DNA loads in control wells − DNA loads in test well)/(average of DNA loads in control wells)] × 100. NT, not tested.
TABLE 1.
MICs of M. genitalium strains as determined by TaqMan 5′ nuclease real-time PCR
| Strain or MIC parameter | MIC (mg/liter)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Doxycycline | Tetracycline | Erythromycin | Clarithromycin | Azithromycin | Ciprofloxacin | Levofloxacin | Moxifloxacin | |
| G37 | 0.5 | 0.5 | 0.125 | 0.031 | 0.008 | 8 | 2 | 0.125 |
| M30 (early) | 0.5 | 0.25 | 0.125 | 0.031 | 0.008 | 8 | 2 | 0.125 |
| M2282 | 0.25 | 0.25 | 0.063 | 0.016 | 0.004 | 4 | 1 | 0.125 |
| M2300 | 0.25 | 0.25 | 0.125 | 0.031 | 0.008 | 16 | 4 | 0.125 |
| M2321 | 0.5 | 0.5 | 0.063 | 0.016 | 0.004 | 8 | 4 | 0.25 |
| M2341 | 1 | 4 | 0.25 | 0.031 | 0.008 | 2 | 1 | 0.25 |
| M6090 | 0.125 | 0.125 | 0.063 | 0.016 | 0.004 | 2 | 1 | 0.063 |
| M6151 | 0.25 | 0.25 | 0.125 | 0.031 | 0.008 | 4 | 1 | 0.125 |
| M6312 | 0.25 | 0.25 | 0.063 | 0.031 | 0.004 | 8 | 2 | 0.125 |
| R5G | 0.125 | 0.25 | 0.063 | 0.016 | 0.004 | 1 | 0.5 | 0.063 |
| R6G | 0.25 | 0.5 | 0.063 | 0.031 | 0.008 | 0.5 | 0.25 | 0.125 |
| R65G | 0.5 | 2 | 0.125 | 0.016 | 0.004 | 1 | 0.5 | 0.125 |
| R66G | 0.25 | 0.5 | 0.125 | 0.031 | 0.004 | 2 | 1 | 0.125 |
| R67G | 0.25 | 0.25 | 0.031 | 0.016 | 0.002 | 2 | 1 | 0.031 |
| R53G | 0.125 | 0.5 | 0.031 | 0.008 | 0.002 | 4 | 2 | 0.5 |
| R69G | 0.25 | 0.25 | 0.125 | 0.063 | 0.004 | 4 | 1 | 0.125 |
| R70G | 0.25 | 0.5 | 0.125 | 0.063 | 0.008 | 1 | 1 | 0.125 |
| R74G | 0.5 | 2 | 0.063 | 0.031 | 0.004 | 2 | 1 | 0.125 |
| MIC50 | 0.25 | 0.25 | 0.063 | 0.031 | 0.004 | 2 | 1 | 0.125 |
| MIC90 | 0.5 | 2 | 0.125 | 0.063 | 0.008 | 8 | 4 | 0.25 |
Comparison between results obtained by the cell culture method and by the conventional method for MIC determination.
For strains which could be adapted to axenic culture, the MICs determined by the cell culture method and by the conventional method were compared (Table 2). The MICs determined by the cell culture method were similar to the initial MICs by the conventional method for DOX (median, 0.375 versus 0.25 mg/liter; P = 0.6) and TET (median, 0.25 versus 0.25 mg/liter; P = 0.19) but significantly higher for ERY (median, 0.125 versus 0.004 mg/liter; P = 0.008) and LVX (median, 1.5 versus 0.5 mg/liter; P = 0.03).
TABLE 2.
