Abstract
Chronic pulmonary infections with Pseudomonas aeruginosa are the primary cause of morbidity and mortality in patients with cystic fibrosis (CF). The macrolide antibiotics exhibit immunomodulatory and antivirulence activity. Clinical trials with azithromycin in CF have demonstrated significant improvements in pulmonary function and decreased hospitalizations. The purpose of this study was to compare the pharmacokinetics (PK) of azithromycin in patients with CF and controls. The study was conducted as an open-label, parallel, two-period crossover study involving 12 healthy volunteers and 12 patients with CF. Period 1 examined the serum PK following a single oral and intravenous dose, while period 2 examined the intracellular PK following multiple-dose oral administration. CF subjects differed significantly from controls based on weight (53.1 versus 71.0 kg; P < 0.01) and body mass index (19.7 versus 23.2; P < 0.01), respectively. Ninety-two percent of CF patients were pancreatic insufficient and were receiving pancreatic enzymes. The rate (time to reach maximum serum drug concentration, 3.0 versus 3.0 h; P = 0.78) and extent of absorption (absolute bioavailability, 34.2 versus 42.8%; P = 0.37) were similar in patients with CF and controls, respectively. Distribution to the tissues (rate of drug transfer from the central to the peripheral compartment, 1.22 versus 0.759 h−1; P = 0.03) and elimination (rate of elimination from the central compartment, 0.693 versus 0.492 h−1; P < 0.01) were more rapid in the healthy volunteers than in the CF subjects, respectively. Mononuclear cell concentrations (15.2 ± 6.0 mg/liter) far exceeded the maximum serum drug concentration (∼50-fold), demonstrating significant intracellular accumulation. These results indicate no alteration in dosage of azithromycin is necessary in patients with CF taking pancreatic enzymes.
Chronic pulmonary infections with Pseudomonas aeruginosa are the primary cause of morbidity and mortality in patients with cystic fibrosis (CF). The development of P. aeruginosa biofilms within the lungs of patients with CF creates a significant challenge due to evasion of host responses as well as antibiotic resistance (5, 9). In addition, the biofilms incite an intense host inflammatory response which contributes to the progressive destruction of the airways (3). The macrolide antibiotics have received much attention over the past several years due to the discovery of their antivirulence activity. In particular, the macrolides disrupt the cell-to-cell signaling processes (quorum sensing) responsible for the formation of Pseudomonas biofilms (10, 24). In addition, the macrolides exhibit anti-inflammatory activity through inhibition of NF-κB and the down regulation of proinflammatory cytokines, a reduction in neutrophil accumulation and migration, and neutrophil oxidant production (11, 13, 22, 23, 27). A recent randomized, double-blind clinical trial conducted in CF patients chronically infected with P. aeruginosa demonstrated a significant improvement in lung function and a reduced risk of pulmonary exacerbations in patients receiving azithromycin (AZM) at 500 mg three times weekly over the 6-month study period (19).
The pharmacokinetics (PK) of a number of drugs have shown to be altered in patients with CF. In particular, the bioavailability of lipid-soluble vitamins and oral cyclosporine (not microemulsion formulations) is reduced in patients with CF, which is attributed to reduced pancreatic lipase release (4, 7, 12, 14, 15, 18, 26). In addition, the volume of distribution of some drugs is altered when normalized for body weight due to the reduced adipose tissue in patients with CF. The clearance of certain antibiotics is increased in patients with CF. It has been speculated that the mechanism of the enhanced clearance of certain antibiotics in CF may be related to upregulation of MDR1. Both CFTR (CF transmembrane conductance regulator) and MDR1 are members of the ATP binding cassette family and appear to be coregulated (25). Upregulation of P-glycoprotein in patients with CF may reduce the bioavailability and possibly alter the cellular accumulation of azithromycin, a known substrate for this transporter (21). The purpose of this study was therefore to compare the serum and cellular (peripheral blood mononuclear cells [PBMCs]) exposures of azithromycin in CF subjects with those of healthy volunteers to determine if there are any alterations in the pharmacokinetics in patients with CF.
MATERIALS AND METHODS
Study design.
