Abstract
We investigated the fungicidal activity of caspofungin (CAS) and amphotericin B (AMB) against 16 clinical isolates of Candida glabrata. The minimum fungicidal concentrations (MFCs) of CAS were similar to those of AMB, ranging from 2.0 to >8.0 μg/ml. Time-kill assays performed on selected isolates showed that AMB was fungicidal at concentrations four times the MIC while CAS was not. A neutropenic-mouse model of disseminated infection was utilized to determine the residual fungal kidney burden. While doses as low as 0.3 and 1 mg/kg of body weight/day of CAS and AMB, respectively, were effective at reducing the counts with respect to controls, organ sterilization was reached when both drugs were administered at 5 mg/kg/day. Our study reveals that, similar to AMB, CAS has the potential for a fungicidal effect in vivo against this difficult-to-treat fungal pathogen.
One of the most desirable properties of a given antimicrobial agent is represented by its ability to “kill” the microorganism. Theoretically, this important characteristic would allow a more rapid and a complete organ clearance during an infection. While for several antibacterial agents activity has been widely documented, among the antifungal agents currently in use in clinical practice only amphotericin B (AMB) is believed to exert a fungicidal activity against many yeasts and filamentous fungi (13). This important property makes this polyene still the mainstay of antifungal therapy for several life-threatening fungal infections.
The new echinocandin compound caspofungin (CAS) has the potential for fungicidal activity against Candida spp. and has been shown to be better tolerated than AMB deoxycholate (1, 4, 5, 7, 13).
Candida glabrata has recently emerged as a significant pathogen, and there are increasing numbers of reports showing its important role in causing either superficial or deep-seated infections (6, 8, 12, 14). Systemic infections due to C. glabrata are characterized by a high mortality rate, and they are difficult to treat due to a reduced susceptibility of this species to azole drugs, especially to fluconazole (12, 14). A fungicidal drug for infections due to this emerging pathogen would be beneficial.
Therefore, in this study we compared the in vitro and in vivo activities of CAS and AMB against C. glabrata.
MATERIALS AND METHODS
Isolates.
A total of 16 clinical isolates of Candida glabrata were used in this study. The isolates were recovered from the gastrointestinal, respiratory, and urinary tracts; blood; and other sterile body fluid specimens. Each strain represented a unique isolate from a patient. Yeast isolates were identified at the species level by conventional morphological and biochemical methods (9) and stored at −70°C in 10% glycerol. Before the initiation of the study, yeast isolates were subcultured on antimicrobial-agent-free medium to ensure viability and purity.
Drugs.
CAS was used as a commercial preparation (Cancidas; Merck Sharp & Dohme) for both in vitro and in vivo experiments. It was dissolved in sterile distilled water and in sterile saline solution for in vitro and in vivo studies, respectively. AMB was used as pure powder (Sigma) for in vitro studies and as a commercial preparation (Fungizone; Bristol-Myers Squibb) for in vivo studies. It was dissolved in dimethyl sulfoxide and in sterile saline solution for in vitro and in vivo studies, respectively.
Broth dilution.
Antifungal susceptibility testing was performed by a broth microdilution method in accordance with the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) recommendations (10). The final concentrations of both drugs ranged from 0.0078 to 8 μg/ml. Plates were incubated at 35°C for 48 h. Readings were performed spectrophotometrically with a Dynatech Instruments MR700 plate reader set at 490 nm. CAS and AMB MICs were considered as the first concentrations of the antifungal agent at which the turbidity in the well was, respectively, 50% or 90% less than that in the control well (10, 11). After MICs were read, 100-μl samples from each well above the MIC were withdrawn and plated in duplicate onto 150-mm Sabouraud dextrose agar plates. Inoculated plates were incubated at 35°C, and minimum fungicidal concentrations (MFCs) were recorded after 48 h. The MFC was defined as the lowest concentration of drug that caused total inhibition of growth.
Killing curves.
