Abstract
The pharmacokinetics of amphotericin B lipid complex (ABLC) were investigated in neonates with invasive candidiasis enrolled in a phase II multicenter trial. Sparse blood (153 samples; 1 to 9 per patient, 1 to 254 h after the dose) and random urine and cerebrospinal fluid (CSF) samples of 28 neonates (median weight [WT], 1.06 kg; range, 0.48 to 4.9 kg; median gestational age, 27 weeks; range, 24 to 41 weeks) were analyzed. Patients received intravenous ABLC at 2.5 (n = 15) or 5 (n = 13) mg/kg of body weight once a day over 1 or 2 h, respectively, for a median of 21 days (range, 4 to 47 days). Concentrations of amphotericin B were quantified as total drug by high-performance liquid chromatography. Blood data for time after dose (TAD) of <24 h fitted best to a one-compartment model with an additive-error model for residual variability, WT0.75 (where 0.75 is an exponent) as a covariate of clearance (CL), and WT as a covariate of volume of distribution (V). Prior amphotericin B, postnatal age, and gestational age did not further improve the model. The final model equations were CL (liters/h) = 0.399 × WT0.75 (interindividual variability, 35%) and V (liters) = 10.5 × WT (interindividual variability, 43%). Noncompartmental analysis of pooled data with a TAD of >24 h revealed a terminal half-life of 395 h. Mean concentrations in the urine after 1, 2, and 3 weeks ranged from 0.082 to 0.430 μg/ml, and those in CSF ranged from undetectable to 0.074 μg/ml. The disposition of ABLC in neonates was similar to that observed in other age groups: weight was the only factor that influenced clearance. Based on these results and previously published safety and efficacy data, we recommend a daily dosage between 2.5 and 5.0 mg/kg for treatment of invasive Candida infections in neonates.
Invasive Candida infections are important causes of infectious morbidity and mortality in premature neonates (10, 34, 36). The reported frequency in very-low-birth-weight infants, the neonatal population at highest risk, is as high as 20%, with a case fatality rate as high as 50% despite the best available treatment (33, 41, 43). Current therapeutic options with documented antifungal efficacy include amphotericin B deoxycholate (DAMB) alone or in combination with flucytosine, fluconazole, and the small liposomal formulation of amphotericin B (19, 23, 39). While treatment with DAMB is frequently limited by the compound's inherent nephrotoxicity (6), fluconazole, a generally well-tolerated, highly water-soluble, renally cleared drug, is not always active (40) and accurate dosing is difficult in very premature neonates that often display extreme variations in the extracellular fluid compartment and urinary output (42). Similarly, while liposomal amphotericin B appears to be well tolerated and effective (30, 31), no pharmacokinetic data exist for neonates and dosing is therefore still determined by maximally tolerated dosages. For these reasons, there clearly is a need for improved, pharmacokinetically based antifungal therapies in neonates with invasive Candida infections.
Amphotericin B lipid complex (ABLC) is a unique lipid formulation and is composed of dimiristoyl phosphatidylcholin/dimiristoyl phospatidylglycerol in a 1:1 molar ratio of lipid to amphotericin B and forms large ribbon-like structures. Following intravenous administration, ABLC is rapidly and efficiently taken up by cells of the mononuclear phagocytic system, mainly of the liver, spleen, and lungs, resulting in a larger volume of distribution (V) than that of conventional DAMB but a lower peak maximum concentration of the drug in the serum and lower values in the blood for the area under the concentration-time curve (25, 27). Based on a series of large, open-label, noncomparative clinical trials (44, 46), ABLC was approved by the Food and Drug Administration for the treatment of adult and pediatric patients with invasive fungal infections who are intolerant of or refractory to DAMB therapy. In addition, a prospective, randomized multicenter trial in 231 patients ≥16 years of age with invasive Candida infections has demonstrated that a dosage of 5 mg/kg of ABLC was less nephrotoxic and as effecitve as DAMB at a dosage of 0.6 to 1.0 mg/kg of body weight (WT) (E. J. Anaissie, M. H. White, O. Uzun, C. Singer, G. P. Bodey, N. Azarnia, G. Lopez-Berestein, and D. Matzke, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LM21, 1995).
ABLC may be a useful therapeutic option for neonates with invasive Candida infections. However, little is known about the disposition and the appropriate dosage of ABLC in this population. A recently completed phase II multicenter clinical trial has demonstrated the safety and efficacy of ABLC in neonates with invasive Candida infections at dosages of 2.5 and 5.0 mg/kg (F. C. Adler-Shohet, A. C. Arrieta, L. A. Ross, J. L. Rowen, N. O'Donnell, H. Waskin, and J. M. Lieberman, Abstr. 2001 Annual IDSA Meeting, abstr. 645, 2001). This study found that treatment with ABLC was well tolerated and effective against neonatal invasive Candida infections, with documented mycological eradication and complete clinical responses achieved by 22 (73.3%) of the 30 patients enrolled in this trial. Herein we report the population pharmacokinetics of ABLC in whole blood and drug concentrations in urine and cerebrospinal fluid (CSF) in neonates enrolled into this phase II multicenter clinical trial. To the best of our knowledge, this is the first investigation that provides a pharmacokinetics-based dosing rationale for a lipid formulation of amphotericin B in neonates.
