Abstract
Nosocomial pneumonia is a severe complication after cardiac surgery (CS). Levofloxacin, a fluoroquinolone, qualifies for the therapy of postoperative pneumonia. However, penetration properties of levofloxacin into the lung tissue could be substantially affected by CS: atelectasis, low cardiac output after CS, high volume loads, and inflammatory capillary leak potentially influence drug distribution. The aim of our study was to gain information on interstitial antibiotic concentrations in lung tissue in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Therefore, six patients undergoing elective CS participated in this prospective study. A dose of 500 mg of levofloxacin was administered intravenously in addition to standard antibiotic prophylaxis immediately after the end of surgery. Time versus concentration profiles of levofloxacin in the interstitial lung tissue and plasma were determined. A microdialysis technique was used for lung interstitial concentration measurements. The microdialysis procedure was well tolerated in all patients and no adverse events were observed. The median area under the concentration curve (AUC) of levofloxacin in interstitial lung fluid was 18.6 μg · h/ml (range, 10.1 to 33.6). The median AUC for tissue (AUCtissue) of unbound levofloxacin/AUCtotal in plasma was 0.6 (range, 0.4 to 0.9). The median unbound AUCtissue/MIC was 2.4 (range, 1.3 to 4.2) for Pseudomonas aeruginosa. Our study demonstrated the feasibility and safety of microdialysis in human lung tissue in vivo after CS. The unbound AUC/MIC ratio revealed that levofloxacin used in the described manner was borderline sufficient for the treatment of nosocomial pneumonia caused by Klebsiella pneumoniae and insufficient for the treatment of pneumonia caused by Pseudomonas aeruginosa, because the breakpoint of 30 to 40 for AUC/MIC could not be reached by the conventionally used dosage schema in our post-CS setting. Penetration was lower than in previous reports.
Postoperative pneumonia is a potentially devastating complication after cardiac surgery (CS), associated with a mortality of 10 to 20% (1, 6). Patients presenting with chronic obstructive pulmonary disease or bronchitis are at an increased risk to develop this feared complication (2, 16, 21, 22, 24). Antibiotic therapy fails in some patients suffering from postoperative pneumonia, whereas in other patients it is successful. The reasons for this variability in the success of antibiotic treatment of postoperative pneumonia are unknown (5).
A variety of mechanisms for antibiotic treatment failure are discussed. Atelectasis associated with ventilation/perfusion mismatch (15) is likely to influence the distribution of antibiotics in the interstitial lung tissue. Additionally, common therapeutic procedures during CS could theoretically substantially affect plasma and lung tissue concentrations of antibiotics in patients undergoing cardiopulmonary bypass (CPB) by the following mechanisms: (i) changes in micro- and macrocirculatory blood flow, (ii) increased volume of drug distribution, and (iii) capillary leak mediated by extracorporeal circulation.
Despite many theories, antibiotic concentration has rarely been measured in human lung tissue after major surgery such as CS.
Therefore, we decided to measure fluoroquinolone concentrations in human lung tissue after CS. Levofloxacin was chosen as the antibiotic because it is effective in the treatment of nosocomial pneumonia caused by susceptible pathogens (9, 13, 17). As a measuring method, we used in vivo microdialysis, an innovative clinical technique, most suitable to measure interstitial antibiotic concentrations in a clinical setting (14, 18). However, in vivo microdialysis has not been used in a complicated clinical setting such as open-heart surgery. Therefore, it was our aim to test in an initial step whether this technique can be applied without serious risk during CS. If in vivo microdialysis was found to be feasible during CS, our study could provide the unbound, i.e., microbiologically active, concentration of levofloxacin in the interstitial lung tissue and thus could lead to sufficient adjustment of dosing of the drug.
MATERIALS AND METHODS
Study design.
The study was approved by the local ethics committee. All patients were given a detailed description of the study, and their written informed consent was obtained prior to the study. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission.
Patients.
A total of six patients were studied. All patients were undergoing elective coronary artery bypass grafting (CABG) during CPB. Inclusion criteria were as follows: female or male >19 years of age with a body mass index (BMI) between 20 and 35. Conventional antibiotic prophylaxis was not affected by the study. Preoperative demographic, hemodynamic, and laboratory data of study patients are presented in Table 1.
