Abstract
Using 15 unrelated Enterococcus faecium isolates as donors, we demonstrated that ampicillin resistance was transferable to an E. faecium recipient containing a pbp5 deletion for all but four strains. The transfers occurred at low frequencies (generally ca. 10−9 transconjugants/recipient CFU), consistent with chromosome-to-chromosome transfer. pbp5 transfer occurred within large genetic regions, and insertion into the recipient genome occurred most commonly into the recipient SmaI restriction fragment that had been created by the previous pbp5 deletion. Restriction mapping of the region upstream of pbp5 revealed a commonality of fragment sizes among the clinical isolates from the United States which differed significantly from those of three strains that were isolated from turkey feces. These data prove conclusively that E. faecium pbp5 is a transferable determinant, even in the absence of a coresiding vancomycin resistance mobile element. They also suggest that the spread of high-level ampicillin resistance among U.S. E. faecium strains is due in part to the transfer of low-affinity pbp5 between clinical isolates.
Among the more intriguing aspects of the emergence and spread of glycopeptide resistance in Enterococcus faecium is the almost universal association of vancomycin resistance with clinically significant levels of resistance to ampicillin (16). The mechanisms for resistance to glycopeptides and ampicillin are distinct. Resistance to glycopeptides is most often conferred by the acquisition of glycopeptide-resistant VanA or VanB operons, both of which are incorporated within mobile elements (7). Resistance to ampicillin is conferred most commonly by expression of low-affinity penicillin-binding protein 5 (PBP5) (20). The genetic determinant encoding PBP5 (pbp5) is located in the bacterial chromosome and considered to be intrinsic to the species. Elevated levels of ampicillin resistance found in clinical E. faecium isolates are most commonly associated with point mutations in PBP5 that lower the affinities for β-lactam antibiotics (11, 15, 21).
Although monoclonal outbreaks of glycopeptide-resistant E. faecium have been reported, many areas of endemicity of highly resistant strains report several distinct clones expressing resistance (5, 10). Polyclonal glycopeptide resistance has often been attributed to the movement of transposons Tn1546 (1, 17) and Tn5382/1549 (2, 6, 9), which encode VanA- and VanB-type resistance, respectively. The movement of these transposons has undoubtedly contributed to the rapidly rising prevalence of vancomycin resistance in U.S. hospitals. The nearly universal association of vancomycin resistance with ampicillin resistance in the United States implies that ampicillin-resistant E. faecium strains are polyclonal as well. The existence of multiple clones of ampicillin-resistant E. faecium raises the possibility that the chromosomally encoded pbp5 of E. faecium is transferable.
In prior work, we demonstrated the transferability of pbp5 from E. faecium strains in which the VanB mobile element Tn5382/1549 was inserted immediately downstream of pbp5 (2). As Tn5382/1549 bears significant similarities to conjugative transposons, the possibility remains that this transposon in some way fueled the transfer of both vancomycin and ampicillin resistance. The present study was undertaken to determine whether we could demonstrate the transferability of pbp5 alone from additional E. faecium strains of diverse origins, including those that are susceptible to vancomycin.
MATERIALS AND METHODS
Bacterial strains.
The E. faecium strains used in this study were derived from a variety of sources and are detailed in Table 1. Strain E. faecium TX0016 is the clinical isolate used to determine the sequence of the E. faecium genome (incomplete sequence available from the Baylor College of Medicine [ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Efaecium/] and the Joint Genome Institute [http://genome.jgi-psf.org/draft_microbes/entfa/entfa.home.html]). It was kindly provided to us by Barbara E. Murray. The recipient strain for transfer of pbp5 was E. faecium strain D344SRF (13), an ampicillin-susceptible derivative of E. faecium strain D344R in which pbp5 was spontaneously deleted from the chromosome and was subsequently selected for resistance to fusidic acid and rifampin by sequential plating on high concentrations of each antibiotic. MICs for ampicillin and vancomycin were determined by agar dilution using techniques as defined in the CLSI guidelines, except that brain heart infusion agar was used instead of Mueller-Hinton agar. Transconjugants expressing resistance to ampicillin or vancomycin exceeding that for strain D344SRF (0.25 to 0.5 μg/ml and 2 to 4 μg/ml, respectively) were deemed resistant to those antibiotics.
