Abstract
Single benzamidine group-carrying compounds were shown to interact with the Trypanosoma brucei P2 aminopurine transporter. Replacement of the amidine with a guanidine group decreased affinity. Trypanocidal activity was evident, but compounds were equally toxic against trypanosomes lacking the P2 transporter, which indicates additional uptake routes for monobenzamidine-derived compounds.
Human African trypanosomiasis is endemic in sub-Saharan Africa. Around 300,000 individuals are afflicted (3). Current drugs have problems including accessibility, administration, and disease stage specificity; most cause side effects of variable severity (3). The development of new trypanotoxic compounds is vital.
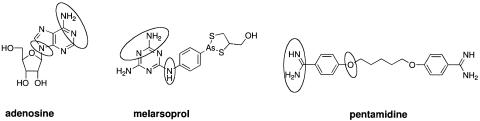
We are developing an approach to deliver toxins to trypanosomes via a specific plasma membrane transporter, P2, that is usually involved in aminopurine accumulation (6, 15). The substrate structural motif recognized by the P2 transporter has been characterized (Fig. 1) (4, 8, 10); it recognizes, binds, and internalizes compounds carrying a recognition motif in the form of a benzamidine or melamine residue. This includes the arsenical and diamidine classes of drugs in common use today (4).
FIG. 1.
Substrates for the P2 transporter. The P2 recognition motif is circled: an amidine moiety, an aromatic ring, and a heteroatom.
Previous studies, adding melamine-based transport units to polyamine (14, 19) or nitroheterocyclic (1, 18) toxic residues, have given rise to trypanocidal compounds. Interestingly these studies indicate that transporters in addition to P2 might also accumulate such compounds; hence, resistance through loss of this transporter need not be an issue. Two other transporters, HAPT1 and LAPT1, have been shown to mediate pentamidine transport in Trypanosoma brucei (9), although the full complement of transporters capable of mediating uptake of melamine- or benzamidine-bearing compounds has yet to be established. In principle, monobenzamidine moieties would be predicted to interact with the P2 transporter, but to date studies have focused on diamidine type compounds (2, 7, 11, 16).
A series of compounds (5) synthesized originally to mimic peptide interactions involving the human immunodeficiency virus gp120 protein carry the P2 recognition motif in the form of a monobenzamidine moiety. It was of interest to determine how they interacted with the P2 transporter and also to determine whether they had any capacity to kill trypanosomes. The ability of these compounds to interact with the P2 transporter was assessed using the well-established oil-stop method to measure tritiated adenosine (0.05 μM) uptake via that transporter in the presence of a range of concentrations (0.1 mM to 100 nM) of each inhibitor. In general the compounds were found to have high affinities for the P2 transporter (Table 1), of the same magnitude as the transporter's natural substrate adenosine, and generally around an order of magnitude higher than the affinities of a series of novel melamine-carrying compounds (1, 14, 18, 19). This could indicate a structural preference for the benzamidine group in P2 interactions, and, this being the case, future compounds developed as potential P2-selective trypanocides could benefit from carrying a benzamidine residue.
TABLE 1.
