Abstract
Caspofungin had diminished activity in vitro against Candida albicans at concentrations of 8 to 32 μg/ml. This phenomenon was markedly attenuated in a Δmkc1/Δmkc1 deletion mutant and by the addition of cyclosporine to the wild type. Short exposure to these caspofungin concentrations resulted in MKC1 up-regulation, suggesting roles of cell wall integrity and calcineurin pathways.
The safety profile and clinical efficacy of caspofungin (CAS) has raised questions as to whether its effectiveness could be further improved by administering higher dosages. However, in vitro studies as well as some dosage escalation studies in animals have reported a paradoxical attenuation of CAS activity at higher drug concentrations (5, 17, 21, 23). These concentrations are comparable to plasma CAS levels achieved in humans at recommended doses (22). The mechanism of the attenuated CAS activity at higher concentrations and its clinical relevance are unknown. Studies of the genetically amenable yeast Saccharomyces cerevisiae have suggested links with both the intracellular protein kinase C (PKC) cell wall integrity and calcineurin pathways (1, 4, 7, 13, 18, 20). In view of the evolutionary conservation of several key cellular processes, including homeostatic responses toward drug-induced damage of the fungal cell wall (2, 19), we hypothesized that the cell wall integrity and calcineurin pathways may play an important role in the paradoxical attenuation of CAS activity at supra-MIC exposures. To this end, we examined expression levels of MKC1, a central kinase of the PKC pathway in Candida albicans, and the effects of MKC1 gene deletion on the paradoxical activity observed with CAS. We also explored the importance of the calcineurin pathway in the attenuation of CAS activity at high doses by testing isolates in the presence of the calcineurin inhibitor cyclosporine.
(This research was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 30 October to 2 November 2004 [abstract M-1682].)
We tested Candida albicans strain ATCC 90028, a C. albicans Δmkc1/Δmkc1 homozygous mutant (Δmkc1::hisG-URA3-hisG/Δmkc1::hisG-Δura3::imm434/Δura3::imm434) (15), and the corresponding isogenic wild-type strain CAI4 (Δura3::imm434/Δura3::imm434) (3). Fresh stock solutions were prepared by dissolving caspofungin acetate powder (Merck & Co., Inc., Whitehouse Station, NJ) in 0.85% saline. Fresh stocks of cyclosporine (Sigma, St. Louis, MO) were prepared in 100% ethanol and further diluted in RPMI 1640 buffered with 0.165 M morpholinepropanesulfonic acid (MOPS; pH 7.0). 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT)-based in vitro viability studies were conducted at CAS concentrations from 0 to 1,024 μg/ml using a modification of the method reported by Meletiadis et al. (12). All isolates were also tested in the presence of CAS plus cyclosporine (0.625 μg/ml). Absorbance was read at 492 nm, and readings were converted to percent absorbance, with the growth control set at 100% and the medium control at 0%.
For gene expression analysis, C. albicans strain ATCC 90028 was adjusted to an inoculum of ∼5 × 105 CFU/ml and incubated at 37°C with shaking to the midlogarithmic growth phase. Yeast cells were then exposed to CAS (0, 0.03, 1.0, 16.0, and 64.0 μg/ml) for 10 min. This brief exposure time was chosen in view of the fact that the transcription of SLT2 mRNA in S. cerevisiae (the homologue of MKC1 in C. albicans) in response to CAS is rapid and transient (18). Cells were harvested, and total RNA was extracted using QIAGEN RNeasy Protect mini kits (QIAGEN, Valencia, CA). Reverse transcription was performed (GeneAmp RNA PCR kit; Applied Biosystems, Inc., Foster City, CA), and relative gene expression was determined using real-time PCR (ABI PRISM 7000 sequence detection system) with primers and probes specific for DNA encoding MKC1 (GenBank accession no. X76708) (14). Relative gene expression levels were calculated by the 2−ΔΔCT method using 18S rRNA as the housekeeping gene (9). One-way analysis of variance with Bonferroni's correction for multiple comparisons was used to assess differences in CAS activity. Changes in gene expression were compared by analysis of variance with Tukey's posttest. All experiments were performed in at least triplicate on separate days.