TABLE 1.
Bacterial strains and plasmids used in these experiments
| Bacterial strain or plasmid | Relevant antimicrobial resistance | Description |
|---|---|---|
| E. faecium strains | ||
| C68 | Apr Vmr | Clinical isolate initially used to define sequences downstream of pbp5 (2) |
| D344R | Apr Vms, Eryr, Tcr | Ampicillin-resistant clinical isolate (21) |
| D344SRF | Aps (pbp5 negative) Eryr Tcr, Fusr Rifr | PBP5−, ampicillin-susceptible derivative of strain D344R (11) |
| TX0016 | Low-level Apr, Vms | Ampicillin-resistant clinical isolate whose partial genome sequence is available (Baylor College of Medicine [ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Efaecium]; Joint Genome Institute [http://genome.jgi-psf.org/draft_microbes/entfa/entfa.home.html]) |
| D14 | Apr Vms | Ampicillin-resistant clinical isolate originally isolated from Providence, R.I. (4), clonally distinct from strains D24, D25, and D29 |
| D24 | Apr Vms | Ampicillin-resistant clinical isolate originally isolated from Richmond, Va. (4), clonally distinct from strains D14, D25, and D29 |
| D25 | Apr Vms | Ampicillin-resistant clinical isolate originally isolated from Durham, N.C. (4), clonally distinct from strains D14, D24, and D29 |
| D29 | Apr Vms | Ampicillin-resistant clinical isolate originally isolated from Calif. (4), clonally distinct from strains D14, D24, and D25 |
| TJ386 | Apr Vmr | Ampicillin- and vancomycin-resistant clinical isolate originally isolated from Philadelphia, Pa. (kindly provided by Henry Fraimow [18]) |
| WB312 | Apr Vmr | Ampicillin- and vancomycin-resistant clinical isolate originally isolated from Scranton, Pa. (kindly provided by Henry Fraimow [18]) |
| WC176 | Apr Vmr | Ampicillin- and vancomycin-resistant clinical isolate originally isolated in Westchester, N.Y. (kindly provided by Henry Fraimow [18]) |
| T420 | Low-level Apr, Vms | Strain derived from turkey feces (did not yield transconjugants) (19) |
| T450 | Low-level Apr, Vms | Strain derived from turkey feces (did not yield transconjugants) (19) |
| T471 | Low-level Apr, Vms | Strain derived from turkey feces (did yield transconjugants) (19) |
| T636 | Low-level Apr, Vms | Strain derived from turkey feces (did yield transconjugants) (19) |
| T642 | Low-level Apr, Vms | Strain derived from turkey feces (did yield transconjugants) (19) |
| GE-1 | Aps (pbp5 negative) Fusr Rifr | Recipient for enterococcal mating experiments, referenced here because it was the recipient strain used in the mating experiment that created CV133 (2) |
| CV133 | Apr Vmr | Ampicillin- and vancomycin-resistant transconjugant resulting from mating between C68 and GE-1 (2); genomic DNA was the source for probe 4 as depicted in Fig. 1; in this region, it is identical to the genome of C68 |
| Plasmids | ||
| PBCSK(−) | Cmr | Cloning vector (Stratagene) |
| pCWR570 | Cmr | 18-Kb PstI fragment of CV133 genomic DNA cloned into pBCSK− |
| PCWR576 | Cmr | 5-Kb XbaI digestion fragment of insert from pCWR570 cloned into pBCSK− |
Conjugation experiments.
Matings were performed using a technique designed to detect low-frequency events as previously described (12) using ampicillin (2 to 10 μg/ml), fusidic acid (25 μg/ml), and rifampin (50 μg/ml) in the agar for the selection of transconjugants. Plates were examined daily for 4 days to detect the appearance of colonies. Colonies that appeared on the mating plates were restreaked on selective plates to confirm ampicillin resistance. Matings were performed one to three times per mating pair. Transfer frequencies were calculated as numbers of transconjugants per recipient CFU. Selected colonies were subjected to detailed analysis by pulsed-field gel electrophoresis (PFGE) to confirm that they were indeed transconjugants. Specific rates were calculated only if colonies from a mating were shown by PFGE to be true transconjugants.