Inhibition of adenosine transport and toxicity of benzamidine-derived compounds
| Compound | Inhibition of adenosine transport (μM)a | Toxicity in vitro (IC50 [μM])
|
Resistance factorb | IC50 (μM) for:
|
|||
|---|---|---|---|---|---|---|---|
| 427 | TbAT1 | ΔTbat1 | T. brucel rhodesiense | L6 cells | |||
| 1 | 9.3* | 57 | 52 | 79 | 1.52 | 15.9 | 140 |
| 2 | 8.05* | 109 | 111 | 128 | 1.15 | 81 | 71 |
| 3 | 0.38* | 54 | 33 | 44 | 1.33 | 12.8 | 167 |
| 4 | 0.38* | 56 | 37 | 60 | 1.62 | 31.1 | 203 |
| 5 | 0.81* | 10 | 6 | 7 | 1.06 | 6.2 | 16.3 |
| 6 | 0.33* | 66 | 58 | 71 | 1.22 | 49.8 | 129 |
| 7 | 0.21* | 14 | 10 | 28 | 2.80 | 11.2 | 52 |
| 8 | 1.01* | 13 | 11 | 14 | 1.27 | 8.4 | 46 |
| 9 | 1.57* | 7 | 8 | 12 | 1.50 | 11.1 | 14.7 |
| Adenosine | 0.92† | NDf | ND | ND | ND | ND | ND |
| Melarsoprol | 0.54‡ | 0.13c | 0.053d | 0.12d | 2.3 | 0.006e | 7.8e |
| Pentamidine | 0.43‡ | 0.04c | 0.011d | 0.026d | 2.36 | 0.00135 | 2.53 |
| Diminazene aceturate | 2.36‡ | 0.99c | 0.30d | 5.77d | 19.23 | 0.0088 | 93.5 |
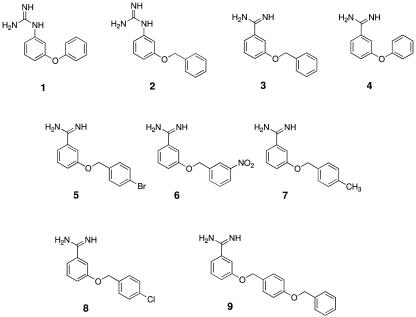
Compounds 1 and 2 are identical to compounds 4 and 3, respectively, other than that they carry a guanidine group in place of the amidine (Fig. 2). This factor alone apparently accounts for 24-fold and 21-fold differences in apparent affinity for the P2 transporter. An obvious explanation for this is not apparent; however, in the guanidine derivatives, the relative orientation of the P2 recognition motifs may be suboptimal, due to extra nitrogen of the guanidine holding this guanidine moiety at an angle. Furthermore the charge on the guanidine is more delocalized.
FIG. 2.
Structures of the compounds evaluated.
Compounds 5 and 8 have an additional halogen attached to the end benzene ring, and this appears to slightly reduce their apparent affinities for P2. Compound 9 also has a lower apparent affinity for P2. With three benzene rings in this compound, it may be the bulk of this compound that is interfering with substrate/transporter interactions.
Compounds were also tested against the bloodstream form T. brucei brucei (strain 427), a T. brucei line lacking the P2 transporter (tbat1−/−) and its wild-type parent line (16), against T. brucei rhodesiense, and also against mammalian L6 cells as previously reported (14). Cells were cultivated in HMI-9 medium containing 20% fetal calf serum (13) at 37°C in a humidified CO2 environment. The Alamar Blue assay (17) was used to determine 50% inhibitory concentration (IC50) values.
All compounds displayed some toxicity towards T. brucei 427 and T. brucei rhodesiense (STIB900). There was good correlation in the growth inhibition of both species of trypanosomes. Compounds were also evaluated against L6 cells as a measure of toxicity. Compounds showed up to a 10-fold selectivity for the parasite compared to the mammalian cells.
The P2 knockout line (tbat1−/−) showed at most a 2.6-fold resistance factor; most experiments yielded under-1.5-fold-reduced activity compared with the wild type. This indicates that the P2 transporter is not the only route into trypanosomes for these compounds. There may be other transporters involved, for example, the high- and low-affinity pentamidine transporters (HAPT1 and LAPT1), or the compounds may enter the cells by diffusion or other transporters. An alternative explanation could be that interaction with cell surface targets is responsible for activity.
Two compounds in particular, 3 and 6, show similar affinity for P2 as adenosine; however, they demonstrate very little trypanotoxicity. Clearly, uptake alone is not sufficient for trypanocidal activity. Two further compounds, 5 and 8, have affinities for P2 of the same magnitude as adenosine and are active against T. brucei in vitro (Table 1). The increase in toxicity in compounds 5 and 8 may be associated with the addition of a halogen group in the para position of the benzyl ring. This could relate to a specific interaction of these drugs with a target molecule, and these observations will be central to the design of new compounds.
