Abstract
Cruzain is an essential cysteine protease of Trypanosoma cruzi and a therapeutic target for Chagas' disease. Eight dogs were infected with T. cruzi; three were treated with an inhibitor of cruzain, K777, for 14 days. Treatment with K777 abrogated myocardial damage by T. cruzi, as documented by histopathological lesion scores and serum troponin I levels.
Trypanosoma cruzi is the causative agent of Chagas' disease, the parasitic infection that remains the leading cause of heart disease in Latin America. Trypanosoma cruzi also produces cardiac disease in dogs and is a significant veterinary problem from Argentina north to the southwest United States (6). Current therapy for Chagas' disease is not always effective and is limited by frequent and severe side effects. A vinyl sulfone (K777) cysteine protease inhibitor, N-methylpiperazine-Phe-homoPhe-vinylsulfone-phenyl, has been shown to cure T. cruzi infection both in a cell culture screen and in a mouse model of Chagas' disease (5). This inhibitor is orally bioavailable, and toxicology studies to date have suggested it is significantly safer than current therapy (7). To confirm the efficacy of this compound in a second animal model, eight clinically normal female beagle dogs were infected by inoculation of 5 × 106 strain Tulahuen T. cruzi trypomastigotes/kg body weight. Three dogs received 14 days of K777 at a dose of 50 mg/kg administered orally every 12 h. Five dogs received no treatment. Response to infection and treatment was monitored by serum cardiac troponin I levels (TnI), histology, tissue PCR, cardiac electrocardiograms (ECGs), and echocardiograms following necropsy.
Eight clinically normal female beagle dogs (aged 16 weeks; mean weight, 7.6 kg [range, 6.3 to 8.6 kg]) were used. All dogs were vaccinated against Bordetella bronchiseptica, parainfluenza, parvovirus infection, distemper, and hepatitis. All procedures performed on the dogs were reviewed and approved by Cornell University's Institutional Animal Care and Use Committee. The Tulahuen strain of T. cruzi is maintained in AJ mice (Jackson Laboratories, Bar Harbor, Maine) (8). Metacyclic trypomastigotes were harvested from mouse blood with high parasitemias and used to infect African green monkey kidney (Vero) cell cultures. Organisms were harvested from the cell culture media after the first passage, washed three times (8,000 g, 15 min) in phosphate-buffered saline (PBS), and suspended to a concentration of 5 × 107/ml of PBS.
Dogs were inoculated subcutaneously between the shoulder blades with 5 × 106 organisms/kg body weight. Group 1 (three dogs) received treatment (dose, 50 mg/kg administered orally every 12 h) continuing for 14 days beginning the day of infection. Group 2 dogs (five dogs) were infected but received no treatment (untreated infected control). Prior to infection (day 0) and on days 14 and 21 postinfection (PI), blood was taken for a complete blood count and biochemical panel; urine was collected for a routine urinalysis (Cornell State Diagnostic Laboratory). On days 0, 6, 12, 15, 18, and 21 PI, blood was taken by venipuncture into an EDTA tube for PCR assessment of parasitemia as described previously (1). Blood was also drawn on days 6, 12, 18, and 21 PI to determine parasitemia and blood cultures. Parasitemia was determined by diluting 5 μl of blood in 45 μl of 0.83% NH4Cl to lyse red blood cells prior to counting in a modified Neubauer hemocytometer.
Blood cultures were performed on 5 ml of heparinized blood from each dog. The blood was centrifuged (1,000 × g, 5 min, room temperature) to collect the plasma, and the parasites were precipitated and washed three times (8,000 × g, 15 min, 4°C) in PBS, the final pellet being resuspended in 7 ml LIT medium. Cultures were examined weekly for 10 weeks for the presence of epimastigote growth. Anti-T. cruzi antibodies were measured on serum collected from each dog on days 0, 6, 12, 15, and 21 PI by use of an enzyme-linked immunosorbent assay as described previously (2). Echocardiograms and ECGs were performed on each dog on days 0, 14, and 21 PI as described previously (4). Serum for TnI determinations was collected on days 0, 6, 12, 15, and 21 PI from each dog. Troponin I was measured using the Axsym system at the Albert Einstein College of Medicine (J. Piscitelli). Serum was collected from all dogs receiving treatment (group 1) on day 2 PI at 1 h and 3 h posttreatment for determinations of drug levels.
On day 21 PI, the dogs were euthanized and the hearts removed and examined for gross pathological changes. Representative sections of predetermined areas of the four chamber walls, septum, and left coronary artery were selected and either fixed in neutral buffered 10% formalin for histopathology and immunohistochemistry or immediately frozen in liquid nitrogen for PCR. Cardiac sections were stained with hematoxylin and eosin and prepared for immunohistochemistry using an anti-T. cruzi antibody. The severity of inflammation in affected tissues was scored on a scale of 1 to 5 as described previously (3). A score of 1 indicated normal tissue. A score of 2 indicated one or fewer foci of inflammatory cells/field (×400). A score of 3 indicated two or more inflammatory foci/field. A score of 4 indicated generalized coalescing of foci of inflammation or disseminated inflammation with minimal cell necrosis and retention of tissue integrity. A score of 5 indicted diffuse inflammation with severe tissue necrosis, interstitial edema, hemorrhage, and loss of tissue integrity. The total number of pseudocysts in each section was recorded from the immunohistochemical slides.