TABLE 1.
Preoperative demographic, hemodynamic, and laboratory data
| Parametera | Patient
|
Mean ± SD | Median | Range | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Age (yr) | 72 | 73 | 62 | 55 | 43 | 56 | 60.2 ± 11.4 | 64 | 43-73 |
| FVC (%) | 79 | 89 | 93 | 67 | 73 | 86 | 81.2 ± 9.9 | 83 | 67-93 |
| FEV1 (%) | 73 | 70 | 74 | 59 | 70 | 74 | 70 ± 5.6 | 72 | 59-74 |
| Serum creatinin (mg/dl) | 1.4 | 0.8 | 1.1 | 0.9 | 1.0 | 0.8 | 1 ± 0.2 | 1.0 | 0.77-1.4 |
| SaO2 (%) | 96 | 99 | 99 | 97 | 98 | 99 | 98 ± 1.2 | 98 | 96-99 |
| Hemoglobin (μg/dl) | 14.7 | 13.4 | 14.2 | 15.9 | 14.5 | 13 | 14.3 ± 1.0 | 14.4 | 13-15.9 |
| CRP (mg/dl) | 0.5 | 0.5 | 1.5 | 1.5 | 0.9 | 0.9 | 1.0 ± 0.5 | 0.9 | 0.5-1.5 |
| APACHE II score | 10 | 5 | 6 | 11 | 7 | 6 | 8 ± 2.4 | 7 | 5-11 |
| BMI (kg/m2) | 23.6 | 25.1 | 25 | 22.8 | 28.4 | 26.7 | 25.3 ± 2.0 | 25.1 | 22.8-28.4 |
FVC, forced vital capacity; FEV1, forced expiratory volume in one second; SaO2, blood oxygen saturation; CRP, C-reactive protein; APACHE II score, acute physiology and chronic health evaluation II score.
In vivo measurement.
Microdialysis was used for in vivo measurement of interstitial lung concentrations of levofloxacin (18). Microdialysis is based on sampling of analytes from the extracellular space fluid by means of a semipermeable membrane at the tip of a microdialysis probe. Samples of the perfusion medium were collected in predefined time intervals. For most analytes, equilibrium between extracellular tissue fluid and the perfusion medium is incomplete, therefore the concentration in tissue (Ctissue) is higher than Cdialysate (Ctissue > Cdialysate). The factor by which the concentrations are interrelated is termed “relative recovery.”
In vivo probe calibration.
To obtain extracellular concentrations from dialysate concentrations, the microdialysis probe calibration was performed in each patient according to the retrodialysis method (12). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, we added levofloxacin to the perfusion medium, and the disappearance rate (delivery) through the membrane was taken as the in vivo recovery.
Blood samples were kept on ice for a maximum of 60 min and were centrifuged at 4°C and 4,000 rpm for 10 min; cells were discharged and plasma was obtained. Plasma samples and dialysate samples were snap-frozen at −20°C and thereafter stored at −80°C until analysis.
Experimental study conduct.
