Abstract
A novel potent trypanocidal diterpene, komaroviquinone, was reduced by Trypanosoma cruzi old yellow enzyme (TcOYE) to its semiquinone radical. The reductase activity in trypanosome lysates was completely immunoabsorbed by anti-TcOYE antibody. Since TcOYE is expressed throughout the T. cruzi life cycle, komaroviquinone is an interesting candidate for developing new antichagasic drugs.
Trypanosoma cruzi is a parasitic protozoan transmitted to mammalian hosts by blood-sucking triatomine bugs (12). Infections by T. cruzi, known as Chagas' disease, pose a major public health problem in endemic countries in Central and South America (20) and result in a life-threatening, acute, and/or chronic disease with severe complications. This situation is worsened by the lack of effective vaccines and undesirable side effects of antichagasic drugs in use, such as nifurtimox and benznidazole, in addition to the emergence of parasite resistance to these drugs. Therefore, developing new chemotherapeutic agents becomes an urgent need.
Our search for trypanocidal compounds by screening traditional medicinal plants used in Uzbekistan led to the isolation of four diterpenes from Dracocephalum komarovi Lipsky (18, 19). Among those diterpene compounds, komaroviquinone displayed the strongest trypanocidal activity against epimastigotes, the replicative form in the insect vector of T. cruzi (18, 19). Thus, we decided to study the trypanocidal properties of komaroviquinone against trypomastigotes, the nondividing and infective form circulating in the blood, and amastigotes, the intracellular replicative form within the mammalian host, of T. cruzi.
Epimastigotes, trypomastigotes, and amastigotes of T. cruzi (Tulahuen strain) were cultivated as reported previously (8, 11, 15). Trypanocidal activity against epimastigotes was determined by incubation with propidium iodide solution (5 μg/ml in phosphate-buffered saline [PBS]), followed by FACScan cytometry (Becton Dickinson). The trypanocidal activity against trypomastigotes was measured after the incubation of parasite cells (2 × 106 to 3 × 106 cells/ml of Eagle minimum essential medium) with test compounds for 24 h at 37°C. Viable trypomastigotes were counted as described previously (11). The cytotoxicity of compounds against human HeLa and KB 3-1 cell lines was examined by a modified MTT [3-(4,5-dimethy-2-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (10). HeLa cell infection activity was determined by infection with trypomastigotes that subsequently differentiated into amastigotes within HeLa cells (11, 12).
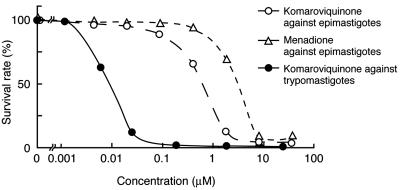
Trypanocidal activity of komaroviquinone is summarized in Table 1 and Fig. 1. Komaroviquinone inhibited the survival of epimastigotes of T. cruzi in a concentration-dependent manner from 0.1 to 10 μM, showing a 50% inhibitory concentration (IC50) value of 1 μM which was 10-, 30-, and 4-fold lower than those of reference trypanocidal agents nifurtimox, benznidazole, and menadione, respectively. Komaroviquinone inhibited the survival of trypomastigotes more potently than that of epimastigotes in a concentration-dependent manner from 3 to 30 nM, displaying an IC50 value of 9 nM, which was 100-fold lower than that of epimastigotes. The inhibition of HeLa cell infection with trypomastigotes by komaroviquinone showed the same IC50 value (9 nM) as that of the survival inhibition, which was 33-, 190-, and 330-fold lower than that of nifurtimox, benznidazole, and menadione, respectively. However, komaroviquinone at its highest concentration (3 μM) did not inhibit the intracellular growth of amastigotes within HeLa cells under our experimental conditions (data not shown). Furthermore, komaroviquinone showed low toxicities against human HeLa and KB 3-1 cells, as indicated by IC50 values of 20 and 17 μM, respectively. The selective toxicity of komaroviquinone between the parasite and host cells was calculated to be about 2,200, which is 6.5-, 38-, and 190-fold higher than that of nifurtimox, benznidazole, and menadione, respectively. These results clearly indicate that komaroviquinone is the most potent and selective trypanocidal compound among the tested drugs. This makes komaroviquinone a good candidate for drug development. Since blood transfusion is the second route of transmission of Chagas' disease, with the prevalence of infected blood donors varying from 0.10% to 62.1% in endemic areas of Latin America (9), trypanocidal agents such as gentian violet are used in blood banks to clear trypomastigotes from donated blood. Komaroviquinone, which shows an IC50 value for trypomastigotes 4 orders of magnitude lower than that of gentian violet (245 μM) (2), can be considered as a valuable tool for such use.