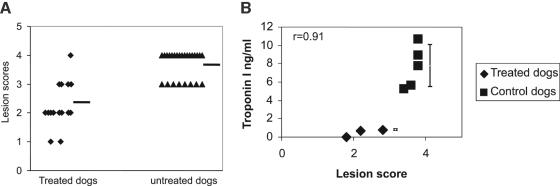
At no time did any of the dogs develop a parasitemia that was detectable by hemocytometer. Nevertheless, parasites were cultured from the blood of every dog at least once and PCR results were positive for all dogs. All dogs developed anti-T. cruzi antibody titers by day 15 PI. Titers did not differ greatly between dogs. At no time were ECG or echocardiographic abnormalities detected in either treated or untreated dogs. Treated dogs showed a significant amelioration of cardiac pathology, as assessed by both TnI and histopathology, both of which correlate directly with cardiac damage. In the untreated dogs, TnI increased slowly until day 15 PI and then peaked at day 21 PI (median; 7.7 ng/ml [range, 5.6 to 10.6]). In contrast, treated dogs showed smaller rises by day 21 PI (median, 0.7 ng/ml [range, 0 to 8]) (P < 0.01) (Fig. 1B). One treated dog showed no elevations of TnI throughout the experimental period. Median (range) lesion scores were 2.2 (1.8 to 2.9) for the treated dogs versus 3.7 (3.6 to 4) for the untreated dogs (P < 0.01) (Fig. 1A). Lesion scores and TnI were significantly (P = 0.02) different between treated and untreated dogs, and TnI were significantly (P = 0.007) correlated (r = 0.95) with lesion scores. (Fig. 1B) The untreated dogs had large numbers of parasite cysts in the right ventricle, whereas two of the three treated dogs had very few parasites within the cardiac muscle. Drug treatment produced no significant alterations in complete blood count, biochemistry, or urinalysis panels, and there were no adverse clinical signs detected. Specifically, there was no elevation in alanine aminotransferase levels (range, 27 to 77 μm/liter; normal range, 25 to 106 μm/liter) at day 15 or 21 PI. One treated dog experienced a mild but reversible thrombocytopenia on day 14 PI. Drug levels in the blood of treated dogs ranged between 1 and 2 μg/ml (1.6 to 3.3 μM) at 1 h postdose.
FIG. 1.
(A) Dispersion plot for actual scores of the inflammatory lesions in each group (bars represents mean values). Each symbol represents a lesion. Multiple lesions may occur in each heart. Since dogs were infected prior to commencing treatment, all dogs were expected to have lesions initially, but those in untreated dogs all progressed to a high score, while the five lesions in each treated dog were scored as ≤3 with one exception. (B) Correlation plot of lesion scores (x axis) and troponin I level (y axis). r = 0.91. Troponin I shown as mean ± standard deviation (bar). Note that treated dogs have minimal cardiac cell leakage of troponin I at 21 days postinfection.
This pilot study was carried out to confirm the efficacy of cysteine protease inhibitor treatment of Chagas' disease in beagle dogs. An oral dose of a vinyl sulfone inhibitor at 50 mg/kg administered orally every 12 h produced significant amelioration of cardiac disease, as demonstrated by an improvement in histopathology and TnI without significant toxicity. The positive correlation of TnI with cardiac lesion scores suggests that determination of TnI is a more sensitive assay than ECGs or echocardiograms to predict cardiac damage in dogs and probably in humans.
Acknowledgments
This work was supported by National Institutes of Allergy and Infectious Diseases TDRU grant AI35707 and the Sandler Family Supporting Foundation.
The vinyl sulfone inhibitor was a gift of Jim Palmer of Axys (now Celera) Pharmaceuticals. We thank Yuan Ming Zhou for carrying out the PCR assays.
REFERENCES
- 1.Aviala, H., A. M. Goncalves, N. S. Nehme, C. M. Morel, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR amplified minicircle variable region sequence. Mol. Biochem. Parasitol. 42:175-188. [DOI] [PubMed] [Google Scholar]
- 2.Barr, S. C., V. A. Dennis, and T. R. Klei. 1991. Serological and blood culture survey of four dog populations for Trypanosoma cruzi from south Louisiana. Am. J. Vet. Res. 52:570-573. [PubMed] [Google Scholar]
- 3.Barr, S. C., P. S. Schmidt, C. C. Brown, and T. R. Klei. 1991. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 52:2033-2039. [PubMed] [Google Scholar]
- 4.Barr, S. C., R. A. Holmes, and T. R. Klei. 1992. Electrocardiographic and echocardiographic features of trypanosomiasis in dogs inoculated with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 53:521-527. [PubMed] [Google Scholar]
- 5.Engel, J. C., P. S. Doyle, I. Hsieh, and J. H. McKerrow. 1998. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J. Exp. Med. 188:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meurs, K. M., M. A. Anthony, M. Slater, and M. W. Miller. 1998. Chronic Trypanosoma cruzi infection in dogs: 11 cases (1987-1996). J. Am. Vet. Med. Assoc. 213:497-500. [PubMed] [Google Scholar]
- 7.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 8.Trischmann, T., H. Tanowitz, M. Wittner, and B. Bloom. 1978. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred stains of mice. Exp. Parasitol. 45:160-168. [DOI] [PubMed] [Google Scholar]