For CS, the following procedures routinely took place before study administration. For maintenance of fluid balance, 10 ml/kg of body weight Ringer's lactate solution was administered via a peripheral venous line before induction of anesthesia. After routine monitoring (electrocardiogram, pulsoxymetry, and invasive blood pressure monitoring), general anesthesia was induced via a peripheral vein. A median sternotomy was performed in all patients. All patients received heparin sodium (300 IU/kg) for CPB, and activated clotting time was kept above 400 seconds (Hemochrom 400; International Technidyne Corp., Edison, NJ). The extracorporeal circuit consisted of a membrane oxygenator (Monolyth; Sorin Biomedica Cardio, Saluggia, Italy), an open venous reservoir system (Monolyth; Sorin Biomedica Cardio, Saluggia, Italy), and a polyvinyl chloride tubing. Ringer's lactate solution (1,500 ml), mannitol 20% (100 ml), and heparin sodium (5,000 IU) were used to prime the circuit. During extracorporeal circulation, body core temperature was maintained at 36°C (normothermic CPB). Mechanical ventilation was terminated after cardioplegic arrest. For study purposes, the surgeon inserted a microdialysis probe into the pulmonary tissue by the following procedure. The surface of the skin localized on the chest wall was punctured by a 20-gauge intravenous plastic cannula after routine surgical skin surface disinfection. The tip of the flexible microdialysis catheter was then inserted through this cannula. Employing a gutter-like 1.4-mm slit needle (supplied in a CMA-60 microdialysis set; CMA Microdialysis, Stockholm, Sweden), the tip of the probe was inserted into the reinflated lung under visual control. The slit needle was retracted and peeled off the tube of the microdialysis catheter. Thereafter, the microdialysis probe was perfused with degassed Ringer's solution. After final inspection for air leakage or bleeding at the insertion site, the sternum was closed conventionally. After the end of surgery, the microdialysis probe was connected and perfused with Ringer's solution at a flow rate of 1.5 μl/min. This was performed by a microinfusion pump (CMA Microdialysis, Stockholm, Sweden). After a 30-min baseline sampling period, a dose of 500 mg of levofloxacin was administered intravenously by means of an infusion pump over a period of 30 min. Blood samples were drawn from the radial artery for 8 h to determine concentrations of levofloxacin initially in 30-min intervals and in 60-min intervals after 2 h. In vivo probe calibration was performed thereafter for a 30-min period. Simultaneously with blood sampling, microdialysis samples were collected. The probes were removed immediately after the end of sampling.
Determination of levofloxacin in microdialysis and plasma samples.
Total levofloxacin levels in plasma and free concentrations in microdialysates were measured by reversed-phase high-performance liquid chromatography (19). The plasma proteins were removed via methanol precipitation. Both sample matrices were diluted with Ringer's solution 1:10 or 1:1,000 prior to the injection. Levofloxacin was added as an internal standard to both sample matrices. The high-performance liquid chromatography system consisted of a System Gold solvent delivery module 126, an automatic sampler 508 (Beckman-Coulter, Fullerton, CA), and a fluorescence detector FP-920 operated at 310/467 nm (Jasco, Tokyo, Japan). The separations were carried out with a BDS Hypersil C18 column (Thermo Hypersil-Keystone, Bellefonte, PA) at 45°C. The mobile phase consisted of a phosphate buffer and 1.5% acetonitrile, adjusted to pH 3 with tetrabutylammonium hydroxide. Calibration standards for plasma samples were prepared by spiking drug-free plasma with levofloxacin. For the quantitation of microdialysis samples, levofloxacin standard solutions in Ringer's solution were used. The assay was sensitive for levofloxacin with a limit of quantification of 0.02 μg/ml in plasma and in microdialysates.
Data analysis and calculations.
Statistical analysis was performed using a commercially available computer program (Statistica; StatSoft, Inc., Tulsa, OK). Data are presented as means and standard errors of the means. Median values and ranges were used when data were not normally distributed.
Pharmacokinetic data were fitted by a commercially available computer program (Kinetica 2.0.2; Innaphase, Philadelphia, PA). The time versus levofloxacin concentration profiles for plasma and interstitial lung tissue were measured, and the following pharmacokinetic parameters were determined: maximum drug concentration (Cmax), time to maximum drug concentration (Tmax), the area under the concentration curve (AUC), and the AUC for plasma/AUC for tissue (AUCplasma/AUCtissue) ratio as a value for drug penetration into the interstitial compartment.
RESULTS
All studied patients underwent uncomplicated CABG during CPB. Additionally, insertion of microdialysis probes in pulmonary tissue was uncomplicated in all patients. Adverse events or clinical complications related to the microdialysis procedure were not observed.
Preoperative demographic, hemodynamic, and laboratory data are given in Table 1. The average age of patients was 60.2 ± 8 years. Patients presented with a weight of 85 ± 11.5 kg, a height of 169.4 ± 7.8 cm, and had a BMI of 25.3 ± 2.0 kg/m2.