MATERIALS AND METHODS
Patients.
The study was performed at four U.S. study sites under a protocol approved by the institutions' respective internal review boards. Written informed consent was obtained from the parent or legal guardian of the patient prior to study entry. Male and female infants ≤90 days of age with invasive Candida infections were eligible for enrollment. Infants were excluded if they had a documented history of hypersensitivity to amphotericin B, an anticipated survival time of less than 3 days, or an infection with Candida lusitaniae or if they had undergone treatment with an unlicensed drug or therapy with more than two doses of another systemic antifungal agent within 7 days prior to enrollment in the study. Any other medications were permitted except for the concomitant administration of systemic antifungal azoles. The definition of invasive Candida infection included a minimum of one positive culture from blood or cerebrospinal fluid or a positive culture from a site temporally associated with at least one out of a panel of predefined signs and symptoms and the absence of another documented infectious or noninfectious cause. Isolated candiduria was not considered to be an invasive infection.
Administration of study drug.
The study was designed as a multicenter, open-label, sequential-dose escalation trial of intravenous ABLC for the treatment of invasive Candida infections. The first cohort of 15 children received ABLC at a dosage of 2.5 mg/kg once daily over 60 min, and the second cohort of 15 children received ABLC at a dosage of 5.0 mg/kg once daily over 120 min. Both cohorts were evaluated for pharmacokinetics, safety, and antifungal efficacy. Enrollment of patients into the second cohort was permitted after 10 patients had completed therapy at the 2.5-mg/kg dosage in compliance with protocol-defined safety criteria. The protocol-defined duration of treatment was at least 14 days for candidemia and at least 3 weeks for Candida meningitis or other deep tissue infections. ABLC was provided by The Liposome Company (Princeton, NJ) as the approved product ABELCET marketed in the United States. ABLC was prepared and administered according to the manufacturer's recommendations in 5% dextrose at a final concentration of 1 mg/ml.
Pharmacokinetic sampling.
Blood specimens (0.2 ml) were collected in heparinized tubes following the first dose of ABLC and weekly thereafter until the discontinuation of the drug. On day 1 of therapy with ABLC, infants were randomly assigned to have specimens collected within two of the following three time periods: 0 to 3 h, 6 to 9 h, and 17 to 24 h after administration. Thereafter, a weekly blood sample was collected for the duration of therapy. In patients who remained hospitalized after the completion of therapy, additional blood samples were obtained within 24 h and 1 week after discontinuation of ABLC. Urine specimens were obtained weekly using a catheter or a bagged specimen. Cerebrospinal fluid was obtained as clinically indicated. Samples of whole blood, urine, and CSF were immediately processed and stored at −70°C.
Analytical method.
Sample extraction was performed by precipitation of blood cells and proteins using 1:3 (vol/vol) methanol-blood or 1:1 (vol/vol) methanol-urine or -CSF, followed by incubation of the samples for 30 min at 4°C, centrifugation at 2,000 × g for 10 min, transfer of the methanolic supernatant into a 0.22-μm Durapore filter tube, and centrifugation at 4,000 × g for 4 min for injection. Standards and quality controls were similarly prepared by adding known amounts of amphotericin B to pooled, drug-free normal human blood, urine, and commercially available CSF standards (Instrumentation Laboratories, Lexington, MA). Blank samples of these matrices also were extracted and submitted to assay to assess the absence of interfering peaks.
Concentrations of ABLC in blood, urine, and CSF were determined as concentrations of total (bound and free) amphotericin B by an in-house-validated, reversed-phase high-performance liquid chromatographic method (20). A commercially available amphotericin B preparation dissolved in 50:50 (vol/vol) methanol-dimethyl sulfoxide was used as the reference standard (Sigma, St. Louis, MO). The mobile phase was methanol-acetonitrile-0.0025 M Na-EDTA (500:350:200), delivered at 1.6 ml/min. The injection volume was 75 μl. Amphotericin B eluted at 3.5 to 4.5 min using a C18 analytical column (Waters, Milford, MA), preceded by a NewGuard C18 precolumn (Perkin Elmer, Norwalk, CT), and was detected at 382 nm by UV absorbance.
Quantitation was based on the peak-height-concentration response of the external calibration standard. Twelve-point standard curves (0 to 5.0 μg/ml) were linear, with R2 values of ≥0.999. The lower limits of quantitation were 0.05 μg/ml in whole blood and 0.01 μg/ml in urine and CSF. The mean recovery rates from whole blood in comparison to those from the spiked mobile phase ranged from 84.9 to 91% and were close to 100% for urine and CSF. Accuracies were within ±9%, and intra- and interday variability (precision) was <7.5% for all matrices. The maximum time of sample storage after processing was 24 months. Validation data generated in our laboratory indicate the stability of polyenes in biological specimens of dosed subjects at −70°C for at least 10 months (21).
Pharmacokinetic analysis.