TABLE 1.
Inhibitory effect (IC50 in μM) and selective toxicity of komaroviquinone and trypanocidal agents on T. cruzi and mammalian cells
| Effect or toxicity | Komaroviquinone | Nifurtimox | Benznidazole | Menadione |
|---|---|---|---|---|
| Survival of epimastigotes | 1.0 | 10.0a | 30.0b | 4.0 |
| Survival of trypomastigotes | 0.009 | |||
| Infection of HeLa cells with trypomastigotes | 0.009 | 0.3 | 1.7 | 3.0 |
| Growth of HeLa cells | 20.0 | 103.0c | >100 | 38.0d |
| Growth of KB 3-1 cells | 17.0 | 52.0d | ||
| Selective toxicitye | 2,222 | 343 | >59 | 13 |
FIG. 1.
Concentration-dependent inhibition of epimastigote survival by komaroviquinone and menadione and inhibition of trypomastigote survival by komaroviquinone. Parasite cell count in the untreated control culture was considered 100%. Values are averages of two separate determinations.
Trypanocidal activity of several types of natural quinones has been partly attributed to reactive oxygen species generated by the reduction of drugs (4, 14). We have previously reported that T. cruzi old yellow enzyme (TcOYE) is involved in the generation of reactive oxygen species (6) which have been shown to kill parasites (5, 21). We therefore tested whether TcOYE catalyzes the reduction of komaroviquinone. TcOYE was heterologously expressed in Escherichia coli and purified to apparent homogeneity as described previously (6). Under anaerobic conditions, komaroviquinone was reduced by recombinant TcOYE in the presence of NADPH. The Km and Vmax values of TcOYE for the reduction of komaroviquinone (30 μM and 360 nmol/min/mg, respectively) were in the same range as those for the reduction of nifurtimox (19 μM and 350 nmol/min/mg, respectively) and menadione (13 μM and 420 nmol/min/mg, respectively). However, these agents showed significantly different values of IC50 for the inhibition of HeLa cell infection with trypomastigotes (Table 1). This discrepancy may be due to differences in cell membrane permeability to agents and/or to the presence of agent-inactivating factors in the parasite cell.
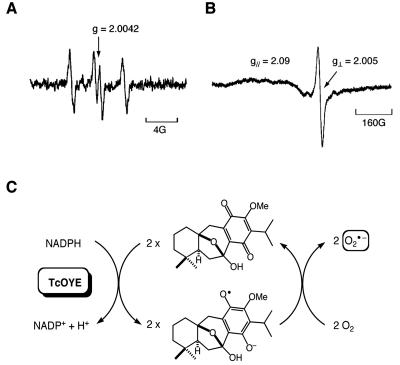
Electron spin resonance (ESR) analysis (6) revealed that a signal corresponding to a semiquinone free radical was observed during the incubation of komaroviquinone with recombinant TcOYE in the presence of NADPH under anaerobic conditions (Fig. 2A). The semiquinone radical in turn reduced O2 to the superoxide anion radical O2.− when conditions were switched to aerobic mode (Fig. 2B). As expected, the semiquinone anion radical was also generated upon incubation of T. cruzi epimastigote lysates with komaroviquinone under anaerobic conditions (data not shown). Thus, TcOYE-catalyzed reduction of komaroviquinone turns out to be a one-electron transfer process in which komaroviquinone is continuously regenerated through a redox-cycling system (Fig. 2C). These data suggest that reactive oxygen species, known for their damaging actions on cellular components (3, 5), are involved in the trypanocidal mechanism of komaroviquinone.