Comparison between MICs of M. genitalium strains as determined by the cell culture method and conventional broth dilution test
| Strain | MIC (mg/liter)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Doxycycline
|
Tetracycline
|
Erythromycin
|
Levofloxacin
|
|||||||||
| Cell culturea | Conventionalb
|
Cell culturea | Conventionalb
|
Cell culturea | Conventionalb
|
Cell culturea | Conventionalb
|
|||||
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |||||
| G37 | 0.5 | 0.125 | 4 | 0.5 | 0.5 | 2 | 0.125 | 0.004 | 0.031 | 2 | 2 | 4 |
| M30 | 0.5 | 0.25 | 4 | 0.25 | 0.125 | 1 | 0.125 | 0.004 | 0.004 | 2 | 0.5 | 2 |
| M2282 | 0.25 | 0.031 | 0.25 | 0.25 | 0.031 | 0.031 | 0.063 | 0.002 | 0.002 | 1 | 0.5 | 0.5 |
| M2300 | 0.25 | 0.25 | 1 | 0.25 | 0.031 | 0.25 | 0.125 | 0.004 | 0.008 | 4 | 0.5 | 1 |
| M2321 | 0.5 | 0.25 | 4 | 0.5 | 0.5 | 2 | 0.063 | 0.002 | 0.02 | 4 | 1 | 2 |
| M2341 | 1 | 2 | 4 | 4 | 2 | 4 | 0.25 | 0.063 | 0.063 | 1 | 0.5 | 2 |
| M6090 | 0.125 | 0.25 | 2 | 0.125 | 0.25 | 1 | 0.063 | 0.008 | 0.02 | 1 | 0.5 | 1 |
| M6151 | 0.25 | 0.25 | 2 | 0.25 | 0.25 | 2 | 0.125 | 0.004 | 0.02 | 1 | 1 | 1 |
TaqMan 5′ nuclease real-time PCR. The MIC value was defined as the lowest concentration of antibiotics causing 99% inhibition at 3 weeks after the incubation of M. genitalium with Vero cells.
The MIC value was defined as the lowest concentration of antibiotics showing no change of color at the initial and final readings. The initial MIC was read when a change of color in the control wells was first observed, and the final MIC was read when no further color change could be observed.
DISCUSSION
The antibiotic susceptibility pattern of M. genitalium has been examined in only a few studies (2, 8, 13, 28), and a limited number of strains have been included. Bebear and Renaudin (2, 28) applied an agar dilution method, whereas Hannan (13) and Duffy (8) used broth dilution. Only in the study by Hannan (13) were the more recently isolated Danish strains M2288, M2300, M2321, and M2341 included. This is in contrast to the other studies that tested the G37 strain and other strains that have been shown to be genetically very closely related to it, such as the respiratory tract isolates (18, 22, 24).
Compared with previous studies, our results differ somewhat in their conclusions. Studies examining only the G37 strain and the respiratory tract isolates (2, 8, 28) find M. genitalium susceptible to the tetracyclines, whereas we and Hannan (13) find a number of strains that would be considered resistant or at least with decreased susceptibility to the tetracyclines. The attainable concentration of DOX after standard dosage has been determined in the human prostate and was found to be <2 mg/liter (27). Consequently, strains with MICs of >2 mg/liter would be considered having decreased susceptibility. Obviously, this threshold has not been evaluated on a patient basis in M. genitalium-infected individuals. However, since the cure rate after DOX therapy is only about 35%, it seems reasonable to expect the breakpoint to be significantly lower than 2 mg/liter. Among the macrolides, AZM is the most potent drug, having a two- to threefold-lower MIC than CLR and a four- to eightfold-lower MIC than ERY. In the present study, we did not find macrolide-resistant strains even after the expanded panel of strains was examined. Reports on sporadic clinical treatment failure after AZM therapy, however, have begun to accrue, and the cell culture method for MIC determination may prove useful in documenting the correlation between clinical treatment failure and in vitro susceptibility. In accordance with earlier studies, we found the MICs for the quinolones to be relatively high. This was particularly the case for the older fluoroquinolones such as CIP and to a lesser extent LVX. The MICs for LVX were two- to threefold lower than those of CIP; this has also been reported for Mycoplasma pneumoniae and Ureaplasma spp. (37). However the MIC of M2300 and M2321 for LVX was 4 mg/liter, and these two strains would be considered fluoroquinolone resistant. However, the MICs for MXF of these two strains were 0.125 mg/liter and 0.25 mg/liter, respectively, and thus this newer fluoroquinolone with an extended gram-positive spectrum would be expected to be clinically effective. The potent antimicrobial activity of MXF has also been reported for M. pneumoniae, (12), and recently, clinical reports showing that MXF was effective in recurrent or persistent NGU due to M. genitalium occurring after treatment with tetracyclines or macrolides have been presented (C. S. Bradshaw, S. M. Tabrizi, T. R. Read, C. A. Hopkins, M. B. Moss, S. M. Garland, and C. K. Fairley, Program Abstr. Int. Soc. Sex. Transm. Dis. Res., abstr. MO-401, 2005). This is in good agreement with our clinical observations, which have shown a good microbiological and clinical cure rate after MXF treatment in persistent or recurring urethritis.