The development of the population pharmacokinetic model for ABLC in blood is provided in detail as supplemental material. In brief, data were analyzed by nonlinear mixed-effect modeling using NONMEM software (version 5.0, level 1.0; University of California, San Francisco, CA) (7). This model accounts for population parameter variability (between subjects) and residual variability (random effects), as well as parameter differences predicted by covariates (fixed effects). The first-order method was used during the model-building process. Covariate selection was performed by general additive modeling (GAM) with the Xpose program, version 3.010 (29), run within the S-PLUS statistical package (MathSoft Corporation, Seattle, WA).
RESULTS
Patients.
A total of 30 infants with invasive Candida infections were enrolled in the study, of which 26 (87%) had Candida spp. detected in blood. Twenty-eight of the 30 patients underwent pharmacokinetic sampling. The demographic characteristics of the 28 infants are shown in Table 1. There were 18 boys and 10 girls, with a median gestational age of 27 weeks (range, 24 to 41 weeks) and a median birth weight of 910 g (range, 460 to 4,600 g). Fifteen infants received ABLC at 2.5 mg/kg, and 13 infants received ABLC at 5.0 mg/kg once daily for a median duration of 21 days (range, 4 to 47 days). At the start of treatment, the median postgestational age was 27 days (range, 8 to 89 days) and the median weight was 1,060 g (range, 480 to 4,900 g). Fifteen patients had received at most two dosages of DAMB prior to the first dose of ABLC. No statistically significant differences were found between patients receiving 2.5 mg/kg/day and patients receiving 5 mg/kg/day with respect to their gestational age, birth weight, sex, age, and weight at baseline and prior therapy with DAMB.
TABLE 1.
Demographic and treatment characteristics of 28 neonates with invasive candidiasis and pharmacokinetic sampling following ABLC therapy
| Parameter and unitb | Valuea
|
||
|---|---|---|---|
| ABLC at 2.5 mg/kg (n = 15) | ABLC at 5.0 mg/kg (n = 13) | All patients (n = 28) | |
| Gender (no. of males/no. of females) | 11/4 | 7/6 | 18/10 |
| Gestational age (wk) | 27 (24-40) | 28 (24-41) | 27 (24-41) |
| Birth wt (g) | 820 (470-4,600) | 980 (460-4,400) | 910 (460-4,600) |
| Age at BL (days) | 23 (10-89) | 31 (8-73) | 27 (8-89) |
| Wt at BL (g) | 970 (480-4,900) | 1,300 (840-4,400) | 1,060 (480-4,900) |
| No. of patients with prior DAMB (yes/no) | 10/5 | 5/8 | 15/13 |
| No. of days on therapy with ABLC | 21 (4-47) | 20 (5-35) | 21 (4-47) |
Single numbers are medians, and numbers in parentheses are ranges.
BL, baseline (start of therapy with ABLC).
Concentrations of amphotericin B in blood.
A total of 153 blood samples were obtained, corresponding to a median of 5 (range, 1 to 15) blood samples per patient. Following the first dose of ABLC, mean AMB concentrations (±standard errors of the means) in infants dosed with 2.5 mg/kg of ABLC decreased from 0.249 ± 0.05 μg/ml during the first sampling interval to 0.111 ± 0.01 μg/ml during the third sampling interval; those in infants dosed with 5 mg/kg decreased from 0.651 ± 0.35 μg/ml during the first sampling interval to 0.220 ± 0.04 μg/ml during the third sampling interval. Mean AMB concentrations were higher following the administration of the 5 mg/kg dose than following the 2.5-mg/kg dose. During therapy with ABLC and at the end of treatment (Table 2), the average amphotericin B concentrations (6 to 13 data points per sampling interval and cohort) did not increase with the duration of treatment and ranged from 0.173 ± 0.02 to 0.277 ± 0.03 μg/ml in the 2.5-mg/kg cohort and from 0.407 ± 0.07 to 0.498 ±0.07 μg/ml in the 5-mg/kg cohort. Similar to what occurred on the first day of therapy, mean concentrations in each sampling period were consistently higher in the 5-mg/kg cohort.
TABLE 2.
Random concentrations of amphotericin B in blood and urine
| Specimenb | Dosage (mg/kg) | Mean concn of AMB (μg/ml) ± SEMa
|
|||
|---|---|---|---|---|---|
| Wk 1 | Wk 2 | Wk 3 | EOT | ||
| Blood | 2.5 | 0.226 ± 0.02 | 0.173 ± 0.02 | 0.277 ± 0.03 | 0.180 ± 0.04 |
| 5.0 | 0.458 ± 0.04 | 0.498 ± 0.07 | 0.460 ± 0.08 | 0.407 ± 0.07 | |
| Urine | 2.5 | 0.082 ± 0.02 | 0.145 ± 0.04 | 0.205 ± 0.04 | |
| 5.0 | 0.430 ± 0.26 | 0.280 ± 0.11 | 0.310 ± 0.12 | ||
Samples were obtained at random time points during weeks 1, 2, and 3 and at the end of therapy (EOT).
The total number of blood samples was 80 (6 to 13 samples per interval and cohort) and that of urine samples was 53 (5 to 13 samples per interval and cohort).
