Abstract
Human immunodeficiency virus type 1 (HIV-1) is not eliminated from patients even after years of antiretroviral therapy, apparently due to the presence of latently infected cells. Here we describe the development of a cell-based system of latency that can be used for high-throughput screening aimed at novel drug discovery to eradicate HIV-1 infection.
Highly active antiretroviral therapy (HAART) has led to a significant improvement in the care and survival of patients infected with human immunodeficiency virus type 1 (HIV-1). However, even after long-term suppression of viral replication with HAART, the virus rapidly rebounds after therapy is discontinued (2, 3). A key contributor to viral rebound appears to be a reservoir of latently infected cells, including CD4+ memory T cells. The half-life of the latently infected population is quite long, and it is estimated that it would take over 60 years of HAART to eliminate this population (4). Therefore, lifelong HAART would be required to control infection in patients.
A potentially fruitful approach to eliminating virus infection would be to identify small compounds with pharmacological properties that allow these molecules to access latently infected cell reservoirs in order to activate latent proviruses and to use them in conjunction with HAART to flush out the virus. There is precedent for small molecule activation of latent HIV-1. For example, valproic acid (VPA) was found to be able to activate latent virus in a model cell culture system (18). Subsequently, a clinical study was performed in which four patients were treated with VPA for 4 to 6 weeks, and three of the four patients demonstrated a significant decrease in the number of latently infected T cells (8). Although latent virus was not totally cleared from any of the patients, the evidence indicates that an approach using pharmacological agents might ultimately be developed that would result in clearance of the virus from infected individuals. Here a cell-based assay is described that can be utilized in high-throughput screening (HTS) to discover novel compounds capable of activating latent HIV-1.
To develop an HIV-1 latency model for HTS, the secretable alkaline phosphatase (seap) gene, encoding a truncated form of placental alkaline phosphatase, was used (1). It provides a very sensitive chemiluminescent assay when used in conjunction with the alkaline phosphatase substrate CSPD [disodium 4-chloro-3-(methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo {3.3.1.13,7}decan}-4-yl)phenyl phosphate] and as such is amenable to HTS. Since a hallmark of active replication is production of the HIV-1 late proteins including the viral envelope polyprotein (Env), the seap gene was inserted in the env position to serve as an indicator of late gene expression (Fig. 1). Disruption of the env gene by insertion of seap also adds a level of safety to the system by preventing production of the essential Env polyprotein. In order to impart an additional level of safety, 2.5 kbp of the pol gene was also deleted (Fig. 1). The enhanced green fluorescent protein (egfp) reporter gene was inserted 5′ to the nef start codon such that it should be expressed from the multiply spliced nef mRNA (Fig. 1) (13, 15). The egfp gene was included in the system to provide an early gene expression marker that would allow single-cell analysis of viral infection by flow cytometry, as has been previously reported (5, 7).
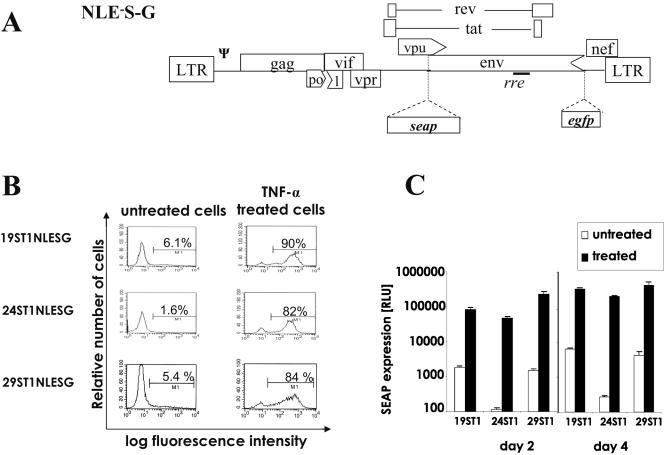
FIG. 1.