The new method for antibiotic susceptibility testing of M. genitalium presented here has two major benefits. First of all, this method can expand the number of M. genitalium strains available for antibiotic susceptibility testing, since M. genitalium strains which cannot be adapted to axenic culture can be tested. In our experience, about 60% of M. genitalium strains could grow in Vero cell culture in about 1 month if the DNA load of M. genitalium in the specimen was more than 1,000 copies per 0.1 ml of SP-4 medium with urethral swab or urine. The second benefit of this method is the objective validation of the MIC using numeric values instead of a color change in broth or colony formation after a certain period of time. When performing antibiotic susceptibility testing by the broth dilution method, the controversial point is when to read the color change in the broth. Hannan reported both the initial and the final MICs (13, 14), but there is no universal agreement about the clinical relevance of the two different values. For antibiotics with mycoplasmacidal effect such as the fluoroquinolones, the two values tend to be more similar than for mycoplasmastatic drugs such as tetracyclines and macrolides. It could be argued that the final MIC would be clinically most relevant, since a temporary suppression of growth would lead to recurrent symptoms after some time. On the other hand, patients with a normal immune system might be able to efficiently combat the infection when the mycoplasmas are in the suppressed state.
MICs for ERY were significantly higher when determined by the cell culture method as compared to the conventional broth dilution test. This could reflect the poorer intracellular concentration of this drug when compared to the newer macrolides, which may be clinically important since M. genitalium may replicate intracellularly (6). This phenomenon has previously been observed for the intracellular pathogen Legionella pneumophila (10). We cannot exclude, however, that the decreasing pH in the cell culture medium caused by the growth of the Vero cells may have decreased the activity of ERY.
Unfortunately, no randomized controlled trials comparing different antibiotics have been published, but clinical experience indicates that the cure rate after treatment with tetracyclines or fluoroquinolones is lower than that after macrolides. Falk et al. reported an open comparison of the clinical efficacies of tetracyclines and AZM (9) and found that M. genitalium could not be eradicated by tetracyclines in 71% of male patients with urethritis and 63% of female patients with cervicitis, whereas AZM appeared to eradicate M. genitalium from all patients investigated. It should be noted, however, that many of the patients treated with AZM had initially received treatment with tetracyclines, although in a subset of the patients, AZM was given as first-line treatment to comparable patients documenting its higher efficacy. Maeda et al. (25) reported that of 12 M. genitalium-positive men treated with LVX who were followed up, 8 were positive after treatment: 1 had persistent NGU, and of the remaining 7, 2 were lost for follow-up but 5 returned with recurrent NGU. Deguchi et al. studied the M. genitalium gyrA and parC genes from six of these men with persistent positive M. genitalium PCR (7) but found the expected mutations in only one of the patients in the pretreatment specimen and in specimens from the same patient and a second patient collected at follow-up. However, since culture was not attempted, no data on the antibiotic susceptibility patterns could be evaluated.