Concentrations of amphotericin B in urine and CSF.
Random urine samples obtained at the end of weeks 1, 2, and 3 of therapy with ABLC (n = 53; 5 to 13 samples per sampling interval and cohort) yielded mean AMB concentrations ranging from 0.082 ± 0.02 to 0.205 ± 0.04 μg/ml in the 2.5-mg/kg dosage cohort and from 0.280 ± 0.11 to 0.430 ± 0.26 μg/ml in the 5.0-mg/kg dosage cohort (Table 2). Concentrations of AMB in CSF, which was obtained from 13 infants at different time points during both the dosing interval and the time course of therapy with ABLC, ranged from undetectable to 0.074 μg/ml without apparent difference between the dosage cohorts.
Population pharmacokinetics in blood.
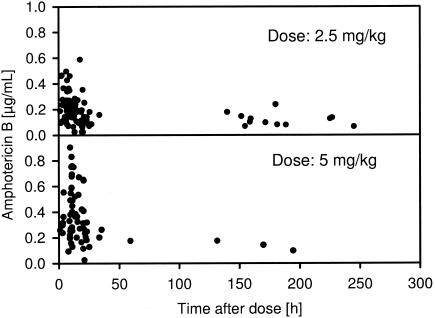
Of the 153 blood samples, 130 were obtained within 24 h after dosing and 23 were obtained from >24 h to 245 h after dosing (Fig. 1). Therefore, model and covariate building were first performed with data for a time after dose (TAD) of ≤24 h, the standard dosing interval. In a second step, all data were analyzed. For modeling, five samples with blood concentrations below the lower limit of quantification (0.05 μg/ml) were defined as one-half of the lower limit of quantification. One additional sample (dose, 5 mg/kg; TAD, 2.2 h; blood concentration, 2.44 μg/ml) was excluded from the analysis, as results obtained with it were outside the range of the data of its respective cohort (4 standard deviations).
FIG. 1.
Plot of observed blood concentrations of amphotericin B at various times after administration of ABLC at 2.5 and 5 mg/kg used for population pharmacokinetic analysis.
Model for data with a TAD of ≤24 h.
Primary analysis revealed that WT was an important determinant of both CL and V. Therefore, WT was included as the primary covariate in the model building. Based on both theoretical reasons (48) and a large body of empirical evidence (4), V was modeled proportionally to WT and CL was modeled proportionally to WT0.75 (where 0.75 is an exponent). Data for a TAD of ≤24 h fitted best to a one-compartment structural model with an exponential-error model for between-subject variability and an additive-error model for residual variability, including WT0.75 as covariate of CL and full WT as a covariate of V.
Potentially significant covariates detected by a GAM analysis of CL were gestational age, postnatal age, and pretreatment with DAMB; pretreatment with DAMB was the only potentially significant covariate of V. Based on residuals of Student's t test and Cook's distance test, none of the patients was diagnosed as an outlier in the GAM analysis.
Backward exclusion of the candidate covariates showed that none of the covariates significantly decreased the objective function on the P level of 0.01 (Table 3). Thus, the basic model was found to be the final model for ABLC: CL (liter/h) = 0.399 × WT0.75 and V (liter) = 10.5 × WT.
TABLE 3.
Summary of covariate population pharmacokinetic models tested for CL and V as proposed by generalized additional modelinga
| Model | Covariate(s) of CL | Covariate of V | Decrease in OFVb | Improvement |
|---|---|---|---|---|
| Basic | ||||
| Full | GA, age, DAMB | DAMB | ||
| Backward | Age, DAMB | DAMB | −5.82 | No |
| exclusion | DAMB | DAMB | −6.33 | No |
| DAMB | −5.59 | No | ||
| DAMB | −1.91 | No |
For all models, WT0.75 is a covariate of CL and WT1 is a covariate of V. Residual variability was modeled using an additional-error model; interindividual variability was modeled using an exponential model. GA, gestational age; age, age at baseline; DAMB, prior treatment with DAMB.
Decrease in the objective function value (OFV) compared with that of the hierarchically preceding covariate model.
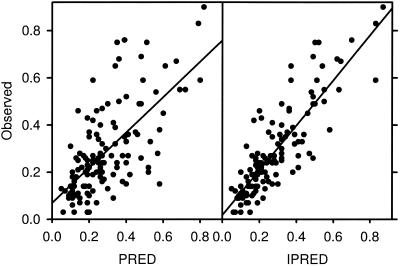
Parameter estimates of the final model and the goodness of fit between the observed and the model-predicted concentrations based on population-derived parameters and individual parameters are provided in Table 4 and in Fig. 2, respectively. Of note, changing the normalization of WT from 1 kg to a centered weight of 70 kg, as proposed by Holford (26), had no impact on the robustness of the parameter estimates.
TABLE 4.
Population pharmacokinetic analysis final parameter estimates for data with a TAD of ≤24 h
| Parameter/unit | Typical value | Relative SEa |
|---|---|---|
| Theta CL (liters/h) | 0.399 | 0.10 |
| Theta V (liters) | 10.5 | 0.15 |
| Residual error (μg/ml) | 0.11 | |
| Interindividual variability CL (%) | 35 | |
| Interindividual variability V (%) | 43 |
Standard error divided by the parameter estimate.