Genetic techniques.
Lysis of E. faecium cells for PFGE was performed as previously described (2). Digestion of genomic DNA with the restriction enzyme SmaI or BstZI (Promega, Madison, Wis.) used 40 U of restriction enzyme in 400 μl of designated restriction buffer. The program for the separation of restriction digests used the autoalgorithm function (Bio-Rad, Hercules, Calif.) with the following details: separation was set for a low of 20 kb and a high of 500 kb (20 kb to 100 kb for BstZI) to run over 24 h, the calibration factor was 1.0, gels consisted of 1% PFC agarose and were run in a bath of 0.5× Tris-borate-EDTA at 14°C with a gradient of 6.0 V/cm, the included angle was 120°, the initial switch time was 2.98 s, and the final switch time was 44.69 s (8.53 s for BstZI) with linear ramping. Extraction of genomic DNA from E. faecium strains for routine restriction digestion was performed as previously described (14). Southern transfer, hybridization using digoxigenin-labeled probes, and detection with chemiluminescence assays were performed by standard techniques as previously described (2). Probes used to hybridize transferred DNA were created by incorporating digoxigenin into PCRs as previously described (2) using the following primers for pbp5: PBP5-2004-1 (5′-GAATCCAGAATTAAGCAGTAATGG-3′), PBP5-2004-2 (5′-CGCAACAGTTGATCCAGC-3′), probe 1 from Fig. 1, 523-F (5′-CTGGGTTCCATCAACGACTG-3′), 523-G (5′-TCATTGATAAGCGCGAGTGC-3′), probe 2 from Fig. 1, 536-1 (5′-GACGTCGAAGATGCGGTACT-3′), 536-2 (5′-AGTATTGCGATGGGGAATGC-3′), probe 3 from Fig. 1, lipase-1 (5′-GAAGGACCGACTGAAGGGATT-3′), and lipase-2 (5′-CGGCTTTTTGTCCCTTCTTG-3′). The probes were based on sequence analysis of pbp5 and its upstream region from E. faecium strain C68 and its transconjugant, strain CV133. The locations of the probes upstream of pbp5 are shown in Fig. 1. The single exception was probe 4, which consisted of a 1.5-kb fragment derived from pCWR576 as detailed in Table 1 and indicated in Fig. 1. This restriction fragment was extracted from the gel and labeled with digoxigenin by using the Klenow fragment as previously described (14).
FIG. 1.
Map of the region extending approximately 62 kb upstream of pbp5 in strains C68 and CV133. Locations of relevant restriction sites are shown. The precise locations of the probes used in the mapping experiments are indicated by the vertical arrows. The expected restriction fragments based on the C68/CV133 map that were hybridized are in white. pbp5 is located at the extreme right end of the map. Just beyond the right end of the map in C68 and CV133 lies Tn5382/1549.
RESULTS AND DISCUSSION
Transfer of pbp5.
Conjugation experiments were performed between the 15 strains listed in Table 2 and strain D344SRF, with selection on ampicillin, fusidic acid, and rifampin. Frequencies of transfer were universally low (Table 2), ranging from a low of 5.3 × 10−10 to a high of 2.56 × 10−7. Four of the 11 donor strains from which transconjugants were isolated expressed vancomycin resistance. In three of these strains, transfer of ampicillin resistance occurred without transfer of vancomycin resistance. Interestingly, one of the three strains from which vancomycin resistance was not transferred along with resistance to ampicillin was C68, in which VanB transposon Tn5382/1549 was integrated immediately downstream of pbp5 and from which the two determinants had been shown to transfer together in the past (vancomycin selection was used in previous experiments). The absence of vancomycin resistance from the transconjugant suggests that Tn5382/1549 may have been excised from its location in the process of transfer (2, 6).
TABLE 2.