FIG. 2.
(A) ESR spectrum of semiquinone anion radical generated after incubation of komaroviquinone with TcOYE under anaerobic conditions with a spectral fission factor (g) value of 2.0042. (B) ESR spectrum of O2.− g// = 2.09 and g⊥ = 2.005 was measured at −155°C and formed under aerobic conditions in the reaction of semiquinone anion radical of komaroviquinone with O2. (C) Redox-cycling scheme showing the one-electron reduction of komaroviquinone catalyzed by TcOYE.
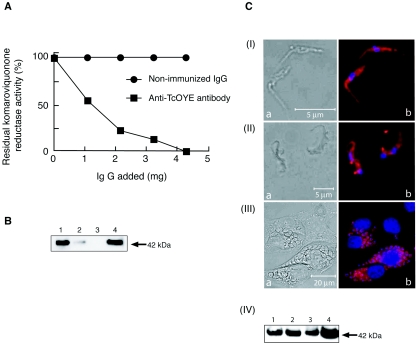
When we incubated hypotonic lysates of T. cruzi epimastigotes with various amounts of polyclonal anti-TcOYE antibody (6), the komaroviquinone reductase activity decreased in a dose-dependent manner and was almost completely immunoabsorbed by excess amounts of the antibody (Fig. 3A). Western blot analysis with the anti-TcOYE antibody revealed that TcOYE in the supernatant of T. cruzi lysates was precipitated by the antibody in a dose-dependent fashion until its complete removal (Fig. 3B). These results clearly indicate that TcOYE is the main source of komaroviquinone reductase activity in T. cruzi, thus implying that trypanocidal activity of komaroviquinone is specifically due to its reduction by the parasite enzyme TcOYE.
FIG. 3.
(A) Immunotitration of the komaroviquinone reductase activity in T. cruzi epimastigote lysates with anti-TcOYE antibody or nonimmunized rabbit immunoglobulin G. Values are averages of two separate determinations. (B) Immunoblot analysis of the lysates after incubation without (lane 1) or with anti-TcOYE antibody at doses of 2.2 mg (lane 2) and 4.3 mg (lane 3). Lane 4, recombinant TcOYE. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and immunostained with anti-TcOYE antibody. (C) Phase-contrast (left) and fluorescent (right) images of (I) epimastigotes, (II) trypomastigotes, and (III) amastigotes in HeLa cells. Nucleus was stained with Hoechst 33342 (blue) and the TcOYE-immunoreactivity was stained with anti-TcOYE antibody and rhodamine-labeled anti-rabbit immunoglobulin G (red). (IV) Immunoblot of the lysates of epimastigotes (lane 1), trypomastigotes (lane 2), and amastigotes (lane 3) with anti-TcOYE antibody. Lane 4, recombinant TcOYE.
We then investigated the expression of TcOYE in different stages of the T. cruzi life cycle. The three stages of parasite cells were fixed with 10% formaldehyde in PBS for 30 min at room temperature, washed with PBS, and incubated with rabbit polyclonal anti-TcOYE antibody (1:500 dilution) followed with rhodamine-labeled anti-rabbit immunoglobulin G (1:100 dilution; Kirkegaard & Perry Laboratories) for 1 h each at room temperature. After nuclear staining with Hoechst 33342 (1:5,000 dilution; Wako Pure Chemical Industries) for 10 min at room temperature, the immunostained cells were observed under a fluorescence microscope (Zeiss). Positive immunofluorescence for TcOYE was found in the cytoplasmic area of epimastigotes (Fig. 3C, panel I), trypomastigotes (Fig. 3C, panel II), and amastigotes (Fig. 3C, panel III). This finding was further confirmed by Western blotting, which detected a 42-kDa band of similar intensity in the lysates of all stages of T. cruzi (Fig. 3C, panel IV). These data are in agreement with the cytosolic localization of TcOYE in T. cruzi epimastigotes (6).