FIG. 2.
Goodness of fit of observed versus predicted concentrations of amphotericin B in blood. Predictions were made based on population (PRED, left panel) or individual (IPRED, right panel) parameters.
Model for all data.
Based on the final model, a two-compartment model was applied in order to model all of the data (additional 23 data points for a TAD of >24 h). However, the number of data points was too small to describe the terminal phase sufficiently. Compartment-free analysis of pooled, observed, dose-normalized data with a TAD of >24 h revealed a terminal half-life of 395 h.
DISCUSSION
The results of this study indicate that the disposition of ABLC in neonates is not different from that observed in other age groups: population pharmacokinetic analysis using blood data for a TAD of <24 h fitted best to a one-compartment model with an additive-error model for residual variability, with WT0.75 as a covariate of CL and WT as a covariate of V. Other potentially significant covariates (prior amphotericin B, postnatal and gestational age on CL, and prior amphotericin B on V) identified by generalized additional modeling did not decrease the objective function value after backward exclusion (P < 0.01). Mean blood concentrations after a dosage of 2.5 mg/kg were lower than corresponding values after a dosage of 5 mg/kg; altogether, the mean blood concentrations did not substantially differ from those reported for adults (1, 2). There was no apparent accumulation of amphotericin B following multiple dosing, and noncompartmental analysis of dose-normalized data with a TAD of >24 h revealed a mean terminal elimination half-life of 395 h. In accordance with experimental data (20; A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, K. Roussillion, M. Hemmings, L. A. Lyman, and T. J. Walsh, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1607, 2001), random sampling demonstrated substantial exposure in urine and low concentrations in the CSF.
Characterizing and understanding the disposition of systemic therapeutics are prerequisites for their safe and effective use. In pediatric patients, developmental changes may have a major impact on the distribution, metabolism, and excretion of drugs, potentially leading to serious untoward effects or suboptimal therapeutic efficacy. Therefore, simple extrapolation of adult dosages is generally not feasible, and separate pharmacokinetic studies are needed to establish the appropriate dosage regimens for further assessment of safety and efficacy (3, 18).
Pharmacokinetic studies of special populations usually require a rigid experimental design with serial sampling of multiple blood samples to fully characterize the concentration-time relationship of one or several dose intervals. Although this conventional approach leads to an accurate and precise estimation of pharmacokinetic parameters, its feasibility and interpretation, however, are limited by several factors, including difficulties in recruitment, patient selection by exclusion criteria, the inability to estimate the magnitude of variability in the target population and to identify and quantify covariates of pharmacokinetic variability, and, particularly relevant in neonates, potentially relevant blood loss (32). Considering a total blood volume of approximately 40 ml in a preterm infant of 500 g, the multiple comorbidities of these patients, and the additional presence of a life-threatening invasive fungal infection, serial blood sampling in this highly vulnerable population is difficult to justify.
Unlike conventional pharmacokinetic studies, population pharmacokinetic analysis allows an estimate of the means and variances of pharmacokinetic parameters directly in the population of interest and an assessment of the relationship between these parameters and specific patient covariates with a minimum of blood sampling from each patient (15, 32). In neonates, population pharmacokinetic parameters can be accurately estimated with fewer than 30 patients with NONMEM software using as few as two concentration-time points for each subject and each dosing interval (15). The use of random sampling within predefined sampling windows allows the accommodation to patient care algorithms and has been shown to be more robust than fixed-sampling designs (24, 28).
In our study, initial population pharmacokinetic model building revealed a significant improvement using weight as a covariate of both V and CL. Based on the general allometric power model, V was modeled proportionally to WT and CL was modeled proportionally to WT0.75 (4, 48). Covariate analysis showed that neither gender, gestational age, birth weight, age at baseline, prior therapy with DAMB, nor dosage and duration of therapy with ABLC further improved the pharmacokinetic model for concentration data with a TAD of ≤24 h. Thus, the final model corresponded to the basic model with WT as the covariate of CL and V. While the residual variability of 35% for CL and 43% for V observed in our study may appear high, similar variabilities have been reported in the neonatal setting by other investigators (15) and may be inherent to multicenter population analyses (32). Apart from factors associated with a less strictly controlled study setting, this variability may be due to intraindividual changes in patient physiology over time and also to the limited number of patients and blood samples included in these analyses. Of note, weight has been identified as a primary covariate of the CL and V of several drugs in neonates, including amikacin (12), tobramycin (17), vancomycin (14, 16), paracetamole (5), and cisapride (37). Few studies have reported further improvement of the models when gestational age, postconceptional age, and postnatal age were included in addition to WT (5, 13, 35). Levels of binding of AMB and ABLC to albumin, lipoproteins, and other proteins may differ between neonates and adults.