Studies aiming at determining the clinical MIC breakpoints of antibiotics have previously been impossible to conduct for M. genitalium; however, by using the techniques presented here, they should be achievable, although very demanding.
Antibiotic susceptibility testing by the use of real-time PCR has been described recently for intracellular bacteria, such as three Rickettsia species (Rickettsia conorii, Rickettsia typhi, and Rickettsia felis) (30), Coxiella burnetii (5), and Tropheryma whipplei (4). The MICs determined using real-time PCR were almost similar to those obtained by conventional methods. Rolain et al. (29) showed that it was possible to speed up the susceptibility tests also for commonly isolated bacteria like Staphylococcus aureus, Haemophilus influenzae, Escherichia coli, and others by real-time PCR. The results were in accordance with those obtained following the CLSI (formerly NCCLS) guidelines. However, the major drawback of this method is the cost. Consequently, at present, we consider this method relevant only for bacteria which are impossible or slow to grow in conventional culture medium, such as M. genitalium and other intracellular bacteria.
Acknowledgments
We thank Groton Laboratories, Pfizer Inc., for azithromycin; Bayer Health Care for ciprofloxacin and moxifloxacin; and Daiichi Pharmacology Company for levofloxacin.
Berit Larsen, Gitte Riisgaard Christensen, and Lisbeth Egelykke Stolpe provided technical assistance with culturing M. genitalium, and Birthe Dohn supported the real-time PCR.
REFERENCES
- 1.Baseman, J. B., S. F. Dallo, J. G. Tully, and D. L. Rose. 1988. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J. Clin. Microbiol. 26:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebear, C. M., H. Renaudin, A. Bryskier, and C. Bebear. 2000. Comparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmas. Antimicrob. Agents Chemother. 44:1980-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björnelius, E., P. Lidbrink, and J. S. Jensen. 2000. Mycoplasma genitalium in non-gonococcal urethritis—a study in Swedish male STD patients. Int. J. STD AIDS 11:292-296. [DOI] [PubMed] [Google Scholar]
- 4.Boulos, A., J.-M. Rolain, and D. Raoult. 2004. Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob. Agents Chemother. 48:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan, R. E., and J. E. Samuel. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J. Clin. Microbiol. 41:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29:301-309. [DOI] [PubMed] [Google Scholar]
- 7.Deguchi, T., S. Maeda, M. Tamaki, T. Yoshida, H. Ishiko, M. Ito, S. Yokoi, Y. Takahashi, and S. Ishihara. 2001. Analysis of the gyrA and parC genes of Mycoplasma genitalium detected in first-pass urine of men with non-gonococcal urethritis before and after fluoroquinolone treatment. J. Antimicrob. Chemother. 48:742-744. [DOI] [PubMed] [Google Scholar]
- 8.Duffy, L. B., D. Crabb, K. Searcey, and M. C. Kempf. 2000. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J. Antimicrob. Chemother. 45(Suppl.):129-133. [DOI] [PubMed] [Google Scholar]
- 9.Falk, L., H. Fredlund, and J. S. Jensen. 2003. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex. Transm. Infect. 79:318-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgeorge, R. B. 1985. The effect of antibiotics on the growth of Legionella pneumophila in guinea-pig alveolar phagocytes infected in vivo by an aerosol. J. Infect. 10:189-193. [DOI] [PubMed] [Google Scholar]
- 11.Gambini, D., I. Decleva, L. Lupica, M. Ghislanzoni, M. Cusini, and E. Alessi. 2000. Mycoplasma genitalium in males with nongonococcal urethritis: prevalence and clinical efficacy of eradication. Sex. Transm. Dis. 27:226-229. [DOI] [PubMed] [Google Scholar]
- 12.Hamamoto, K., T. Shimizu, N. Fujimoto, Y. Zhang, and S. Arai. 2001. In vitro activities of moxifloxacin and other fluoroquinolones against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 45:1908-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannan, P. C. 1998. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J. Med. Microbiol. 47:1115-1122. [DOI] [PubMed] [Google Scholar]