Although TcOYE is expressed at similar levels in all stages of the parasite life cycle, IC50 values of komaroviquinone for the inhibition of T. cruzi viability significantly varied from stage to stage. Unfortunately, komaroviquinone was devoid of any inhibitory effect on intracellular replication of amastigotes within HeLa cells. These discrepancies may be explained either by the inability of these cells to take up the compound or by the rapid degradation of komaroviquinone into inactive metabolites after its incorporation in HeLa cells. Indeed, Torres-Mendoza et al. (16, 17) have shown some diterpenes from Myrospermum frutescens to be more active against the extracellular than intracellular form of T. cruzi.
Here, we have shown that komaroviquinone is a potent trypanocidal agent against trypomastigotes of T. cruzi. To improve its pharmacological parameters, we intend to use komaroviquinone as a lead compound for the rational design of structurally improved derivatives with the ability of remaining stable in the host cell environment, thus inhibiting amastigote cell replication. Future assays of komaroviquinone on animal models of T. cruzi infection will determine the biological activity of komaroviquinone under pathophysiological conditions.
Acknowledgments
We thank K. Nagamune and S. Tamura for their assistance in cytotoxicity studies.
This work was supported in part by a Japan Society for the Promotion of Science postdoctoral fellowship (no. 02271) to Z.K. and a grant-in-aid (no. 16205020) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, to K.O. and S.F.
REFERENCES
- 1.Araya, J. E., I. Neira, S. da Silva, R. A. Mortara, P. Manque, E. Cordero, H. Sagua, A. Loyola, J. Borquez, G. Morales, and J. Gonzalez. 2003. Diterpenoids from Azorella compacta (Umbelliferae) active on Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 98:413-418. [DOI] [PubMed] [Google Scholar]
- 2.del Olmo, E., M. G. Armas, J. L. Lopez-Perez, G. Ruiz, F. Vargas, A. Gimenez, E. Deharo, and A. San Feliciano. 2001. Anti-Trypanosoma activity of some natural stilbenoids and synthetic related heterocyclic compounds. Bioorg. Med. Chem. Lett. 11:2755-2757. [DOI] [PubMed] [Google Scholar]
- 3.Docampo, R., J. N. Lopes, F. S. Cruz, and W. Souza. 1977. Trypanosoma cruzi: ultrastructural and metabolic alterations of epimastigotes by beta-lapachone. Exp. Parasitol. 42:142-149. [DOI] [PubMed] [Google Scholar]
- 4.Docampo, R., F. S. Cruz, A. Boveris, R. P. Muniz, and D. M. Esquivel. 1978. Lipid peroxidation and the generation of free radicals, superoxide anion, and hydrogen peroxide in beta-lapachone-treated Trypanosoma cruzi epimastigotes. Arch. Biochem. Biophys. 186:292-297. [DOI] [PubMed] [Google Scholar]
- 5.Docampo, R., and S. Moreno. 1984. Free radicals in mode of action of chemotherapeutic agents and phagocytic cells on T. cruzi. Rev. Infect. Dis. 6:223-238. [DOI] [PubMed] [Google Scholar]
- 6.Kubata, B. K., Z. Kabututu, T. Nozaki, C. J. Munday, S. Fukuzumi, K. Ohkubo, M. Lazarus, T. Maruyama, S. K. Martin, M. Duszenko, and Y. Urade. 2002. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J. Exp. Med. 196:1241-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maya, J. D., S. Bollo, L. J. Nunez-Vergara, J. A. Squella, Y. Repetto, A. Morello, J. Perie, and G. Chauviere. 2003. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 65:999-1006. [DOI] [PubMed] [Google Scholar]
- 8.Minning, T. A., J. Bua, G. A. Garcia, R. A. McGraw, and R. L. Tarleton. 2003. Microarray profiling of gene expression during trypomastigote to amastigote transition in Trypanosoma cruzi. Mol. Biochem. Parasitol. 131:55-64. [DOI] [PubMed] [Google Scholar]
- 9.Moraes-Souza, H., J. Orlando, and D. Langhi, Jr. 2004. Control of blood transfusion of American trypanosomiasis, p. 479-490. In I. Maudlin, P. H. Holmes, and M. A. Miles (ed.), The trypanosomiases. CABI Publishing, Oxfordshire, United Kingdom.