Noncompartmental pharmacokinetic studies of adults (1, 2) and children 4 to 7 years of age (47) have shown that the disposition of AMB after the administration of ABLC is multiexponential and characterized by a high clearance rate, a large volume of distribution, and a prolonged terminal elimination half-life, but there is no evidence for accumulation in the blood. These pharmacokinetic features are consistent with the rapid removal of the large AMB-loaded lipid aggregates from the blood into mainly the liver, spleen, and lungs, with subsequent slow redistribution of AMB back into the circulation (1, 11, 38, 45). AMB does not undergo relevant hepatic metabolism and is excreted in unchanged form into the urine and feces at a rate that is determined by the fraction of unbound compound (8, 9). This fraction may theoretically be affected by serum albumin and, indirectly, bilirubin concentrations. However, given the small fraction of redistributed drug, these effects are unlikely to have major effects on CL and V. Since AMB does not appear to accumulate in plasma and is not hepatically metabolized, and since <1% of AMB is excreted in unchanged form within 24 h following ABLC dosing (package insert [1995] for amphotericin B lipid complex injection; The Liposome Company, Inc., Princeton, NJ), no predictable major differences in the dispositions of ABLC were to be expected between neonates and adults.
Pharmacokinetic data of ABLC in adults and children beyond the first year of life are limited to noncompartmental analyses of small samples of subjects (1, 2, 47). Mean clearance rates for these subgroups varied considerably and ranged from 6.55 ± 1.41 liter/h in children with hepatosplenic candidiasis treated with 2.5 mg/kg/day (47) and from 17.8 ± 5.2 liter/h to 28.85 ± 4.33 and 32.6 ± 14.0 liter/h in adult patients treated with 5 mg/kg/day for systemic fungal infections, mucocutaneous leishmaniasis, and proven or suspected fungal infections in the state of granulocytopenia (1, 2). When clearance rates are normalized as a function of weight, neonates display an approximately twofold-higher mean weight-normalized population clearance rate than children older than 1 yr of age (0.399 versus 0.218 liter/h/kg [47]), but similar weight-normalized clearance rates were observed in adult subjects (0.272, 0.390, and 0.410 liter/h/kg [1, 2]). In addition, the population value for a V of 10.5 liter/kg in the neonates was comparable to the mean V of 12.4 liter/kg observed in adults receiving 5 mg/kg/day for probable or proven invasive infections, and compartment-free analysis of the dose-normalized pooled data for a TAD of >24 h revealed an estimated elimination half-life of 395 h that was very similar to the 393 h reported for the adults (1). Of note, average blood concentrations were similar to those observed in adults, and like in adults (1, 2), the CL in neonates was independent of dose and duration of treatment within the investigated dosage range of 2.5 to 5.0 mg/kg. Taken together, these data suggest the absence of fundamental differences in the dispositions of ABLC between neonates and adults.
The assessment of drug concentrations in urine and CSF during ABLC therapy bears note. Random sampling of urine demonstrated substantial AMB concentrations in the urine following treatment with ABLC exceeding the MIC of many Candida isolates. This observation is consistent with substantial exposure in urine in an experimental kidney target model of invasive candidiasis (A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, K. Roussillion, M. Hemmings, L. A. Lyman, and T. J. Walsh, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1607, 2001). In this model, however, administration of DAMB (1 mg/kg) resulted in a higher exposure of AMB in the urine, a higher renal clearance rate, a higher amount of AMB excreted into the urine, and a higher recovery of AMB than occurs with ABLC and liposomal amphotericin B (5 mg/kg each). This behavior coincided with the improved clearance from infected kidney tissue. Randomly timed CSF samples showed low or no detectable AMB concentrations after the administration of ABLC. The lack of achievement of CSF concentrations is a finding common to all AMB formulations; nevertheless, AMB is effective in the treatment of fungal infections of the central nervous system (22). Concentration-time-effect relationships of AMB formulations have been investigated in an experimental model of Candida meningoencephalitis (20). In comparison to DAMB and liposomal amphotericin B, ABLC (5 mg/kg/day) achieved a lower exposure of total AMB in plasma and was only partially effective. Thus, carrier effects are pharmacodynamically important for therapy with AMB. However, it is unknown at present whether and how these experimentally derived observations may impact the treatment of patients with invasive Candida infections of the urinary tract and the central nervous system.
In summary, this is the first report that provides a pharmacokinetics-based dosing rationale for a lipid formulation of amphotericin B in neonates with invasive candidiasis. The disposition of ABLC in neonates was not substantially different from that observed in other age groups, and weight was the only factor that influenced the clearance of amphotericin B from blood. Random sampling demonstrated substantial drug exposure in urine and, as with other amphotericin formulations, low concentrations in CSF. Based on the results of this study, previously published safety and efficacy data, and the results of a comparative randomized clinical trial with adults, a dosage between 2.5 and 5.0 mg/kg/day of ABLC administered as an intravenous infusion over 2 h is recommended for the treatment of invasive Candida infections in neonates.