Characteristics of donors and transconjugants and frequency of transfer of ampicillin resistance
| E. faecium donor straina (drug resistance) | Transconjugant (drug resistance) | Transfer frequency |
|---|---|---|
| C68 (Apr Vmr) | CV384 (Apr Vms) | 3.3 × 10−8 |
| TX0016 (Apr Vms) | CV477 (Apr Vms) | 5.3 × 10−10 |
| D14 (Apr Vms) | None | <5 × 10−10 |
| D24 (Apr Vms) | CV465 (Apr Vms) | 7.14 × 10−9 |
| D25 (Apr Vms) | Colonies appeared on plates but did not regrow on ampicillin | Unknown |
| D29 (Apr Vms) | CV420 (Apr Vms) | 1.35 × 10−7 |
| TJ386 (Apr Vmr) | CV426 (Apr Vms) | 5.7 × 10−9 |
| WB312 (Apr Vmr) | CV433 (Apr Vmr) | 1 × 10−9 |
| WC176 (Apr Vmr) | CV437 (Apr Vms) | 2.56 × 10−7 |
| T420 (low-level Apr, Vms) | None | Unknownb |
| T450 (low-level Apr, Vms) | None | <5 × 10−10 |
| T471 (low-level Apr, Vms) | CV343 (low-level Apr, Vms) | 3.3 × 10−9 |
| T636 (low-level Apr, Vms) | CV470 (low-level Apr, Vms) | 3.08 × 10−9 |
| T642 (low-level Apr, Vms) | CV472 (low-level Apr, Vms) | 3.28 × 10−9 |
| D344R (Apr Vms) | CV333 (Apr Vms) | 3.3 × 10−8 |
Recipient strain for all matings was E. faecium D344SRF.
No recipients grew on colony-counting plates, suggesting a donor-killing recipient.
Two clinical strains (D14, D25) and two strains isolated from turkeys (T420, T450) yielded no transconjugants. One clinical strain, D14, yielded no colonies on mating plates during any mating. The second donor, D25, yielded colonies on mating plates initially, but these colonies failed to grow repeatedly when restreaked on identical ampicillin concentrations. Neither T420 nor T450 produced any colonies on mating plates. In fact, one strain (T420) yielded no colonies on plates designed to determine recipient numbers, suggesting that the donor produced a substance that was lethal to the recipient. The 11 remaining strains all produced transconjugants that were verified as such by PFGE.
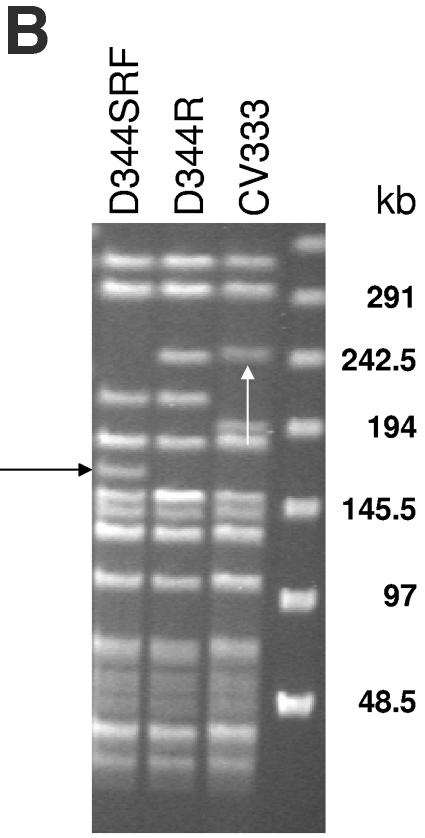
PFGE of eight donor and transconjugant pairs is shown in Fig. 2A. Comparison of the PFGE patterns seen in the eight donor strains (D344R is not well shown in Fig. 2A) confirmed that they are genetically unrelated to each other (a difference of greater than five bands). All of the transconjugants had digestion patterns similar to that of D344SRF, confirming that they are true transconjugants. In all but one transconjugant, a ca.-170-kb SmaI digestion fragment (Fig. 2A) of D344SRF had disappeared and larger bands of various lengths had appeared. In a previous work, we showed that this 170-kb band is created as a result of the deletion of pbp5 and surrounding regions from the D344R genome (11a). A clearer picture of restriction digests of D344R, D344SRF, and their transconjugant CV333 is shown in Fig. 2B. These data suggest that integration of the transferred pbp5 region occurs within the same SmaI restriction fragment from which the pbp5 region had been excised, consistent with either site-specific integration of the transferred region or recombination across homologous genomic regions. Similar results were seen with the three donor strains not shown on this gel (for strain D24, disappearance of the band in one of two transconjugants; for strain T636, disappearance of the band in one of one transconjugant; and for strain T642, disappearance of the band in five of five transconjugants).