Three to five colonies of C. glabrata isolates 4205 and 4293 from a 48-h growth plate were suspended in 10 ml of sterile distilled water, and the turbidity was adjusted using spectrophotometric methods to a 0.5 McFarland standard (approximately 1 × 10 6 to 5 × 10 6 CFU/ml). One milliliter of the adjusted fungal suspension was added to 9 ml of either RPMI 1640 medium buffered with MOPS (morpholinepropanesulfonic acid) or a solution of growth medium plus an appropriate amount of drug. Both CAS and AMB were used at concentrations of 0.5, 1.0, 2.0, and 4.0 μg/ml, which corresponded to one-half, one, two, and four times the MIC, respectively. Test solutions were placed on a shaker and incubated at 35°C. At time points 0, 2, 6, and 24 h following the introduction of the test isolate into the system, 100-μl aliquots were removed from each test solution. After 10-fold serial dilutions, a 50-μl aliquot from each dilution was streaked in triplicate onto Sabouraud dextrose agar plates for colony count determination. Following incubation at 35°C for 48 h, the number of CFU on each plate was determined. The limit of detection was 20 CFU/ml. Fungicidal activity was considered to be achieved when the number of CFU per milliliter was <99.9% compared with the initial inoculum size (13). Experiments were performed in triplicate.
Animal experiments.
CD1 male mice (Charles River, Calco, Lecco, Italy) weighing 25 g were rendered neutropenic by intraperitoneal administration of cyclophosphamide (200 mg/kg of body weight/day) on days −4, +1, and +4 postinfection. They were infected intravenously with C. glabrata 4293 (approximately 1 × 108 cells) given in a 0.2-ml volume. Both drugs were administered intraperitoneally in a 0.2-ml volume starting after 24 h postchallenge at doses of 0.3, 1, 3, and 5 mg/kg/day. Drug efficacy was assessed by determining the number of CFU per kidney pair. Briefly, the mice were sacrificed, the kidneys were homogenized, and diluted and undiluted aliquots, including the entire organ, were grown on Sabouraud dextrose agar for colony count determination. Tissue burden experiments were performed on days 3, 5, and 7 postinfection, which corresponded to a total of 2, 4, and 6 days of therapy, respectively. There were from 7 to 18 animals in each control and treatment group. Animal experiments were conducted with the approval of the University of Ancona Ethics Committee.
Statistical analysis.
MICs and MFCs of both drugs were compared by the Mann-Whitney U test. A P value of <0.05 was considered statistically significant. The Mann-Whitney U test was also used to compare tissue burden counts. Due to multiple comparisons, a P value of <0.025 was considered statistically significant.
RESULTS
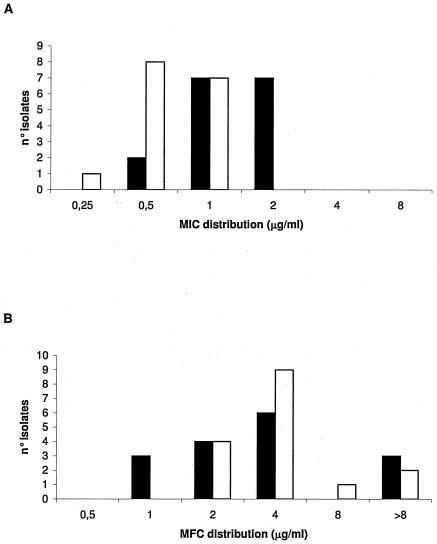
MICs and MFCs of both drugs against 16 clinical isolates of C. glabrata are presented in Fig. 1. CAS MICs ranged from 0.25 to 1.0 μg/ml, while AMB MICs ranged from 0.5 to 2.0 μg/ml. The AMB MIC distribution was significantly higher than that of CAS (P = 0.001). CAS MFCs ranged from 2.0 to >8.0 μg/ml, while AMB MFCs ranged from 1.0 to >8.0 μg/ml. No differences were found in MFC distribution between drugs.
FIG. 1.
CAS (white bars) and AMB (black bars) MICs (A) and MFCs (B) of 16 clinical isolates of Candida glabrata.