Supplementary Material
Acknowledgments
We thank R. E. Port, Krebsforschungszentrum Heidelberg, and H. G. Schäfer, Boehringer Ingelheim, for helpful discussion during pharmacokinetic modeling.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adedoyin, A., C. E. Swenson, L. E. Bolcsak, A. Hellmann, D. Radowska, G. Horwith, A. S. Janoff, and R. A. Branch. 2000. A pharmacokinetic study of amphotericin B lipid complex injection (Abelcet) in patients with definite or probable systemic fungal infections. Antimicrob. Agents Chemother. 44:2900-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adedoyin, A., J. F. Bernardo, C. E. Swenson, L. E. Bolsack, G. Horwith, S. DeWit, E. Kelly, J. Klasterksy, J. P. Sculier, D. DeValeriola, E. Anaissie, G. Lopez-Berestein, A. Llanos-Cuentas, A. Boyle, and R. A. Branch. 1997. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase I and phase II studies. Antimicrob. Agents Chemother. 41:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcorn, J., and P. J. McNamara. 2002. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin. Pharmacokinet. 41:959-998. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, B. J., A. D. McKee, and N. H. Holford. 1997. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin. Pharmacokinet. 33:313-327. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, B. J., G. A. Woollard, and N. H. Holford. 2000. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br. J. Clin. Pharmacol. 50:125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baley, J. E., R. M. Kliegman, and A. A. Fanaroff. 1984. Disseminated fungal infections in very low-birth-weight infants: therapeutic toxicity. Pediatrics 73:153-157. [PubMed] [Google Scholar]
- 7.Beal, S. L., L. B. Sheiner, and A. Boeckmann. 1999. NONMEM user's guide. University of California, San Francisco, Calif.
- 8.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin, D. K., H. Garges, and W. J. Steinbach. 2003. Candida bloodstream infection in neonates. Semin. Perinatol. 27:375-383. [DOI] [PubMed] [Google Scholar]
- 11.Bhamra, R., A. Sa'ad, L. E. Bolcsak, A. S. Janoff, and C. E. Swenson. 1997. Behavior of amphotericin B lipid complex in plasma in vitro and in the circulation of rats. Antimicrob. Agents Chemother. 41:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botha, J. H., P. M. du Preez, R. Miller, and M. Adhikari. 1998. Determination of population pharmacokinetic parameters for amikacin in neonates using mixed-effect models. Eur. J. Clin. Pharmacol. 53:337-341. [DOI] [PubMed] [Google Scholar]
- 13.Botha, J. H., P. M. du Preez, R. Miller, and M. Adhikari. 2003. Population pharmacokinetics of gentamicin in South African newborns. Eur. J. Clin. Pharmacol. 59:755-759. [DOI] [PubMed] [Google Scholar]
- 14.Capparelli, E. V., J. R. Lane, G. L. Romanowski, E. J. McFeely, W. Murray, P. Sousa, C. Kildoo, and J. D. Connor. 2001. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J. Clin. Pharmacol. 41:927-934. [DOI] [PubMed] [Google Scholar]
- 15.Collart, L., T. F. Blaschke, F. Boucher, and C. Prober. 1992. Potential of population pharmacokinetics to reduce the frequency of blood sampling required for estimating kinetic parameters in neonates. Dev. Pharmacol. Ther. 18:71-80. [PubMed] [Google Scholar]
- 16.de Hoog, M., R. C. Schoemaker, J. W. Mouton, and J. N. van den Anker. 2000. Vancomycin population pharmacokinetics in neonates. Clin. Pharmacol. Ther. 67:360-367. [DOI] [PubMed] [Google Scholar]
- 17.Falcao, A. C., D. S. Buelga, M. E. Mendez, M. J. Garcia, and M. Pardo. 2001. Population kinetics of tobramycin in neonates. Ther. Drug Monit. 23:202-208. [DOI] [PubMed] [Google Scholar]
- 18.Fox, E., and F. M. Balis. 2001. Drug therapy in neonates and pediatric patients, p. 293-303. In A. J. Atkinson, Jr., C. E. Daniels, R. L. Dedrick, C. V. Grudzinskas, and S. P. Markey (ed.), Principles of clinical pharmacology. Academic Press, San Diego, Calif.