FIG. 2.
(A) (Left panel) PFGE of SmaI-digested genomic DNA from donor-transconjugant pairs. The first lane contains lambda concatemer size standards (Bio-Rad, Hercules, Calif.) with relevant sizes (in kb) indicated to the left of the gel. The white arrow indicates the SmaI fragment created by deletion of the pbp5 region from D344R, creating D344S. The identities of the different strains are marked above the gel. The ampicillin MIC of each strain is indicated below the gel. (Right panel) Hybridization of Southern transfer of DNA in panel A using an internal fragment of pbp5 as a probe. The lane with the lambda concatemer size standard is not shown. The identities of the strains are marked above the gel. The black arrow indicates the intense hybridization in the well of CV426. (B) SmaI digestion of donor strain D344, recipient D344SRF, and transconjugant CV333. The black arrow to the left indicates the SmaI fragment of D344SRF that was created by excision of the D344R pbp5 region. The white arrow indicates the SmaI restriction fragment of CV333 that hybridizes with the pbp5 probe, representing a portion of the region transferred along with pbp5.
Southern hybridization of DNA transferred from the PFGE gel using an internal amplification product of pbp5 as a probe is shown in Fig. 2A. Similar-sized pbp5-hybridizing SmaI restriction fragments were seen in five of the transconjugants, which corresponded in size to the pbp5-hybridizing SmaI fragments in three of the donor strains (TX0016, D29, TJ386). These include the transconjugant resulting from the mating in which C68 was the donor strain. In this instance, the pbp5-hybridizing band in the transconjugant was roughly 30 kb smaller than that in the donor strain, consistent with the excision of Tn5382/1549, which was 33 kb in length (see above). The donor-transconjugant pair, seen in the last two lanes of Fig. 2A, represents a mating between D344R and D344SRF. In this instance, the size of the hybridizing band in the transconjugant closely approximates the size of the band in D344R (seen faintly on the blot immediately to the left of the CV333 band in Fig. 2A), suggesting that the mating resulted in the reinsertion of the transferred DNA in the same location from which it had been excised. A clearer view of this donor-recipient-transconjugant triad is presented in Fig. 2B.
Two transconjugants did not yield clear bands hybridizing to the pbp5 probe. One transconjugant, CV426, showed very faint hybridization to two gel fragments but revealed intense hybridization to the DNA remaining in the well of that lane in the gel (Fig. 2A), a finding that has been suggested to imply a supercoiled state independent of the bacterial chromosome. The other transconjugant (CV437) that does not hybridize within the restriction fragment region exhibits a weaker hybridization to the DNA in the well. In order to assess the possibility that hybridization to the well in these strains reflected a supercoiled state (without an internal SmaI site), we digested the two transconjugants with restriction enzyme BstZI and hybridized them with pbp5 as well as probe 3 and probe 4. Results of these experiments are shown in Fig. 3. In both instances, strong hybridization to bands within the gel was observed. For transconjugant CV437, all three probes hybridized to the same restriction fragment. In contrast, CV426 demonstrated hybridization to two distinct fragments with both pbp5 and probe 3 and did not hybridize at all to probe 4. These data support the idea that hybridization to the wells after SmaI digestion reflects the supercoiling of a non-SmaI-containing segment of transferred DNA. They also indicate that the transfer event resulting in CV426 did not include the region that hybridized to probe 4 in the donor strain and would indicate that a duplication of the pbp5-probe 3 region had occurred. The duplication of this region may also explain the increased intensity of the well hybridization seen for CV426 in Fig. 2A.