This was an open-label, single- and multiple-dose pharmacokinetic study of azithromycin in patients with CF and healthy volunteers. The study consisted of two periods. Period 1 was designed to compare the absolute bioavailability (F) and serum pharmacokinetics of AZM in CF patients and healthy volunteers. Subjects were randomized to receive the drug by either the intravenous or oral route, with the first dose using a pseudo-random method. Each subject was then crossed over to receive the remaining dosage form after a 1-week washout period. Period 2 was designed to compare the intracellular uptake and efflux of AZM within PBMCs in CF patients and healthy volunteers.
A sample size of 24 subjects (12 CF patients and 12 healthy volunteers) was chosen to detect a 15% difference in bioavailability with 80% probability (α = 0.05) based on an expected standard deviation of 0.125 (14).
Study subjects.
Subjects were included in the study if they met the following criteria: age, ≥18 years and weight within 30% of ideal body weight. In addition, CF subjects required a confirmed diagnosis of cystic fibrosis (positive sweat chloride test or known CF genotype). Subjects were excluded from the study if they had a history of sensitivity to macrolide antibiotics, were pregnant or nursing, were post-lung transplantation, had a history of drug or alcohol abuse within 1 year of screening, use drugs known to alter AZM concentrations (cyclosporine, phenytoin, carbamazepine, or ergot alkaloids within 30 days of screening), had serum transaminase levels ≥three times upper limit of normal, total bilirubin level ≥1.5 times normal, serum creatinine level ≥1.4, or hematocrit of ≤25%. The study protocol was approved by the institutional review board, and all participants provided written informed consent.
Drug administration and sample collection.
In period 1, a single dose of oral or intravenous azithromycin at 500 mg was administered according to the randomization schedule followed by crossover to the remaining dosage form 1 week later. The intravenous doses were prepared from commercial powder (Pfizer Inc.) and diluted in 250 ml of 5% dextrose in water and administered over 60 min. Oral doses of 2× 250 mg (Pfizer Inc.) were given with a glass of water 30 min after a light breakfast. All subjects received the same meal with the single oral dose. CF patients were allowed to take their normal dose of pancreatic enzymes before the meal. After each dose on day 0 or 7, 5-ml blood samples were drawn at 0, 0.5, 1, 2, 3, 6, 12, 28, 52, and 96 h after the oral dose and 0, 1, 1.5, 2, 3, 4, 7, 13, 29, 53, and 97 h after the start of the intravenous infusion. Samples were centrifuged, and the harvested serum was stored at −70°C until analyzed.
Following completion of period 1, all subjects received three doses of azithromycin at 500 mg (2 × 250 mg) given in a three-times-weekly regimen starting on study day 9. Blood samples of 20 ml were obtained in tubes containing heparin on days 11, 14, 16, 25, and 32.
Isolation of mononuclear cells.
Whole blood was gently poured into one 50-ml centrifuge tube. The sample was then diluted with 20 ml of phosphate-buffered saline (PBS). The tube was then inverted to mix three times. Using a sterile syringe and 20-gauge needle, 10 ml of Ficoll-Hypaque was carefully transferred to the bottom of blood sample. The mixture was then centrifuged for 30 min at 1,300 rpm at room temperature. Mononuclear cells were then aspirated using a glass Pasteur pipette, transferred to a 15-ml centrifuge tube, and resuspended with 10 ml of PBS. The tube was inverted to mix three times. The mixture was then centrifuged for 10 min at 1,500 rpm at room temperature. The supernatant was then discarded, and the cell pellet was resuspended, followed by a repeat washing of the cells with 10 ml of PBS. Finally, the supernatant was discarded and the mononuclear cells were resuspended with exactly 0.5 ml of PBS. To determine the cell count, an aliquot of 10 μl was diluted with 90 μl of Turk's solution and vortexed for 3 to 5 s. A 20-μl aliquot was then placed into the hemacytometer, and the cells were counted. The sample was then centrifuged at 13,000 rpm for 5 min, and the cell pellet was stored at −70°C until analyzed.
Intracellular sample preparation.