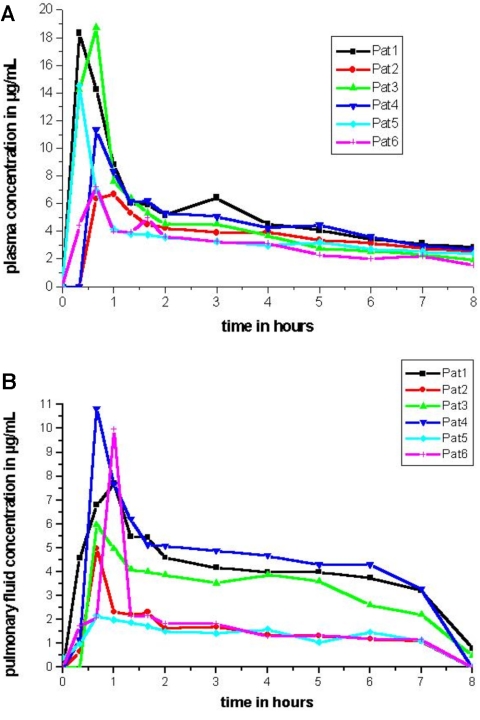
After administration of 500 mg of levofloxacin intravenously, plasma concentration rose rapidly with a median peak of 15.9 μg/ml (range, 6.7 to 18.8 μg/ml) within 20 min (range, 20 to 40 min) after the end of drug infusion (Fig. 1A; Table 2).
FIG. 1.
These panels show the time (hours) versus concentration (μg/ml) profiles of levofloxacin in plasma (A) and in pulmonary fluid (B) of each patient.
TABLE 2.
Main pharmacokinetic parameters of levofloxacin in plasma and pulmonary tissue
| Parameter | Patient
|
Median | Range | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Tmax plasma (min) | 20 | 20 | 20 | 40 | 40 | 20 | 20 | 20-40 |
| Tmax tissue (min) | 60 | 60 | 40 | 40 | 40 | 40 | 40 | 40-60 |
| Cmax plasma (μg/ml) | 18.3 | 17.2 | 6.7 | 18.8 | 11.3 | 14.5 | 15.9 | 6.7-18.3 |
| Cmax tissue (μg/ml) | 7.7 | 10 | 5 | 6 | 10.8 | 2.2 | 6.8 | 2.2-10.8 |
| AUCplasma (μg · h/ml) | 44.2 | 24.3 | 28.9 | 37.3 | 35.9 | 29.2 | 32.6 | 24.3-44.2 |
| AUCtissue (μg · h/ml) | 30.6 | 13.5 | 11.4 | 23.7 | 33.6 | 10.1 | 18.6 | 10.1-33.6 |
| AUCtissue/AUCplasma ratio | 0.7 | 0.6 | 0.4 | 0.6 | 0.9 | 0.4 | 0.6 | 0.4-0.9 |
| AUCtissue/MIC90a ratio | 3.8 | 1.7 | 1.4 | 3.0 | 4.2 | 1.3 | 2.4 | 1.3-4.2 |
The MIC90 of levofloxacin for P. aeruginosa.
Maximum concentration of levofloxacin in pulmonary interstitial space fluid (Cmax tissue) was achieved after a median of 40 min (range, 40 to 60 min) and then fell at a lower rate than plasma concentrations did (Fig. 1B).
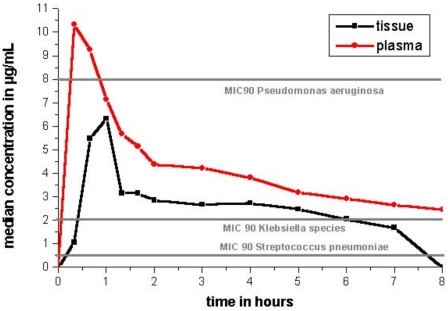
The time versus median values of concentration profiles for levofloxacin in plasma and in pulmonary interstitial space fluid are shown in Fig. 2.
FIG. 2.
This graph shows the time (hours) versus median concentration (μg/ml) of levofloxacin in plasma and interstitial lung tissue and additionally the MIC90s for the potential respiratory pathogens.
The maximum drug concentration of levofloxacin in pulmonary fluid was (median, 6.8 μg/ml; range, 2.2 to 10.8 μg/ml) 38% of maximum plasma levels.
For fluoroquinolones, the pharmacodynamic parameter that best correlates with the outcome is the AUC/MIC ratio. The AUCtissue ratio is shown in Table 2 for single subjects, and other major pharmacodynamic and pharmacokinetic parameters are also shown.