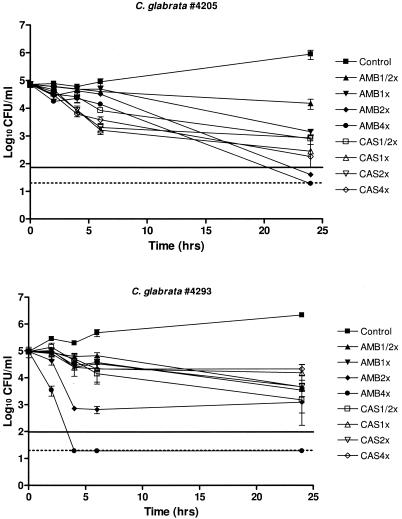
Isolates 4205 and 4293 were then selected for killing experiments (Fig. 2). They were equally susceptible to either CAS or AMB (MICs = 1.0 μg/ml for both drugs). AMB at two and four times the MIC was fungicidal against C. glabrata 4205, while only AMB at four times the MIC was shown to be rapidly fungicidal against C. glabrata 4293. Although, after 24 h of incubation, CAS yielded a reduction of CFU/ml ranging from 1.9 to 2.6 log10 against C. glabrata 4205 and from 0.6 to 1.8 log10 against C. glabrata 4293, a fungicidal effect was never reached.
FIG. 2.
Time-kill studies conducted with C. glabrata 4205 and C. glabrata 4293. The solid lines represent a >99.9% growth reduction compared with the initial inoculum size (fungicidal effect). The limit of detection (20 CFU/ml) is represented by the dotted lines. Each data point represents the mean ± standard deviation for three independent experiments.
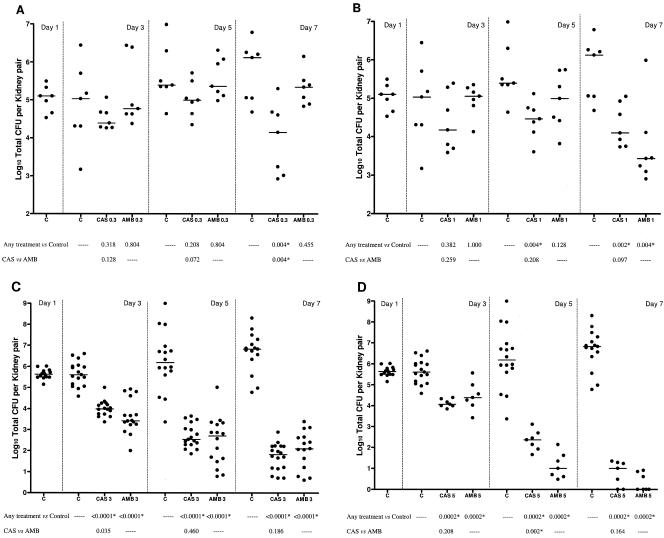
Then, we investigated the potential fungicidal activity of both drugs in an experimental model of murine systemic candidiasis. Neutropenic mice infected intravenously with C. glabrata 4293 were treated with either drug at doses ranging from 0.3 to 5 mg/kg/day, and kidney counts were performed on days 3, 5, and 7 postinfection. The results are reported in Fig. 3. Drugs utilized at 0.3 mg/kg/day were not effective at reducing the fungal burden at any time, with the exception of CAS, which was effective against the controls on day 7 postinfection (P = 0.004). In this time interval, CAS was also more effective than AMB (P = 0.004). Drugs at a dosing of 1 mg/kg/day had no effect on kidney tissue counts at day 3 postinfection. At this dose, CAS (P = 0.004), but not AMB, was effective on day 5, while both drugs were equally active on day 7 (CAS, P = 0.002; AMB, P = 0.004). Drugs at 3 mg/kg/day reduced kidney counts when compared to controls at any time (P < 0.0001). No differences were found between drugs. Drugs given at 5 mg/kg/day were equally effective against the controls at any time (P = 0.0002). AMB was shown to be superior to CAS on day 5 (P = 0.002). In addition, on day 7 postinfection, 29% (2/7) and 57% (4/7) of animals treated with CAS and AMB, respectively, cleared the infection.
FIG. 3.
Kidney tissue burden of neutropenic CD1 mice. For study no. 1 (A and B), the mice were infected intravenously with 1.2 × 108 CFU/mouse of C. glabrata 4293 and treated daily with CAS at 0.3 or at 1 mg/kg/day (CAS 0.3 and CAS 1) and with AMB at 0.3 or at 1 mg/kg/day (AMB 0.3 and AMB 1). For study no. 2 (C and D), the mice were infected intravenously with 2.6 × 108 CFU/mouse and treated daily with CAS at 3 or 5 mg/kg/day (CAS 3 and CAS 5) and with AMB at 3 or 5 mg/kg/day (AMB 3 and AMB 5). Tissue burden experiments for control mice (C) were performed on days 1, 3, 5, and 7 postinfection. Tissue burden experiments for treated mice were performed on days 3, 5, and 7 postinfection, which corresponded to a total of 2, 4, and 6 days of therapy, respectively. The bars represent the medians. The asterisks at the bottom of panels A, B, C, and D indicate P values of <0.025.