Schematic diagram of the HIV-1 transducing vector and characterization of latently infected cell clones. A. NLE−S-G is a pNL4-3-based lentiviral vector that contains all the cis-acting elements required for replication as well as two reporter genes: seap in the env position and egfp positioned 5′ to the start codon of nef. Abbreviations: LTR, long terminal repeat; rre, Rev response element; Ψ, packaging signal. Arrowheads represent partial deletions of viral sequence. Detailed cloning procedures will be supplied upon request. B. 19ST1NLESG, 24ST1NLESG, and 29ST1NLESG cell clones (106/ml) were exposed to TNF-α (50 ng/ml), and 4 days later gfp-expressing cells were analyzed by flow cytometry. The left panels show the histograms of unstimulated cells, and the right panels represent the histogram analysis of TNF-α (50 ng/ml)-stimulated cells. Numbers represent the percentages of gfp-positive cells. C. SEAP in the cultured media of untreated and TNF-α (50 ng/ml)-stimulated 19ST1NLESG, 24ST1NLESG, and 29ST1NLESG cells (106/ml) 2 and 4 days postplating. Results shown are the means and standard deviations of one of three representative experiments. RLU, relative light units.
Next, human T-cell clones derived from SupT1 cells and harboring latent vector provirus were isolated. NLE−S-G vector virus (Fig. 1) was propagated and used to inoculate SupT1 cells as previously described (10-12). A mass population of infected SupT1 cells was maintained for 1 month, by which time most SEAP and GFP expression became extinguished (data not shown). Cell clones were then isolated by limiting dilution according to the standard procedures. Clones were tested to determine if they harbored latent virus that could be stimulated with tumor necrosis factor alpha (TNF-α). Flow cytometry to monitor GFP expression showed that after treatment with TNF-α there was a significant increase in the number of cells expressing GFP within each of three clonal populations (Fig. 1B); however, a relatively small fraction of the cells were not activated. This varied expression indicates that there might be some level of epigenetic regulation of expression (16) and disparate responses of cells to TNF-α stimulation in different phases of the cell cycle (14). All three lines also exhibited a significant induction in SEAP activity after treatment with TNF-α. By day 2 posttreatment, SEAP activity increased by approximately 50-, 400-, and 150-fold in lines 19ST1NLESG, 24ST1NLESG, and 29ST1NLESG, respectively (Fig. 1C). It is noteworthy that the cell lines have been cultured extensively for at least 10 months and still retain the same characteristics, indicating that the cell lines are quite stable over time. The variation in the induction of the three cell clones was primarily due to differences between the background levels of expression (Fig. 1C). Since the proviruses are integrated in different positions in the genome (data not shown), the local site of integration can influence expression from the provirus and may account for the different levels of background expression (6, 9, 17). It was also found that induction of vector virus mRNA levels paralleled those of seap, egfp, and gag gene expression after TNF-α treatment (data not shown). It is also noteworthy that Southern blotting confirmed that each cell clone harbored a single NLE−S-G provirus (data not shown), and standard assays demonstrated that the cell lines were devoid of replication-competent virus (data not shown).
For the assay to be useful in an HTS it must be reliable in a small-well format. Assay optimization and validation require the determination of the Z factor (Z′), a dimensionless statistical characteristic used to assess the quality of data generated in a potential HTS assay (19). The Z′ values for the current latency assay were calculated based on the analysis of SEAP expression from uninduced (negative) and induced (positive) 24ST1NLESG cells in a 96-well format using the equation:
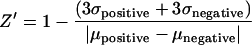 |
where σ represents the standard deviation and μ is the mean of each set of data points.