Median levofloxacin levels exceeded MICs (MIC90s) for the potential respiratory pathogen Streptococcus pneumoniae (MIC90, 0.5 μg/ml) in all patients (10, 11). However, the AUCtissue/MIC ratio was exceeding the critical value of 30 in only three out of six patients. The MIC90 for Klebsiella species (MIC90, 2.0 μg/ml) (11, 23) prophylaxis was exceeded in pulmonary fluid only in three out of six patients. However, the MIC90 for Pseudomonas aeruginosa (MIC90, 8 μg/ml) (11, 23) exceeded pulmonary fluid concentrations of levofloxacin by far (Table 2; Fig. 2).
No correlation between BMI and AUCtissue (r = 0.64; P > 0.05) nor between CPB time and AUCtissue (r = 0.51; P > 0.05) was observed. Additionally, no correlation between fluid balance and AUCtissue (r = 0.56; P > 0.05) was detected in our patients.
Three bypasses were performed in four patients, and the remaining two patients received two grafts. Surgical time was 240 ± 13.8 min (range, 190 to 285 min).
All patients were treated with norepinephrine intravenously (median dosage of 0.048 μg/kg/min; range, 0.012 to 0.13 μg/kg/min). Only two of six patients needed inotropic support with dobutamine (no. 2, 2.23 μg/kg/min; no. 5, 4.76 μg/kg/min).
The mean duration of intensive-care unit stay was 1.9 ± 0.7 days. Discharge from hospital was possible after 8.9 ± 1.6 days.
DISCUSSION
In the present study, we determined lung tissue concentrations of levofloxacin in patients undergoing CABG with CPB by means of in vivo microdialysis. To our knowledge this was the first time that on-line antibiotic tissue concentration measurements were performed successfully in six patients undergoing open-heart surgery. Only a small number of patients were studied, because it was very difficult to convince the local ethical committee that patients are not subjected to substantial additional risk when in vivo microdialysis is performed immediately after open-heart surgery. Since the method was feasible and not associated with adverse events, in vivo microdialysis for determination of tissue concentrations of various drugs seems to be a promising technique for future clinical practice.
Levofloxacin penetrated within 40 min into lung tissue (Table 2). Over the observation period of 8 h, tissue concentrations of levofloxacin were higher than the MIC90 for S. pneumoniae in all subjects. However, for fluoroquinolones, the pharmacodynamic parameter that best correlates with outcome is the AUCtissue/MIC ratio. An unbound AUCtissue/MIC ratio of 30 to 40 is associated with high rates of bacterial killing and thus maximizes the efficacies of fluoroquinolones (8). The AUCtissue/MIC was far below 35 in the majority of our patients (Table 2). The MIC90s for Klebsiella species and P. aeruginosa were far higher than lung tissue concentrations of levofloxacin. Therefore, the usual dosing scheme for levofloxacin seems inadequate for treatment of pneumonia caused by Klebsiella and P. aeruginosa after CPB. However, the AUCtissue of levofloxacin observed in our patients after CS was comparable to the concentrations measured by Zeitlinger et al. in the muscle tissue of sepsis patients (25).
Discussion of the method of in vivo microdialysis.
Most drugs do not exert their effects within the plasma compartment but in defined target tissues. From the scientific and clinical point of view, it is desirable to determine drug levels from the site of action because penetration from the central compartment to the site of action might be very variable depending on various factors (3). For antibiotics the site of action is the interstitial space fluid. Therefore, the most relevant antibiotic concentration is the interstitial antibiotic concentration (7). However, drug concentrations in the interstitial compartment could not be evaluated by routine techniques so far. Nevertheless, this would be of special interest because the dosing of therapeutic and prophylactic antibiotics could be adjusted to the interstitial drug levels in order to guarantee adequate dosing and minimal side effects.
In the six patients taking part in our study, no side effects or adverse events were observed. Therefore, we conclude that measurement of interstitial drug concentration is possible in clinical practice, even if the patient is undergoing complicated surgery such as CABG with CPB. Additionally, our data show that it is important to reevaluate clinical dosing schemas by means of direct in vivo measurements.