Isolated mononuclear cell pellets were suspended in 250 μl of saline and lysed. To 50 μl of the mononuclear cell suspension, 100 μl of ice-cold cell lysing solution consisting of 70:30 methanol and 0.05 mM Tris HCl (vol/vol) was added. The cellular lysate was sonicated for 5 min to ensure thorough cellular breakdown. To this suspension, 50 μl of internal standard, erythromycin (50 ng/ml), was added. The entire solution was protein precipitated through addition of 100 μl of methanol, and the entire solution was centrifuged for 15 min at 13,000 rpm. The supernatant was transferred into injection vials, where 30 μl of the sample was quantified using liquid chromatography tandem mass spectrometry (LC-MS/MS) methods described in the section on analytical determination of azithromycin concentration.
Calibration curves were constructed using mononuclear cell pellets from 3 × 106 cells spiked with a specified amount of azithromycin resulting in concentrations ranging from 0.5 to 100 ng/ml. To validate this method, a standard addition method was employed using one subject's sample to test if components of the matrix could affect the response from the analyte.
The intracellular drug concentration in PBMCs (CPBMC) was determined by the following formula: CPBMC = (measured PBMC concentration/actual cell count for each sample)/(421 fl), where 421 fl is the mean PBMC volume (16).
Serum sample preparation.
To 100 μl of serum, 100 μl of methanol and 100 μl of erythromycin (50 ng/ml) were added, and the entire mixture was vortexed vigorously. An aliquot of 500 μl of acetonitrile was added to precipitate the serum proteins, and then the sample was centrifuged at 13,000 rpm for 15 min. The supernatant was then transferred into an Eppendorf vial, and the entire content was evaporated to dryness using a steady stream of dried air. The residues were reconstituted with 200 μl of 50:50 (vol/vol) methanol and 20 mM ammonium acetate, pH 5.1. The samples were then centrifuged at 13,000 rpm to remove any debris, and the supernatant was transferred into injection vials.
Analytic determination of azithromycin concentrations.
Both serum and intracellular concentrations of azithromycin were analyzed using LC-MS/MS, which consists of an Agilent 1100 high-performance liquid chromatography (HPLC) system coupled to a triple quadrupole mass spectrometer (Sciex API 3+). A reversed-phase C18 Hypurity column (Thermo; Keystone) (4.6 by 50 mm, 5-μm packing) was used to separate the analytes. The mobile phase used to elute the analytes consisted of 70% (vol/vol) methanol in 30% of 20 mM ammonium acetate, adjusted to pH 5 with acetic acid. The flow rate was set at 0.3 ml/min for analysis. The sample was introduced into the MS/MS by an LC pump through an articulated ion spray inlet used in conjunction with a heated turbo probe (turbo ion spray). In this study, ion spray ionization was used to ionize the analytes. In addition, a heated turbo nitrogen stream was used to assist the desolvation process and increase ionization efficiency. The retention times of azithromycin and erythromycin were 2.5 and 3.4 min, respectively. The mass transition was from 749.6 to 591.4 and 734.4 to 576.2 for azithromycin and erythromycin, respectively. The calibration curve range for serum samples was 5 to 1,000 ng/ml, with a lower limit of quantification of 5 ng/ml. The calibration curve range for intracellular samples was 0.5 to 100 ng, with a lower limit of quantification of 0.5 ng. The assay was linear over this range (r2 > 0.99) and demonstrated excellent interday accuracy and precision, with the mean bias less than 4% and coefficients of variation less than 8%.
Data analysis. (i) Pharmacokinetic analysis.
Serum concentrations of AZM were analyzed using noncompartmental and compartmental methods. Parameter estimates from the noncompartmental analysis included the maximum serum drug concentration (Cmax), time to reach Cmax (Tmax), and the area under the serum drug concentration-time curve from time zero to 96 h (AUC0-96). Both Cmax and Tmax were determined from visual inspection of the observed data. The AUC0-96 was calculated using the linear trapezoidal rule.