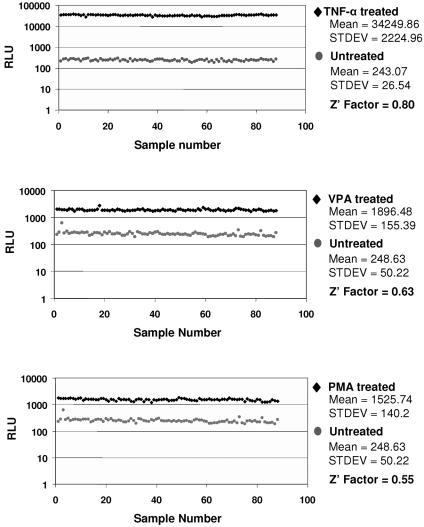
Z′ is a commonly used measure of assay performance and reliability that takes into account both the assay signal dynamic range (signal-to-background ratio) and variation (signal-to-noise ratio) associated with the measured signals. A perfect assay would have a Z′ value of 1, while an excellent assay would score between 0.5 and 1 (19). Using three different activators, Z′ values ranging between 0.55 and 0.8 were obtained, indicating excellent assay reliability (Fig. 2).
FIG. 2.
Z factor determination. Eighty-eight wells of a 96-well plate were seeded with 24ST1NLESG cells (105/well) either in medium alone or in medium containing an inducer in a final volume of 20 μl. The remaining eight wells contained serial dilutions of alkaline phosphatase protein as a positive control for the assay. The results from 88 samples per plate were compiled and used to determine the Z factor. Both uninduced (gray circles) and induced (black diamonds) cells were assayed for SEAP activity after 48 h. Cells were treated with 50 ng/ml TNF-α (A), 1 mM VPA (B), and 100 ng/ml phorbol 12-myristate 13-acetate (PMA) (C). RLU, relative light units.
In summary, we have developed a safe and reliable assay of HIV-1 latency that should be amenable to HTS. Moreover, this is the first HIV-1 latency model that has been developed with the idea to utilize it for HTS to identify novel small molecules that can be employed to eradicate latent virus from infected individuals.
REFERENCES
- 1.Berger, J., J. Hauber, R. Hauber, R. Geiger, and B. R. Cullen. 1988. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene 66:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. J. Shawn, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 3.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 5.Jordan, A., D. Bisgrove, and E. Verdin. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22:1868-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 20:1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutsch, O., E. N. Benveniste, G. M. Shaw, and D. N. Levy. 2002. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J. Virol. 76:8776-8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehrman, G., I. B. Hogue, S. Palmer, C. Jennings, C. A. Spina, A. Wiegand, A. L. Landay, R. W. Coombs, D. D. Richman, J. W. Mellors, J. M. Coffin, R. J. Bosch, and D. M. Margolis. 2005. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 366:549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewinski, M. K., D. Bisgrove, P. Shinn, H. Chen, C. Hoffmann, S. Hannenhalli, E. Verdin, C. C. Berry, J. R. Ecker, and F. D. Bushman. 2005. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J. Virol. 79:6610-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 11.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacchia, A. L., M. E. Adelson, M. Kaul, Y. Ron, and J. P. Dougherty. 2001. An inducible packaging cell system for safe, efficient lentiviral vector production in the absence of HIV-1 accessory proteins. Virology 282:77-86. [DOI] [PubMed] [Google Scholar]
- 13.Purcell, D. F., and M. A. Martin. 1993. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 67:6365-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts, B. D., G. Fang, and S. T. Butera. 1997. Influence of cell cycle on HIV-1 expression differs among various models of chronic infection. Arch. Virol. 142:1087-1099. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, S., B. K. Felber, D. M. Benko, E. M. Fenyo, and G. N. Pavlakis. 1990. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J. Virol. 64:2519-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberger, L. S., J. C. Burnett, J. E. Toettcher, A. P. Arkin, and D. V. Schaffer. 2005. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell 122:169-182. [DOI] [PubMed] [Google Scholar]
- 17.Winslow, B. J., R. J. Pomerantz, O. Bagasra, and D. Trono. 1993. HIV-1 latency due to the site of proviral integration. Virology 196:849-854. [DOI] [PubMed] [Google Scholar]
- 18.Ylisastigui, L., N. M. Archin, G. Lehrman, R. J. Bosch, and D. M. Margolis. 2004. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS 18:1101-1108. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4:67-73. [DOI] [PubMed] [Google Scholar]