The goal of antibiotic therapy is to reach tissue concentrations of the antibiotic exceeding the MIC90 for the most relevant pathogens. It is desirable that all patients receiving the antibiotic exhibit tissue concentrations higher than the MIC90. As shown in Fig. 1C, 500 mg of levofloxacin in patients undergoing CABG with CPB was only sufficient in three out of six patients to reach interstitial concentrations over the MIC90 for the most prevalent pathogens. Therefore, we conclude that levofloxacin administered with the usual dose of 500 mg is not associated with sufficient lung tissue concentrations necessary to be effective against all K. pneumoniae strains. Additionally, the MIC90 of levofloxacin for P. aeruginosa was not reached, by far, immediately after CABG with CPB (Fig. 2). Since 750 mg of levofloxacin is recommended as the maximum dosage (20), it seems reasonable to increase the dosage in the setting we examined. Whether this would lead to an adequate increase in tissue concentration remains to be shown. This is surprising, because levofloxacin should qualify in the mentioned dosage for excellent coverage against gram-positive and gram-negative pathogens (9, 13, 17). However, the results of the present study could have been strongly influenced by changes in macro- and microcirculation, increased volume of drug distribution, capillary leakage, and atelectasis associated with CABG with CPB. However, no correlations (r < 0.5; P > 0.05) between BMI and AUCtissue, CPB time and AUCtissue, and fluid balance and AUCtissue were detected in our patients. The amount of atelectasis formation and capillary leakage were not evaluated because of ethical and technical reasons.
Another important aspect in the interpretation of our data is the fact that many pathogens enter the respiratory system via the bronchial pathway. Several authors have shown that levofloxacin concentration in the epithelial lining fluid is substantially higher than the concentration of levofloxacin in plasma (4, 8). Therefore, “low” levofloxacin concentrations measured in lung interstitial fluid might be less important for infections caused by pathogens entering the lung via the bronchial system than for pathogens entering the lung via the circulation.
The differences of antibiotic penetration were reasonably small (Table 2) compared to interindividual penetration differences in the muscle tissue of sepsis patients (25). Direct comparison with the interstitial concentration of levofloxacin in lung tissue after CS evaluated by other authors is, due to the lack of data, impossible.
In conclusion, closed-chest microdialysis is a feasible and safe method to measure drug concentrations in the human lung in vivo. Especially in high-risk patients, e.g., cystic fibrosis patients, the measurement of interstitial drug concentration seems important to achieve optimal dosing for therapy and prophylaxis. This is strongly supported by our data showing unexpectedly low lung tissue concentrations of levofloxacin in patients undergoing CABG during CPB.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Ariano, R. E., and G. G. Zhanel. 1991. Antimicrobial prophylaxis in coronary bypass surgery: a critical appraisal. Drug Intell. Clin. Pharm. 25:478-484. [DOI] [PubMed] [Google Scholar]
- 2.Bellchambers, J., J. M. Harris, P. Cullinan, H. Gaya, and J. R. Pepper. 1999. A prospective study of wound infection in coronary artery surgery. Eur. J. Cardiothorac. Surg. 15:45-50. [DOI] [PubMed] [Google Scholar]
- 3.Brunner, M., T. Pernerstorfer, B. X. Mayer, H. G. Eichler, and M. Müller. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754-1759. [DOI] [PubMed] [Google Scholar]
- 4.Capitano, B., H. M. Mattoes, E. Shore, A. O'Brien, S. Braman, C. Sutherland, and D. P. Nicolau. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965-973. [DOI] [PubMed] [Google Scholar]
- 5.Carrel, T., E. R. Schmid, L. von Segesser, M. Vogt, and M. Turina. 1991. Perioperative assessment of the likelihood of infection of the lower respiratory tract after cardiac surgery. Thorac. Cardiovasc. Surg. 39:85-88. [DOI] [PubMed] [Google Scholar]
- 6.Cunha, B. A. 2001. Nosocomial pneumonia. Diagnostic and therapeutic considerations. Med. Clin. N. Am. 85:79-114. [DOI] [PubMed] [Google Scholar]
- 7.FDA. Guidance for industry. Developing antimicrobial drugs. General considerations for clinical trials. [Online.] http://www.fda.gov/cder/guidance/2580dft.pdf.