- 10.Murakami, N., H. M. Mostaqul, S. Tamura, S. Itagaki, T. Horii, and M. Kobayashi. 2001. New anti-malarial flavonol glycoside from Hydrangeae Dulcis Folium. Bioorg. Med. Chem. Lett. 11:2445-2447. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima-Shimada, J., Y. Hirota, Y. Kaneda, and T. Aoki. 1994. Quantitative determination of growth of amastigotes and trypomastigotes in an in vitro cultivation of HeLa cells infected with Trypanosoma cruzi. J. Protozool. Res. 4:10-17. [Google Scholar]
- 12.Nakajima-Shimada, J., Y. Hirota, and T. Aoki. 1996. Inhibition of Trypanosoma cruzi growth in mammalian cells by purine and pyrimidine analogs. Antimicrob. Agents Chemother. 40:2455-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutter, L. M., A. L. Cheng, H. L. Hung, R. K. Hsieh, E. O. Ngo, and T. W. Liu. 1991. Menadione: spectrum of anticancer activity and effects on nucleotide metabolism in human neoplastic cell lines. Biochem. Pharmacol. 41:1283-1292. [DOI] [PubMed] [Google Scholar]
- 14.Sepulveda-Boza, S., and B. K. Cassels. 1996. Plant metabolites active against Trypanosoma cruzi. Planta Med. 62:98-105. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson, S., F. Vandekerckhove, U. Frevert, and V. Nussenzweig. 1995. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitology 110:547-554. [DOI] [PubMed] [Google Scholar]
- 16.Torres-Mendoza, D., L. D. Urena-Gonzalez, E. Ortega-Barria, T. L. Capson, and L. Cubilla-Rios. 2003. Five new cassane diterpenes from Myrospermum frutescens with activity against Trypanosoma cruzi. J. Nat. Prod. 66:928-932. [DOI] [PubMed] [Google Scholar]
- 17.Torres-Mendoza, D., L. D. Urena-Gonzalez, E. Ortega-Barria, P. D. Coley, T. A. Kursar, T. L. Capson, K. McPhail, and L. Cubilla-Rios. 2004. Novel cassane and cleistanthane diterpenes from Myrospermum frutescens: absolute stereochemistry of the cassane diterpenes series. J. Nat. Prod. 67:1711-1715. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama, N., F. Kiuchi, M. Ito, G. Honda, Y. Takeda, O. K. Khodzhimatov, and O. A. Ashurmetov. 2003. New icetexane and 20 norabietane diterpenes with trypanocidal activity from Dracocephalum komarovi. J. Nat. Prod. 66:128-131. [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama, N., M. Ito, F. Kiuchi, G. Honda, Y. Takeda, O. K. Khodzhimatov, and O. A. Ashurmetov. 2004. A trypanocidal diterpene with novel skeleton from Dracocephalum komarovi. Tetrahedron Lett. 45:531-533. [Google Scholar]
- 20.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas' disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 21.Viode, C., N. Bettache, N. Cenas, R. L. Krauth-Siegel, G. Chauviere, N. Bakalara, and J. Perie. 1999. Enzymatic reduction studies of nitroheterocycles. Biochem. Pharmacol. 57:549-557. [DOI] [PubMed] [Google Scholar]