FIG. 3.
PFGE agarose separation of genomic DNA from strains CV437 (lanes A) and CV426 (lanes B). Both strains revealed minimal to no hybridization of restriction fragments with the pbp5 probe after digestion with SmaI (Fig. 2A), implying possible supercoiling of the transferred fragment, which remained in the well. In both cases, BstZ1 digestion resulted in entry of the hybridizing fragment into the gel, consistent with a supercoiled form successfully digested with BstZI. Probes used were for pbp5, along with probes 3 and 4 as described in the Materials and Methods. CV426 demonstrated no hybridization to probe 4, indicating that this region of DNA was not involved in the transfer.
These data indicate that pbp5 can be transferred to an E. faecium recipient from a variety of E. faecium strains of diverse origins (human clinical isolates from both North America and Europe, as well as isolates derived from turkey feces), whether or not the donor strain also harbored a mobile vancomycin resistance operon. In this context, the rapid emergence of vancomycin- and ampicillin-resistant E. faecium strains in the United States is very likely the result of not only mobile vancomycin resistance genes but also mobile ampicillin resistance. It should be acknowledged that the mating pairs employed in these experiments differ from those that would be anticipated to occur in nature, where the recipients would all be expected to have their own copy of pbp5. Preliminary mating experiments indicate that transfer of pbp5 from strain C68 into strain D344R occurs, with eventual replacement of the D344R pbp5 region with that acquired from strain C68 (data not shown).
Mapping the upstream pbp5 region.
In an effort to further define the nature of the pbp5/Tn5382/1549 element from strain C68, we cloned and determined the sequence from restriction fragments upstream of pbp5 from strain CV133, a transconjugant resulting from a mating between strain C68 and ampicillin-susceptible recipient strain E. faecium GE-1. We used sequence from these various clones to construct probes to span a region extending approximately 60 kb upstream of pbp5. All probes hybridized to identical-sized fragments in strains C68 and CV133 and did not hybridize to E. faecium strain GE-1, confirming that they were within the transferred region (data not shown). These same probes were used to hybridize the restriction digestions of several clinical ampicillin-resistant E. faecium (D14, D24, D25, and D29) and ampicillin- and vancomycin-resistant E. faecium (C68, TJ386, WB312, and WC176) strains and three E. faecium strains derived from turkey feces (T420, T450, and T471). Only one of these turkey strains (T471) yielded transconjugants in mating experiments. The location of the probes is detailed in Fig. 1. Results from these experiments are shown in Table 3.
TABLE 3.
Hybridization of restriction fragments from selected strains using probes derived from regions upstream of pbp5 in CV133 and C68
| Strain | Size (kb) of hybridizing restriction fragment for probe:
|
|||
|---|---|---|---|---|
| 1 (BamHI-PstI) | 2 (BglII) | 3 (XbaI) | 4 (XbaI) | |
| C68 | 12.5 | 4 | 9 | 5.2 |
| D14 | Ca. 23 | 5.2 | 4.5 | 6 |
| D24 | 12.5 | 4 | 9 | 5.2 |
| D25 | 12.5 | 4 | 9 | 5.2 |
| D279 | 12.5 | 4 | 9 | 5.2 |
| TJ386 | 12.5 | 4 | 9 | 5.2 |
| WB312 | 12.5 | 4 | 9 | 5.2 |
| WC176 | 12.5 | 4 | 9 | 5.2 |
| T420 | 12.5 | 4 | 4.5 | 7 |
| T450 | 9 | 16 | 4.5 | No hybridization |
| T471 | 12.5 | 10 | Ca. 23 | 6.2 |
All of the clinical strains that yielded transconjugants exhibited identical restriction fragment sizes hybridizing to each of the four probes. In addition, the ampicillin-resistant clinical strain that yielded transconjugants that did not grow on repeated inoculation (D25) yielded an identical pattern. In contrast, the ampicillin-resistant clinical strain that never produced any transconjugants (D14) yielded entirely different restriction fragments. The three strains isolated from turkeys for whom restriction mapping was performed (only one of which produced transconjugants) yielded fragments of different sizes than those of the clinical strains, with one strain showing two bands of similar sizes, one strain showing one band of a similar size, and the third strain showing no size similarities. E. faecium strain TX0016 was not included in these experiments, but restriction enzyme site identification using the available genome database indicates that at least three of the four probes will hybridize to fragments identical in size to those observed for strain C68 (data not shown).