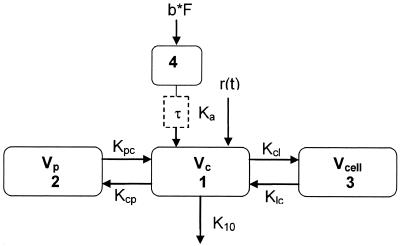
Compartmental PK analysis was performed using ADAPT II software (Biomedical Simulations Resource, University of Southern California, Los Angeles) to provide parameter estimates for serum and intracellular disposition in both groups (CF and healthy volunteers). Parameter estimates included the absolute bioavailability (F), absorption rate constant (Ka), volume of distribution for the central compartment (Vc), rate of drug transfer to and from the central and peripheral compartment (Kcp and Kpc, respectively), rate of drug transfer to and from the central and cellular compartment (Kcl and Klc, respectively), volume of distribution for the cellular compartment (Vcell), and elimination rate from the central compartment (K10). A two-compartment distributive model using the maximum-likelihood method was fit to the serum concentration data. Lag times were fixed for each subject based on the time with the first measurable serum drug concentration. A three-compartment distributive model (Fig. 1) using the median values from the two-compartment model as initial estimates for the serum parameters was then fit to the serum and intracellular concentration data. The maximum a posteriori (MAP) Bayesian method in ADAPT II was then used in an iterative approach and fit to the serum and the intracellular data. The median and standard deviation from each run were incorporated into the model file, and the data from each subject were then reanalyzed. This process continued until the parameter values between iterations stabilized (which occurred after seven iterations).
FIG. 1.
Four-compartment PK model. Numbers indicate the central compartment (no. 1), peripheral compartment (no. 2), cellular compartment (no. 3), and absorptive compartment (no. 4). b, oral bolus; F, bioavailability; τ, oral lag time; Vc, volume of central compartment; Vp, volume of peripheral compartment; Vcell, volume of cellular compartment; Kcp and Kpc, intercompartmental rate constants between central and peripheral compartments; Kcl and Klc, intercompartmental rate constant between central and cellular compartments; r(t) = intravenous infusion rate; K10, elimination rate from central compartment; Ka, absorption rate constant.
(ii) Statistical analysis.
A comparison of demographic characteristics and pharmacokinetic parameters between CF patients and controls was performed using the Mann-Whitney or Fishers exact test, where appropriate. The significance level was 0.05. Analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
The demographic and clinical characteristics of the two groups are shown in Table 1. There were a greater number of males in the healthy volunteer group and a greater number of female CF patients; however, this did not reach statistical significance. The weight and body mass index of the CF patients were significantly less than those of the healthy volunteers, which likely reflects differences in nutritional status between the two groups.
TABLE 1.
Subject characteristics
| Characteristic | Subjectsa
|
P | |
|---|---|---|---|
| CF | Healthy volunteers | ||
| No. of males/females | 4/8 | 9/4 | 0.12 |
| Age (yr)a | 26.0 (6.75) | 30.0 (8.59) | 0.72 |
| Height (in.)a | 64.7 (3.55) | 69.2 (4.02) | 0.08 |
| Weight (kg)a | 53.1 (5.68) | 71.0 (13.3) | <0.01 |
| Body mass indexa | 19.7 (1.58) | 23.2 (2.77) | <0.01 |
| No. with pancreatic insufficiency/total | 11/12 | 0/13 | <0.01 |
Median values are shown, with the standard deviation given in parentheses.
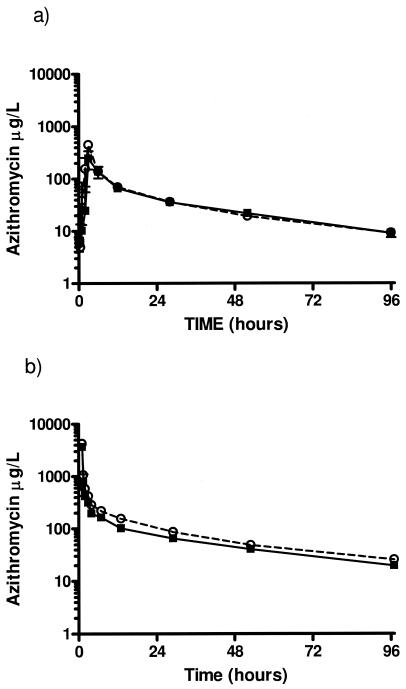
The results of the noncompartmental analysis revealed no significant differences in the pharmacokinetics of oral azithromycin between the CF and normal volunteer subjects (Table 2). The similarity in disposition of oral azithromycin between the two groups is depicted graphically in Fig. 2. Likewise, compartmental PK analysis (Table 3) demonstrated that the disposition of azithromycin is remarkably similar between the two groups, with the exception of the more rapid distribution to the tissues (Kcp, 1.22 versus 0.759; P = 0.03) and elimination (K10, 0.693 versus 0.492, P < 0.01) in the healthy volunteers when compared with the CF subjects, respectively. These relatively small differences are more clearly depicted when examining the serum concentration time profile following the intravenous dose (Fig. 2). Importantly, the Ka and F did not differ between the two groups, indicating the rate and extent of absorption are unaffected in the CF subjects. The cellular PK data demonstrate extensive accumulation within PBMCs (large Kcl/Klc ratio) with significant intracellular binding to cell constituents (large Vcell). The cellular concentration-time curve is flattened relative to the serum due to the long intracellular half-life (Fig. 3). The intracellular concentrations were approximately 100-fold greater than the trough serum drug concentrations, which is consistent with the extensive cellular accumulation.