- 14.Hannan, P. C. 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 31:373-395. [DOI] [PubMed] [Google Scholar]
- 15.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, J. S., E. Björnelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, J. S., R. Ørsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind. 1993. Mycoplasma genitalium: a cause of male urethritis? Genitourin. Med. 69:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannisson, G., Y. Enström, G. B. Löwhagen, V. Nagy, K. Ryberg, S. Seeberg, and C. Welinder-Olsson. 2000. Occurrence and treatment of Mycoplasma genitalium in patients visiting STD clinics in Sweden. Int. J. STD AIDS 11:324-326. [DOI] [PubMed] [Google Scholar]
- 22.Kokotovic, B., N. F. Friis, J. S. Jensen, and P. Ahrens. 1999. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J. Clin. Microbiol. 37:3300-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo, D., W. Xu, G. Liang, S. Wang, Z. Wang, Z. Bi, and W. Zhu. 1999. Isolation and identification of Mycoplasma genitalium from high risk populations of sexually transmitted diseases in China. Chin. Med. J. 112:489-492. [PubMed] [Google Scholar]
- 24.Ma, L., and D. H. Martin. 2004. Single-nucleotide polymorphisms in the rRNA operon and variable numbers of tandem repeats in the lipoprotein gene among Mycoplasma genitalium strains from clinical specimens. J. Clin. Microbiol. 42:4876-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda, S. I., M. Tamaki, K. Kojima, T. Yoshida, H. Ishiko, M. Yasuda, and T. Deguchi. 2001. Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex. Transm. Dis. 28:472-476. [DOI] [PubMed] [Google Scholar]
- 26.Misyurina, O. Y., E. V. Chipitsyna, Y. P. Finashutina, V. N. Lazarev, T. A. Akopian, A. M. Savicheva, and V. M. Govorun. 2004. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides. Antimicrob. Agents Chemother. 48:1347-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oosterlinck, W., E. Wallijn, and J. J. Wijndaele. 1976. The concentration of doxycycline in human prostate gland and its role in the treatment of prostatitis. Scand. J. Infect. Dis. Suppl. 9:85-88. [PubMed] [Google Scholar]
- 28.Renaudin, H., J. G. Tully, and C. Bebear. 1992. In vitro susceptibilities of Mycoplasma genitalium to antibiotics. Antimicrob. Agents Chemother. 36:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolain, J. M., M. N. Mallet, P. E. Fournier, and D. Raoult. 2004. Real-time PCR for universal antibiotic susceptibility testing. J. Antimicrob. Chemother. 54:538-541. [DOI] [PubMed] [Google Scholar]
- 30.Rolain, J.-M., L. Stuhl, M. Maurin, and D. Raoult. 2002. Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob. Agents Chemother. 46:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samra, Z., M. Borin, Y. Bukowsky, Y. Lipshitz, and D. Sompolinsky. 1988. Non-occurrence of Mycoplasma genitalium in clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 7:49-51. [DOI] [PubMed] [Google Scholar]
- 32.Somani, J., V. B. Bhullar, K. A. Workowski, C. E. Farshy, and C. M. Black. 2000. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181:1421-1427. [DOI] [PubMed] [Google Scholar]
- 33.Taylor-Robinson, D., P. M. Furr, and N. F. Hanna. 1985. Microbiological and serological study of non-gonococcal urethritis with special reference to Mycoplasma genitalium. Genitourin. Med. 61:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totten, P. A., M. A. Schwartz, K. E. Sjöström, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 35.Tully, J. G., D. L. Rose, J. B. Baseman, S. F. Dallo, A. L. Lazzell, and C. P. Davis. 1995. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J. Clin. Microbiol. 33:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 37.Waites, K. B., D. M. Crabb, X. Bing, and L. B. Duffy. 2003. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob. Agents Chemother. 47:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]