- 8.Florea, N. R., P. R. Tessier, C. Zhang, C. H. Nightingale, and D. P. Nicolau. 2004. Pharmacodynamics of moxifloxacin and levofloxacin at simulated epithelial lining fluid drug concentrations against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 48:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogarty, C. M., J. G. Sullivan, and M. S. Chattman. 1998. Once a day levofloxacin in the treatment of mild to moderate and severe community-acquired pneumonia in adults. Infect. Dis. Clin. Pract. 7:400-407. [Google Scholar]
- 10.Georgopoulos, A., A. Buxbaum, U. Straschil, the Austrian Bacterial Surveillance Network, and W. Graninger. 1998. Austrian national survey of prevalence of antimicrobial resistance among clinical isolates of Streptococcus pneumoniae 1994-96. Scand. J. Infect. Dis. 30:345-349. [DOI] [PubMed] [Google Scholar]
- 11.Hoogkamp-Korstanje, J. A. A. 1997. In-vitro activities of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J. Antimicrob. Chemother. 40:427-431. [DOI] [PubMed] [Google Scholar]
- 12.Joukhadar, C., H. Derenhdorf, and M. Müller. 2001. Microdialysis, a novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211-219. [DOI] [PubMed] [Google Scholar]
- 13.Klimberg, I. W., C. E. Cox, and C. L. Fowler. 1998. A controlled trial of levofloxacin and lomefloxacin in the treatment of complicated urinary tract infection. Urology 51:610-615. [DOI] [PubMed] [Google Scholar]
- 14.Lönnroth, P., P. A. Jansson, and U. Smith. 1987. A microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol. 253:E228-E231. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson, L., V. Zemgulis, S. Wicky, H. Tyden, S. Thelin, and G. Hedenstiema. 1997. Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 87:1153-1163. [DOI] [PubMed] [Google Scholar]
- 16.Marffraff, G., F. H. Splittgerber, M. Knox, and J. C. Reidmeister. 1999. Mediastinitis after cardiac surgery—epidemiology and current treatment. Eur. J. Surg. Suppl. 584:12-16. [DOI] [PubMed] [Google Scholar]
- 17.Martin, S. J., R. Jung, and C. G. Garvin. 2001. A risk-benefit assessment of levofloxacin in respiratory, skin and skin structure, and urinary tract infections. Drug Safety 24:199-222. [DOI] [PubMed] [Google Scholar]
- 18.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Wenninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neckel, U., C. Joukhadar, M. Frossard, W. Jäger, M. Müller, and B. M. Mayer. 2000. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 463:199-206. [Google Scholar]
- 20.Noel, G. J., D. B. Goodman, S. Chien, B. Solanki, and M. Padmanabhan. 2004. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods correction and periodic and continuous ECG recording. J. Clin. Pharmacol. 44:464-473. [DOI] [PubMed] [Google Scholar]
- 21.Slaughter, M. S., M. M. Olson, J. T. Lee, and H. B. Ward. 1993. A fifteen-year wound surveillance study after coronary artery bypass. Ann. Thorac. Surg. 56:1063-1068. [DOI] [PubMed] [Google Scholar]
- 22.Stahle, E., A. Tammelin, R. Bergstrom, A. Hambreus, S. O. Nystrom, and H. E. Hansson. 1997. Sternal wound complications—incidence, microbiology and risk factors. Eur. J. Cardiothorac. Surg. 11:1146-1153. [DOI] [PubMed] [Google Scholar]
- 23.Wise, R., J. M. Andrews, C. Cross, and L. J. V. Piddock. 1985. The antimicrobial activity of cefpirome, a new cephalosporin. J. Antimicrob. Chemother. 15:449-456. [DOI] [PubMed] [Google Scholar]
- 24.Zacharias, A., and R. H. Habib. 1996. Factors predisposing to median sternotomy complications. Deep vs. superficial infection. Chest 110:1173-1238. [DOI] [PubMed] [Google Scholar]
- 25.Zeitlinger, M. A., P. Dehghanyar, B. X. Mayer, B. S. Schenks, U. Neckel, G. Herkner, A. Georgopoulos, M. Mueller, and C. Joukhadar. 2003. Relevance of soft-tissue penetration by levofloxacin for target site bacterial killing in patients with sepsis. Antimicrob. Agents Chemother. 47:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.