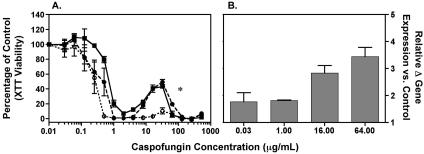
CAS demonstrated marked concentration-dependent activity against both wild-type C. albicans strains (ATCC 90028 and CAI4), with a 95 to 100% reduction in absorbance at 1 to 2 μg/ml and a significant paradoxical increase in viability at CAS concentrations ranging from 8 to 32 μg/ml (16 to 64× MIC) (Fig. 1A). In contrast, the paradoxical effect of CAS against the Δmkc1/Δmkc1 isolate was significantly decreased. In addition, short exposure (10 min) of the C. albicans ATCC 90028 strain to higher CAS concentrations (16 and 64 μg/ml) increased MKC1 transcription compared to what occurred with lower concentrations (Fig. 1B). Our data and those of others (1, 18) show that a rapid and transient induction of genes encoding components of the cell wall integrity pathway occurs in response to CAS. Furthermore, in a large-scale genome-wide screen of S. cerevisiae deletion mutants, four genes of the PKC cell wall integrity pathway (SLG1, BCK1, FKS1, and SMI1/KNR4) were identified as affecting the sensitivity of S. cerevisiae specifically to CAS (10). In contrast, Liu et al., in a recent genome-wide expression profiling study of C. albicans including subinhibitory concentrations of CAS for 180 min found that the induction of PKC genes was not prominent (8). Future genome-wide approaches that compare exposures of C. albicans at different time intervals to either subinhibitory or inhibitory concentrations of CAS associated with the paradoxical effect and the use of other antifungal agents as controls (e.g., azoles, amphotericin B) would be informative to further address the specificity of up-regulation of PKC-encoding genes in the attenuation of cidality following exposure to high concentrations of cell wall-active agents. Assessment of the active phosphorylated Mkc1p protein on the downstream targets of the cell wall integrity pathway upon exposure to CAS may be further informative.
FIG. 1.
(A) In vitro viability assay for XTT. Percentages of viability (means ± standard errors of the means [SEM] as measured at 492 nm) relative to the viability of the control are plotted on the y axis, and increasing concentrations of caspofungin (0 to 1,024 μg/ml) are plotted on the x axis. A decrease in the paradoxical attenuation of caspofungin activity was observed in the Δmkc1/Δmkc1 homozygous-knockout mutant compared to that of C. albicans strain ATCC 90028 and the parent strain CAI4 (*, P < 0.001 for Δmkc1/Δmkc1 at concentrations of 8 to 32 μg/ml). ▪, C. albicans strain ATCC 90028; •, CAI4; ○, Δmkc1/Δmkc1 strain. (B) Relative MKC1 gene expression in Candida albicans strain ATCC 90028 (mean + SEM). Changes in gene expression compared to that of cells not exposed to caspofungin are plotted on the y axis and capsofungin concentrations on the x axis. Expression levels were normalized to 18S rRNA.
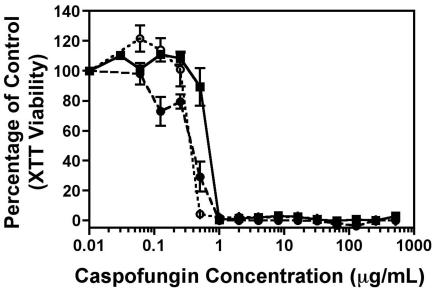
In view of the fact that a functional link might exist between the calcineurin pathway of fungi and the tolerance to antifungal agents (2, 6), we examined whether the addition of cyclosporine, a known inhibitor of the calcineurin pathway, modulates the paradoxical CAS effect. Indeed, the paradoxical effect was completely absent in both wild-type strains at a cyclosporine concentration of 0.625 μg/ml (Fig. 2), while the other portions of the CAS dose-response curve remained unchanged. Future studies examining the use of other calcineurin inhibitors and utilizing genome-wide expression profiling will be of interest.
FIG. 2.