TABLE 2.
Noncompartmental PK parameters
| Parameter | Subjectsa
|
P | |
|---|---|---|---|
| CF | Healthy volunteers | ||
| Cmax (μg/liter) | 567 (307) | 304 (344) | 0.29 |
| Tmax (h) | 3.00 (1.5) | 3.00 (1.81) | 0.78 |
| AUC0-96 (h · μg/liter) | 4,407 (1,507) | 4,365 (1,936) | 0.85 |
| F (%) | 34.2 (13.6) | 42.8 (13.4) | 0.37 |
Median values are shown, with the standard deviation given in parentheses.
FIG. 2.
Median azithromycin serum concentrations versus time following a single oral (a) and a single intravenous (b) dose of 500 mg. Squares, healthy volunteer subjects; circles, cystic fibrosis subjects. Each value represents the median ± standard error (n = 24).
TABLE 3.
Compartmental PK parameters
| Parameter | Subjectsa
|
P | |
|---|---|---|---|
| CF | Healthy volunteers | ||
| Vc (liters) | 64.8 (22.8) | 44.4 (27.4) | 0.31 |
| K10 (h−1) | 0.492 (0.650) | 0.692 (0.691) | <0.01 |
| Ka (h−1) | 0.483 (0.877) | 0.452 (0.274) | 0.60 |
| Lag time (h) | 0.500 (0.498) | 0.500 (1.03) | 0.29 |
| F (%) | 34.8 (13.5) | 40.1 (17.8) | 0.34 |
| Vp (liters) | 412 (232) | 557 (367) | 0.28 |
| Kcp (h−1) | 0.759 (0.394) | 1.22 (0.617) | 0.03 |
| Kpc (h−1) | 0.120 (0.171) | 0.157 (0.0759) | 0.93 |
| Vcell (liters) | 5.40 (3.05) | 4.59 (5.21) | 0.88 |
| Kcl (h−1) | 0.282 (0.459) | 0.671 (0.437) | 0.41 |
| Klc (h−1) | 0.0151 (0.00777) | 0.0109 (0.00604) | 0.98 |
Median values are shown, with the standard deviation given in parentheses.
FIG. 3.
Representative plot with fitted serum (r2 = 0.933) and intracellular (r2 = 0.900) azithromycin concentrations.
DISCUSSION
Azithromycin is now a part of routine care for patients with CF who are chronically infected with P. aeruginosa, based on the positive outcomes observed in a phase III study (19). The present study was conducted to compare the serum and cellular (PBMC) exposures of azithromycin in CF subjects with those in healthy volunteers to determine if the bioavailability and/or serum and cellular pharmacokinetics are altered in patients with CF.
Our primary finding was that the oral absorption of azithromycin in patients with CF is comparable to that of age-matched healthy control subjects. The absorption in both groups was relatively rapid (3 h) following a short lag time (0.5 h). The bioavailability of 35% and 40% in the CF and healthy volunteer groups, respectively, indicates incomplete absorption, similar to the findings of others (6, 8). Importantly, however, the lack of a difference between the two groups suggests that either azithromycin absorption is unaffected by pancreatic insufficiency or supplementation by administration of exogenous enzymes was able to overcome the insufficiency. The fact that many CF patients have difficulty maintaining normal nutrition and require high-dose lipid-soluble vitamin supplementation despite the use of pancreatic enzymes favors the former explanation.