The addition of monobenzamidine moieties to potential toxophiles can clearly be employed in the design of new trypanocidal agents, and future work will focus on the coupling of other potentially toxic moieties to this head group with the aim of selectively targeting the drugs to trypanosomes.
Acknowledgments
This work received support from the Wellcome Trust, Parke Davis, and the Welsh School of Pharmacy.
The EPSRC National Mass Spectrometry Service Centre (Swansea) is acknowledged for accurate mass measurements.
REFERENCES
- 1.Baliani, A., G. Jimenez-Bueno, M. L. Stewart, V. Yardley, R. Brun, M. P. Barrett, and I. H. Gilbert. 2005. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites J. Med. Chem. 48:5570-5579. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, M. P., Z. Q. Zhang, H. Denise, C. Giroud, and T. Baltz. 1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73:223-229. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, M. P., R. J. S. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 4.Barrett, M. P., and A. H. Fairlamb. 1999. The biochemical basis of arsenical-diamidine crossresistance in African trypanosomes. Parasitol. Today 15:136-140. [DOI] [PubMed] [Google Scholar]
- 5.Boussard, C., T. Klimkait, N. Mahmood, M. Pritchard, and I. H. Gilbert. 2004. Design, synthesis and evaluation of potential inhibitors of HIV gp120-CD4 interactions. Bioorg. Med. Chem. Lett. 14:2673-2676. [DOI] [PubMed] [Google Scholar]
- 6.Carter, N. S., and A. H. Fairlamb. 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361:173-175. [DOI] [PubMed] [Google Scholar]
- 7.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153-28157. [DOI] [PubMed] [Google Scholar]
- 8.Carter, N. S., M. P. Barrett, and H. P. de Koning. 1999. A drug resistance determinant in Trypanosoma brucei. Trends Microbiol. 7:469-471. [DOI] [PubMed] [Google Scholar]
- 9.De Koning, H. P. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59:586-592. [DOI] [PubMed] [Google Scholar]
- 10.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 11.De Koning, H. P., L. F. Anderson, M. L. Stewart, R. J. S. Burchmore, L. J. Wallace, and M. P. Barrett. 2004. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 48:1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enanga, B., M. R. Ariyanayagam, M. L. Stewart, and M. P. Barrett. 2003. Activity of megazol, a trypanocidal nitroimidazole, is associated with DNA damage. Antimicrob Agents Chemother. 47:3368-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei bloodstream forms in a medium containing a low concentration of serum-protein without feeder cell-layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 14.Klenke, B., M. Stewart, M. P. Barrett, R. Brun, and I. H. Gilbert. 2001. Synthesis and biological evaluation of s-triazine substituted polyamines as potential new anti-trypanosomal drugs. J. Med. Chem. 44:3440-3452. [DOI] [PubMed] [Google Scholar]
- 15.Maser, P., C. Sutterlin, A. Kralli, and R. Kaminsky. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242-244. [DOI] [PubMed] [Google Scholar]
- 16.Matovu, E., M. L. Stewart, F. Geiser, R. Brun, P. Maser, L. J. M. Wallace, R. J. Burchmore, J. C. K. Enyaru, M. P. Barrett, R. Kaminsky, T. Seebeck, and H. P. de Koning. 2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2:1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminski, and R. Brun. 1997. The Alamar blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 18.Stewart, M. L., G. J. Bueno, A. Baliani, B. Klenke, R. Brun, J. M. Brock, I. H. Gilbert, and M. P. Barrett. 2004. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob. Agents Chemother. 48:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tye, C. K., G. Kasinathan, M. P. Barrett, R. Brun, V. E. Doyle, A. H. Fairlamb, R. Weaver, and I. H. Gilbert. 1998. An approach to use an unusual adenosine transporter to selectively deliver polyamine analogues to trypanosomes. Bioorg. Med. Chem. Lett. 8:811-816. [DOI] [PubMed] [Google Scholar]