In vitro viability assay for XTT. Percentages of viability (means ± standard errors of the means as measured at 492 nm) relative to the viability of the control are plotted on the y axis, and increasing concentrations of caspofungin (0 to 1,024 μg/ml) in the presence of cyclosporine (0.625 μg/ml) are plotted on the x axis. ▪, C. albicans strain ATCC 90028; •, CAI4; ○, Δmkc1/Δmkc1 strain.
Our data indicate that, following exposure of C. albicans to high CAS concentrations, calcineurin-mediated and PKC-mediated signaling pathways act to regulate the functionally redundant cellular events important in resisting the toxic effects of CAS. In fact, cell wall integrity and calcineurin pathways have been shown to perform independent but related functions in S. cerevisiae (13). Alternatively, other indirect mechanisms might be operative in the phenomenon of the attenuated cidality of high concentrations of CAS. Up-regulation of 1,3-β-glucan synthesis, increases in the chitin content of the cell wall, and increased export of cell wall components for cell wall repair (10, 11, 16, 20, 24) need further investigation.
These preliminary data should invite further studies of the role of the PKC and calcineurin pathways in the orchestrated regulation of cell wall-sensing pathways, the stress response, and the activities of the echinocandins.
Acknowledgments
A University of Texas M. D. Anderson faculty E. N. Cobb Scholar Award Research Endowment was given to D.P.K. R.E.L. received research support from and served as a consultant to Merck & Co., Pfizer, Astellas, Enzon, and Schering-Plough; D.P.K. received research support from and served as a consultant to Merck & Co., Pfizer, Astellas, Enzon, and Schering-Plough; and R.A.P. received research support from Merck & Co., Bristol Myers Squibb, Pfizer, Ortho McNeil, and Enzon.
Isolates (CAI4 and the Δmkc1/Δmkc1 isolate) were kindly provided by Jesús Pla, Universidad Complutense de Madrid, Madrid, Spain, and P. David Rogers, University of Tennessee Health Science Center.
REFERENCES
- 1.Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. S. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. [DOI] [PubMed] [Google Scholar]
- 2.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemenway, C. S., and J. Heitman. 1999. Calcineurin. Structure, function, and inhibition. Cell Biochem. Biophys. 30:115-151. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim, A. S., J. C. Bowman, V. Avanessian, K. Brown, B. Spellberg, J. E. Edwards, Jr., and C. M. Douglas. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-D-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontoyiannis, D. P., R. E. Lewis, N. Osherov, N. D. Albert, and G. S. May. 2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51:313-316. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz, M. B., and C. M. Douglas. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79-86. [DOI] [PubMed] [Google Scholar]
- 8.Liu, T. T., R. E. Lee, K. S. Barker, L. Wei, R. Homayouni, and P. D. Rogers. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 49:2226-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
9.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the
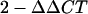 method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar] - 10.Markovich, S., A. Yekutiel, I. Shalit, Y. Shadkchan, and N. Osherov. 2004. Genomic approach to identification of mutations affecting caspofungin susceptibility in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3871-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazur, P., N. Morin, W. Baginsky, M. el-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-D-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura, T., T. Ohmoto, D. Hirata, E. Tsuchiya, and T. Miyakawa. 1996. Genetic evidence for the functional redundancy of the calcineurin- and Mpk1-mediated pathways in the regulation of cellular events important for growth in Saccharomyces cerevisiae. Mol. Gen. Genet. 251:211-219. [DOI] [PubMed] [Google Scholar]
- 14.Navarro-Garcia, F., R. Alonso-Monge, H. Rico, J. Pla, R. Sentandreu, and C. Nombela. 1998. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology 144:411-424. [DOI] [PubMed] [Google Scholar]
- 15.Navarro-García, F., M. Sánchez, J. Pla, and C. Nombela. 1995. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 15:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osherov, N., G. S. May, N. D. Albert, and D. P. Kontoyiannis. 2002. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinoso-Martin, C., C. Schuller, M. Schuetzer-Muehlbauer, and K. Kuchler. 2003. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 20.Smits, G. J., H. van den Ende, and F. M. Klis. 2001. Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology 147:781-794. [DOI] [PubMed] [Google Scholar]
- 21.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]