The segments within which pbp5 transfers are likely to be very heterogeneous, given the marked differences in restriction maps between the clinical isolates and those isolated from turkey feces. In light of this variability, it is also interesting that the region upstream of pbp5 in both vancomycin-resistant and -susceptible clinical isolates appears to be identical by restriction mapping. These data suggest that there may be one version of a transferable element that is primarily responsible for transfer in U.S. clinical strains and that this element was present and spreading before the emergence and spread of vancomycin resistance within the genus. If this one “super-transfer” element also encodes functions that confer virulence on the bacterium, then the increased importance of E. faecium as a nosocomial pathogen and its increased resistance to ampicillin could be explained by a single mechanism (3, 8). We are in the process of conducting a more detailed analysis of the transferred region from strain C68.
Some insight into the potential size of the mobile element can be derived from our recent work analyzing the mechanism by which pbp5 was deleted from the D344R chromosome to yield D344S (11a). In that strain, a ca.-178-kb region extending from approximately 78 kb upstream of pbp5 to 100 kb downstream has been deleted. The deletion event resulted from the interaction of heterologous Tn916-like transposons. One of these elements, likely to be nearly identical to Tn916, was not present at the same position of the C68 chromosome. The other, designated Tn5386, was not present in our clinical strains. However, it is conceivable that other Tn916-like elements present in these clinical strains, or perhaps even smaller insertion sequence elements, can prompt the excision of smaller or larger segments of the E. faecium genome that contain pbp5. Our observation that insertion occurred most commonly into the SmaI site vacated by pbp5 suggests some consistency to the process of insertion. However, the fact that insertion into this fragment does not always occur and that transconjugant CV426 contains two copies of both pbp5 and the region hybridized by probe 3, while at the same time lacking the region hybridized by probe 4, suggests substantial potential for variability.
In conclusion, we have shown that pbp5, the gene that confers high-level β-lactam resistance in E. faecium, is located within transferable elements and can be transferred from a range of E. faecium donor strains to an E. faecium recipient. These results shed new light on the molecular epidemiology of multidrug-resistant E. faecium strains both in the United States and around the world. It will be important to precisely characterize the transferable element that appears to be common to several different strains to better allow us to determine the specific impact of this element on the spread of high-level ampicillin resistance within the species.
Acknowledgments
This study was supported by a Merit Review from the Department of Veterans Affairs and by a grant from the National Institute of Allergy and Infectious Diseases (AI 045626) to L.B.R.