In comparing the serum PK between the two groups significant differences were noted for K10 and Kcp. The elimination from the central compartment (K10) is dependent on clearance and the volume of central compartment; therefore, one reason for the difference could be due to alterations in either the volume of the central compartment, clearance, or both between healthy volunteers and CF patients. Although the difference in the volume of the central compartment (Vc) between the two groups was not statistically significant, CF patients exhibited a slightly larger Vc. Therefore, the difference in K10 is likely due to a combination of a reduced clearance and larger Vc in the CF subjects. The primary route of elimination for azithromycin is through biliary excretion. Hepatobiliary disease is relatively common in patients with CF and is the second leading cause of death. Hepatomegaly is present in 30% of children and young adult CF patients and is associated with pancreatic insufficiency (20). Although all subjects were screened with standard liver function tests and were excluded if they had hepatobiliary abnormalities, it is possible that some of the CF patients may have had subclinical hepatobiliary disease resulting in reduced biliary excretion of azithromycin. The larger Vc in patients with CF likely represents the differences in body composition between the two groups. Patients with CF typically have reduced adipose tissue due to malnutrition secondary to pancreatic insufficiency. The greater amount of adipose tissue in the healthy subjects would therefore account for the more rapid (Kcp) and greater tissue distribution (larger Vp and smaller Vc) than those of the CF subjects.
The macrolide antibiotics exhibit pharmacokinetic properties characterized by high and sustained concentrations within tissues and intracellular compartments and conversely low concentrations in serum (1, 2, 6, 8, 28). Our results were consistent with this finding demonstrating extensive tissue distribution (Kcp/Kpc ratio = 6.3 and 7.8) and slow release within PBMCs (Kcl/Klc ratio = 18.7 and 61.6) in CF and healthy subjects, respectively. Several studies have evaluated the intracellular concentrations of azithromycin in healthy volunteers and patients (1, 2, 6, 17, 28). The results of these studies differ slightly from one another due to variations in the dosage regimen administered, the timing of the measurements, as well as the specific cell type analyzed. Three of the studies measured intracellular concentrations from monocytes or mononuclear cells (1, 17, 28). The intracellular concentrations reported in these studies are higher than what we found in the present study and demonstrate wide variability. The most likely explanation for the variability is due to differences in cellular isolation techniques which may result in differences in the population of cells isolated. In addition, some drug may have been lost during the repeat washing of the cells. Regardless, the results of the present study demonstrate extensive tissue and cellular accumulation with no significant differences between patients with CF and control subjects. The clinical significance of the sustained intracellular concentrations of azithromycin in patients with CF demonstrating chronic infections with P. aeruginosa requires further investigation.
Conclusions.
The bioavailability and rate of absorption appear to be unaltered in patients with CF. Based on the results of our study, no alteration in dosage of AZM is therefore necessary in patients with CF taking pancreatic enzymes. In addition, the pharmacokinetics of azithromycin have been found to be characterized by extensive accumulation and slow release within the intracellular and tissue compartments without a significant difference in CF patients compared to non-CF subjects.
Acknowledgments
This research was supported by the Webb Cystic Fibrosis Research Award and the John T. Nicoloff General Clinical Research Center (grant M01-RR-00043 from NIH/NCRR).