- 19.Frattarelli, D. A., M. D. Reed, G. P. Giacoia, and J. V. Aranda. 2004. Antifungals in systemic neonatal candidiasis. Drugs 64:949-968. [DOI] [PubMed] [Google Scholar]
- 20.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Calenadrio, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative central nervous system distribution and antifungal activity of lipid formulations of amphotericin B in rabbits. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 21.Groll, A. H., D. Mickiene, K. Werner, S. C. Piscitelli, and T. J. Walsh. 1999. High-performance liquid chromatographic determination of liposomal nystatin in plasma and tissues for pharmacokinetic and tissue distribution studies. J. Chromatogr. B 26:51-62. [DOI] [PubMed] [Google Scholar]
- 22.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 23.Groll, A. H., and T. J. Walsh. 2003. Antifungal agents, p. 3075-3108. In R. D. Feigin, J. Cherry, G. Demmler, and S. Kaplan (ed.), Textbook of pediatric infectious diseases, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 24.Hashimoto, Y., and L. Steiner. 1991. Designs for population pharmacodynamics: value of pharmacokinetic data and population analysis. J. Pharmacokinet. Biopharm. 19:333-353. [DOI] [PubMed] [Google Scholar]
- 25.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):S133-S144. [DOI] [PubMed] [Google Scholar]
- 26.Holford, N. H. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329-332. [DOI] [PubMed] [Google Scholar]
- 27.Janknegt, R., S. de Marie, I. A. Bakker-Woudenberg, and D. J. Crommelin. 1992. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin. Pharmacokinet. 23:279-291. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson, E., J. Wade, and M. Karlsson. 1996. Comparison of some practical sampling strategies for population pharmacokinetic studies. J. Pharmacokinet. Biopharm. 24:245-263. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM.Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 30.Juster-Reicher, A., O. Flidel-Rimon, M. Amitay, S. Even-Tov, E. Shinwell, and E. Leibovitz. 2003. High-dose liposomal amphotericin B in the therapy of systemic candidiasis in neonates. Eur. J. Clin. Microbiol. Infect. Dis. 22:603-607. [DOI] [PubMed] [Google Scholar]
- 31.Juster-Reicher, A., E. Leibovitz, N. Linder, M. Amitay, O. del-Rimon, S. Even-Tov, B. Mogilner, and A. Barzilai. 2000. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection 28:223-226. [DOI] [PubMed] [Google Scholar]
- 32.Kastrissios, H., and M. J. Ratain. 2001. Screening for sources of interindividual pharmacokinetic variability in anticancer drug therapy: utility of population analysis. Cancer Investig. 19:57-64. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman, D., R. Boyle, K. C. Hazen, J. T. Patrie, M. Robinson, and L. G. Donowitz. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 345:1660-1666. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman, D. 2004. Fungal infection in the very low birthweight infant. Curr. Opin. Infect. Dis. 17:253-259. [DOI] [PubMed] [Google Scholar]
- 35.Kimura, T., H. Kokubun, M. Nowatari, N. Matsuura, K. Sunakawa, and H. Kubo. 2001. Population pharmacokinetics of panipenem in neonates and retrospective evaluation of dosage. J. Antimicrob. Chemother. 47:51-59. [DOI] [PubMed] [Google Scholar]
- 36.Leibovitz, E. 2002. Neonatal candidosis: clinical picture, management controversies and consensus, and new therapeutic options. J. Antimicrob. Chemother. 49(Suppl. 1):69-73. [DOI] [PubMed] [Google Scholar]
- 37.Odoul, F., C. Le Guellec, A. Henrot, E. Saliba, J. C. Levron, M. C. Saux, G. Paintaud, and E. Autret-Leca. 2002. Population pharmacokinetics of cisapride in neonates. Eur. J. Clin. Pharmacol. 58:507-513. [DOI] [PubMed] [Google Scholar]
- 38.Olsen, S. J., M. R. Swerdel, B. Blue, J. M. Clark, and D. P. Bonner. 1991. Tissue distribution of amphotericin B lipid complex in laboratory animals. J. Pharm. Pharmacol. 43:831-835. [DOI] [PubMed] [Google Scholar]
- 39.Rowen, J. L., J. M. Tate, et al. 1998. Management of neonatal candidiasis. Pediatr. Infect. Dis. J. 17:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowen, J. L., J. M. Tate, N. Nordoff, L. Passarell, and M. R. McGinnis. 1999. Candida isolates from neonates: frequency of misidentification and reduced fluconazole susceptibility. J. Clin. Microbiol. 37:3735-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saiman, L., E. Ludington, J. D. Dawson, J. E. Patterson, S. Rangel-Frausto, R. T. Wiblin, H. M. Blumberg, M. Pfaller, M. Rinaldi, J. E. Edwards, R. P. Wenzel, W. Jarvis, et al. 2001. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 20:1119-1124. [DOI] [PubMed] [Google Scholar]
- 42.Saxen, H., K. Hoppu, and M. Pohjavuori. 1993. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin. Pharmacol. Ther. 54:269-277. [DOI] [PubMed] [Google Scholar]
- 43.Saxen, H., M. Virtanen, P. Carlson, K. Hoppu, M. Pohjavuori, M. Vaara, J. Vuopio-Varkila, and H. Peltola. 1995. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr. Infect. Dis. J. 14:776- 781. [DOI] [PubMed] [Google Scholar]
- 44.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 45.Walsh, T. J., A. J. Jackson, J. W. Lee, M. Amantea, T. Sein, J. Bacher, and L. Zech. 2000. Dose-dependent pharmacokinetics of amphotericin B lipid complex in rabbits. Antimicrob. Agents Chemother. 44:2068-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh, T. J., N. L. Seibel, C. Arndt, R. E. Harris, M. J. Dinubile, A. Reboli, J. Hiemenz, and S. J. Chanock. 1999. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr. Infect. Dis. J. 18:702-708. [DOI] [PubMed] [Google Scholar]
- 47.Walsh, T. J., P. Whitcomb, S. Piscitelli, W. D. Figg, S. Hill, S. J. Chanock, P. Jarosinski, R. Gupta, and P. A. Pizzo. 1997. Safety, tolerance, and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob. Agents Chemother. 41:1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West, G. B., J. H. Brown, and B. J. Enquist. 1997. A general model for the origin of allometric scaling laws in biology. Science 276:122-126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.