REFERENCES
- 1.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carias, L. L., S. D. Rudin, C. J. Donskey, and L. B. Rice. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirurgi, V. A., S. E. Oster, A. A. Goldberg, and R. E. McCabe. 1992. Nosocomial acquisition of β-lactamase-negative, ampicillin-resistant enterococcus. Arch. Int. Med. 152:1457-1461. [PubMed] [Google Scholar]
- 4.Donobedian, S. M., J. W. Chow, J. M. Boyce, R. E. McCabe, S. M. Markowitz, P. E. Coudron, A. Kuritza, C. L. Pierson, and M. J. Zervos. 1992. Molecular typing of ampicillin-resistant, non-β-lactamase-producing Enterococcus faecium isolates from diverse geographic areas. J. Clin. Microbiol. 30:2757-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskey, C. J., J. R. Schreiber, M. R. Jacobs, R. Shekar, F. Smith, S. Gordon, R. A. Salata, C. Whalen, and L. B. Rice. 1999. A polyclonal outbreak of predominantly VanB vancomycin-resistant enterococci in Northeast Ohio. Clin. Infect. Dis. 29:573-579. [DOI] [PubMed] [Google Scholar]
- 6.Garnier, F., S. Taourit, P. Glaser, P. Courvalin, and M. Galimand. 2000. Characterization of transposon Tn1549, conferring VanB-type resistance in Enterococcus spp. Microbiology 146:1481-1489. [DOI] [PubMed] [Google Scholar]
- 7.Gold, H. S. 2001. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin. Infect. Dis. 33:210-219. [DOI] [PubMed] [Google Scholar]
- 8.Grayson, M. L., G. M. Eliopoulos, C. B. Wennersten, K. L. Ruoff, P. C. DeGirolami, M.-J. Ferraro, and R. C. Moellering, Jr. 1991. Increasing resistance to β-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob. Agents Chemother. 35:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanrahan, J., C. Hoyen, and L. B. Rice. 2000. Geographic distribution of a large mobile element that transfers ampicillin and vancomycin resistance between Enterococcus faecium strains. Antimicrob. Agents Chemother. 44:1349-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris, J. G., Jr., D. K. Shay, J. N. Hebden, R. J. McCarter, Jr., B. E. Perdue, W. Jarvis, J. A. Johnson, T. C. Dowling, L. B. Polish, and R. S. Schwalbe. 1995. Enterococci resistant to multiple antimicrobial agents, including vancomycin: establishment of endemicity in a university medical center. Ann. Int. Med. 123:250-259. [DOI] [PubMed] [Google Scholar]
- 11.Rice, L. B., S. Bellais, L. L. Carias, R. Hutton-Thomas, R. A. Bonomo, P. Caspers, M. G. Page, and L. Gutmann. 2004. Impact of specific pbp5 mutations on expression of β-lactam resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 48:3028-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Rice, L. B., L. L. Carias, S. Marshall, S. D. Rudin, and R. Hutton-Thomas. 2005. Tn5386, and novel Tn916-like mobile element in Enterococcus faecium D344R that interacts with Tn916 to yield a large genomic deletion. J. Bacteriol. 187:6668-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice, L. B., and L. L. Carias. 1998. Transfer of Tn5385, a composite, multiresistance element from Enterococcus faecalis. J. Bacteriol. 180:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice, L. B., S. H. Marshall, and L. L. Carias. 1992. Tn5381, a conjugative transposon identifiable as a circular form in Enterococcus faecalis. J. Bacteriol. 174:7308-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybkine, T., J.-L. Mainardi, W. Sougakoff, E. Collatz, and L. Gutmann. 1998. Penicillin-binding protein 5 sequence alterations in clinical isolates of Enterococcus faecium with different levels of β-lactam resistance. J. Infect. Dis. 178:159-163. [DOI] [PubMed] [Google Scholar]
- 16.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database —USA. Clin. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 17.Schouten, M. A., R. J. Willems, W. A. Kraak, J. Top, J. A. Hoogkamp-Korstanje, and A. Voss. 2001. Molecular analysis of Tn1546-like elements in vancomycin-resistant enterococci isolated from patients in Europe shows geographic transposon type clustering. Antimicrob. Agents Chemother. 45:986-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorisdottir, A. S., L. L. Carias, S. H. Marshall, M. Green, M. J. Zervos, C. Giorgio, L. A. Mermel, J. M. Boyce, A. A. Medeiros, H. Fraimow, and L. B. Rice. 1994. IS6770, an enterococcal insertion-like element useful for determining the clonal relationship of clinical enterococcal isolates. J. Infect. Dis. 170:1539-1548. [DOI] [PubMed] [Google Scholar]
- 19.Welton, L. A., L. A. Thal, M. B. Perri, S. Donabedian, J. McMahon, J. W. Chow, and M. J. Zervos. 1998. Antimicrobial resistance in enterococci isolated from turkey flocks fed virginiamycin. Antimicrob. Agents Chemother. 42:705-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson, R., C. LaBouguenec, L. Gutmann, and T. Horaud. 1985. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to penicillin. J. Gen. Microbiol. 131:1933-1940. [DOI] [PubMed] [Google Scholar]
- 21.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]