To see whether the colonies grown from cultured organs of mice treated for 6 days with drugs at 3 and 5 mg/kg/day represented resistant mutants, two single colonies from each isolation plate were randomly selected and tested for CAS and AMB susceptibility and the results were compared to those observed for the parent isolate. All strains tested maintained a susceptibility pattern identical or similar (within ±1 double dilution) to the parent isolate for both drugs (data not shown).
DISCUSSION
In this study, we investigated the fungicidal activity of CAS and AMB against C. glabrata. To this aim, two in vitro approaches and an in vivo model of systemic candidiasis were utilized. Although not yet standardized, the determination of MFC is the simplest method to investigate the potential fungicidal activity of a given drug (13). Literature data reported so far indicate that AMB MFCs for isolates of C. glabrata are significantly higher than those generally observed for Candida albicans. A recent study analyzing the MFCs of AMB for bloodstream Candida spp. found that the MFCs inhibiting 90% of the isolates of C. glabrata and C. albicans were 16 and 1 μg/ml, respectively (3). Our data agree with those reported by the literature, having found AMB MFCs ranging from 1.0 to >8.0 μg/ml. Similarly, our CAS MFCs ranged from to 2.0 to >8.0 μg/ml. Recently, Espinel-Ingroff determined CAS MFCs of up to 100 clinical yeasts and found that those obtained for strains of C. glabrata ranged from 1.0 to 4.0 μg/ml (7). Overall, these data seem to indicate that, in terms of MFCs, both drugs have similar fungicidal activities against this species of Candida.
Time-kill studies of antimicrobial agents provide a more dynamic assessment of the interaction between drugs and a given organism. It has been reported that this methodology might have greater clinical utility than the static MFC determination (13). Killing experiments conducted so far with the echinocandin derivative involved Candida spp. other than C. glabrata. Early data showed that CAS was rapidly fungicidal at concentrations ranging from one-half to four times the MICs against isolates of C. albicans and Candida tropicalis (1). Similar to findings reported by Cantón et al. for C. glabrata (2), we found that AMB was fungicidal at concentrations four times the MIC. Surprisingly, CAS did not exert a fungicidal activity.
Another approach for demonstrating the fungicidal activity is the determination of the residual fungal tissue burden in neutropenic-animal models of disseminated infection. Therefore, we utilized the kidney counts of infected animals to analyze the rate and extent of organ clearance determined by either CAS or AMB. Our in vivo results partially mirrored those obtained in vitro. Actually we found that both drugs were rapidly effective at reducing the fungal burden against untreated animals. Of note, CAS was active at a dose as low as 0.3 mg/kg/day while AMB was shown to be effective starting from 1 mg/kg/day. Organ sterilization occurred after 6 days of therapy with the highest drug doses utilized in this study (i.e., 5 mg/kg/day). In general, these data indicate that the eradication of C. glabrata in a neutropenic host is difficult to reach. However, similar to AMB, CAS has the potential for an in vivo fungicidal effect against this difficult-to-treat fungal pathogen. The dose-dependent in vivo antifungal effect shown by both drugs suggests the need for dose adjustments to maximize drug activity.
REFERENCES
- 1.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantón, E., J. Pemán, M. Gobernado, A. Viudes, and A. Espinel-Ingroff. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 48:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón, E., J. Pemán, A. Viudes, G. Quindós, M. Gobernado, and A. Espinel-Ingroff. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 45:203-206. [DOI] [PubMed] [Google Scholar]
- 4.Colombo, A. L., J. Perfect, M. DiNubile, K. Bartizal, M. Motyl, P. Hicks, R. Lupinacci, C. Sable, and N. Kartsonis. 2003. Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:470-474. [DOI] [PubMed] [Google Scholar]
- 5.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 6.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS antifungal surveillance program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]