REFERENCES
- 1.Amsden, G. W., and C. L. Gray. 2001. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 47:61-66. [DOI] [PubMed] [Google Scholar]
- 2.Amsden, G. W., A. N. Nafziger, and G. Foulds. 1999. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob. Agents Chemother. 43:163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonfield, T. L., M. W. Konstan, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 4.Bouquet, J., M. Sinaasappel, and H. Neijens. 1988. Malabsorption in cystic fibrosis: mechanisms and treatment. J. Pediatr. Gastroenterol. Nutr. 7(Suppl. 1):S30-S35. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. 2001. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 9:50-52. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, E., M. E. Klepser, T. B. Klepser, C. H. Nightingale, and L. G. Hunsicker. 2000. Comparison of the serum and intracellular pharmacokinetics of azithromycin in healthy and diabetic volunteers. Pharmacotherapy 20:657-661. [DOI] [PubMed] [Google Scholar]
- 7.Feranchak, A. P., M. K. Sontag, J. S. Wagener, K. B. Hammond, F. J. Accurso, and R. J. Sokol. 1999. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J. Pediatr. 135:601-610. [DOI] [PubMed] [Google Scholar]
- 8.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 9.Henry, R. L., C. M. Mellis, and L. Petrovic. 1992. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 12:158-161. [DOI] [PubMed] [Google Scholar]
- 10.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe, A., and M. Rosenthal. 2002. Macrolides in the respiratory tract in cystic fibrosis. J. R. Soc. Med. 95(Suppl. 41):27-31. [PMC free article] [PubMed] [Google Scholar]
- 12.James, D. R., G. Owen, I. A. Campbell, and M. C. Goodchild. 1992. Vitamin A absorption in cystic fibrosis: risk of hypervitaminosis A. Gut 33:707-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi, T., K. Hagiwara, Y. Honda, K. Gomi, T. Kobayashi, H. Takahashi, Y. Tokue, A. Watanabe, and T. Nukiwa. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J. Antimicrob. Chemother. 49:745-755. [DOI] [PubMed] [Google Scholar]
- 14.Knoop, C., I. Vervier, P. Thiry, M. De Backer, J. M. Kovarik, A. Rousseau, P. Marquet, and M. Estenne. 2003. Cyclosporine pharmacokinetics and dose monitoring after lung transplantation: comparison between cystic fibrosis and other conditions. Transplantation 76:683-688. [DOI] [PubMed] [Google Scholar]
- 15.Lark, R. K., G. E. Lester, D. A. Ontjes, A. D. Blackwood, B. W. Hollis, M. M. Hensler, and R. M. Aris. 2001. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am. J. Clin. Nutr. 73:602-606. [DOI] [PubMed] [Google Scholar]
- 16.Nibbering, P. H., T. P. Zomerdijk, A. J. Corsel-Van Tilburg, and R. Van Furth. 1990. Mean cell volume of human blood leukocytes and resident and activated murine macrophages. J. Immunol. Methods 129:143-145. [DOI] [PubMed] [Google Scholar]
- 17.Olsen, K. M., G. S. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters, S. A., and C. J. Rolles. 1993. Vitamin therapy in cystic fibrosis: a review and rationale. J. Clin. Pharm. Ther. 18:33-38. [DOI] [PubMed] [Google Scholar]
- 19.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Cambell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1748-1756. [DOI] [PubMed] [Google Scholar]
- 20.Sokol, R. J., and P. R. Durie. 1999. Recommendations for management of liver and biliary tract disease in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 28(Suppl. 1):S1-S13. [DOI] [PubMed] [Google Scholar]
- 21.Sugie, M., E. Asakura, Y. L. Zhao, S. Torita, M. Nadai, K. Baba, K. Kitaichi, K. Takagi, K. Takagi, and T. Hasegawa. 2004. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 48:809-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeoka, K., T. Ichimiya, and T. Yamasaki. 1998. The in vitro effect of macrolides on the interaction of human polymorphonuclear leukocytes with Pseudomonas aeruginosa in biofilm. Chemotherapy 44:190-197. [DOI] [PubMed] [Google Scholar]
- 23.Tamaoki, J., J. Nakata, E. Tagaya, and K. Konno. 1996. Effects of roxithromycin and erythromycin on interleukin 8-induced neutrophil recruitment and goblet cell secretion in guinea pig tracheas. Antimicrob. Agents Chemother. 40:1726-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tateda, K., R. Comte, J.-C. Pechere, T. Köhler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trezise, A. E., R. Ratcliff, T. E. Hawkins, M. J. Evans, T. C. Freeman, P. R. Romano, C. F. Higgins, and W. H. Colledge. 1997. Co-ordinate regulation of the cystic fibrosis and multidrug resistance genes in cystic fibrosis knockout mice. Hum. Mol. Genet. 6:527-537. [DOI] [PubMed] [Google Scholar]
- 26.Tsang, V. T., A. Johnston, F. Heritier, N. Leaver, M. Hodson, and M. Yacoub. 1994. Cyclosporin pharmacokinetics in heart-lung transplant recipients with cystic fibrosis. Effects of pancreatic enzymes and ranitidine. Eur. J. Clin. Pharmacol. 46:261-265. [DOI] [PubMed] [Google Scholar]
- 27.Wales, D., and M. Woodhead. 1999. The anti-inflammatory effects of macrolides. Thorax 54:S58-S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildfeuer, A., H. Laufen, and T. Zimmermann. 1996. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob. Agents Chemother. 40:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]