Abstract
Preexisting humoral immunity to adeno-associated virus (AAV) vectors may limit their clinical utility in gene delivery. We describe a novel caprine AAV (AAV-Go.1) capsid with unique biological properties. AAV-Go.1 capsid was cloned from goat-derived adenovirus preparations. Surprisingly, AAV-Go.1 capsid was 94% identical to the human AAV-5, with differences predicted to be largely on the surface and on or under the spike-like protrusions. In an in vitro neutralization assay using human immunoglobulin G (IgG) (intravenous immune globulin [IVIG]), AAV-Go.1 had higher resistance than AAV-5 (100-fold) and resistance similar to that of AAV-4 or AAV-8. In an in vivo model, SCID mice were pretreated with IVIG to generate normal human IgG plasma levels prior to the administration of AAV human factor IX vectors. Protein expression after intramuscular administration of AAV-Go.1 was unaffected in IVIG-pretreated mice, while it was reduced 5- and 10-fold after administration of AAV-1 and AAV-8, respectively. In contrast, protein expression after intravenous administration of AAV-Go.1 was reduced 7.1-fold, similar to the 3.8-fold reduction observed after AAV-8administration in IVIG-pretreated mice, and protein expression was essentially extinguished after AAV-2 administration in mice pretreated with much less IVIG (15-fold). AAV-Go.1 vectors also demonstrated a marked tropism for lung when administered intravenously in SCID mice. The pulmonary tropism and high neutralization resistance to human preexisting antibodies suggest novel therapeutic uses for AAV-Go.1 vectors, including targeting diseases such as cystic fibrosis. Nonprimate sources of AAVs may be useful to identify additional capsids with distinct tropisms and high resistance to neutralization by human preexisting antibodies.
Adeno-associated viruses (AAVs) are members of the Parvoviridae family (27, 28). AAV genomes consist of a single-stranded DNA molecule of approximately 4.7 kb that encodes two genes, rep and cap, which are flanked by inverted terminal repeat (ITR) sequences. The rep gene encodes a set of four proteins that mediate viral replication and site-specific integration and also modulate gene transcription from the AAV, host cell, and helper virus promoters. The cap gene encodes a set of three progressively longer capsid proteins, VP1, VP2, and VP3. The ITRs are palindromic DNA structures that allow replication of the linear viral genome and also act as packaging signals.
AAVs are replication defective and require coinfection by helper viruses, typically adenovirus or herpesvirus, for productive infection. AAV vectors are DNA transfer vehicles which are constructed by packaging therapeutic genes, bounded by AAV ITRs, into AAV capsids. All viral sequences except for the nontranscribed ITR sequences are eliminated. To date, all AAV vectors used in clinical trials are derived from AAV-2, the most extensively characterized AAV (17). More recently, other AAVs have been identified from primates (4, 5, 15, 16, 25, 26, 28, 30, 35). Nonprimate AAVs also have been found, including equine (11), ovine (7), avian (2, 12), bovine (32), and snake (13) AAVs. Vectors made from these novel AAVs have distinct tropisms, dose response profiles, and serum neutralization susceptibilities (3, 18).
The potential utility of AAV-2 vectors for treating serious human diseases including hemophilia A and B (8), Parkinson's disease (1, 19, 31), heart failure (9, 21), and cystic fibrosis (14), among others, has been established in animal models. However, the presence of human preexisting antibodies reactive with primate AAV serotypes may reduce the clinical usefulness of vectors made from these serotypes (17). In particular, a significant proportion of humans have antibodies that neutralize AAV serotypes 1 to 6 (R. Surosky and P. Colosi, personal communication). Recent experiments have demonstrated that the injection of human antibodies into mice to generate sera with low neutralizing titers significantly reduced transduction with AAV-2 vectors (31a). To address the problem of human preexisting humoral immunity to primate serotypes, we screened adenovirus preparations from several nonprimate species to find novel AAV capsids having little or no preexisting immunity in humans. We describe here a new AAV capsid, isolated from a goat, with low preexisting humoral immunity in humans and a potentially useful tissue tropism.
MATERIALS AND METHODS
Cell culture and virus isolation.
Caprine adenovirus preparations with electron microscopic evidence of parvovirus contamination (kindly provided by Lea Ann Hobbs, Respiratory Diseases of Livestock Research Unit, National Animal Disease Center, USDA, Agricultural Research Service, Ames, IA) were processed from caprine ileum at the Diagnostic Virology Laboratory, National Veterinary Services Laboratory, APHIS/USDA (Ames, IA) (29). Porcine trypsin (U.S. origin) was used in all tissue culture processes, and no human cell cultures or products were employed.
Viral DNA isolation and AAV sequence identification.
Four preparations from different cell cultures and passages were processed individually for DNA extraction. Virus-containing supernatant was treated with proteinase K (200 μg/μl) in digestion buffer (10 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], and 0.5% sodium dodecyl sulfate [SDS]) and incubated at 37°C for 1 h. Following phenol-chloroform extraction and ethanol precipitation, the viral DNA was resuspended in Tris-EDTA.
Oligonucleotide primers for PCR screening were selected on the basis of sequence alignments from segments that are highly conserved among known AAVs.
The forward primer 5′-GTGCCCTTCTACGGCTGCGTCAACTGGACCAATGAGAACTTTCC-3′ is complementary to the helicase domain of rep, and the reverse primer 5′-GGAATCGCAATGCCAATTTCCTGAGGCATTAC-3′ is complementary to one of the several DNA binding domains in capsid. The expected size of PCR fragments was 1.5 kb.
All PCRs were performed in 50 μl in an automated Eppendorf Mastercycler Gradient thermocycler (Perkin-Elmer, Boston, MA). Each reaction mixture contained 200 ng of nucleic acid, 1 μM (each) oligonucleotide primer, 1 mM Mn(OAc)2, 200 μM (each) deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP), and 1.0 unit of rTth polymerase XL (Applied Biosystems, Foster City, CA) in 1× XL Buffer II. Ampliwax PCR Gem 100 (Applied Biosystems, Foster City, CA) was used to facilitate hot start.
Cycling conditions were as follows: 2 min of denaturation at 94°C, followed by 35 cycles of 15 s of denaturation at 94°C, 30 s of annealing at 45°C, and 2 min of elongation at 72°C. PCR products (10 μl) were electrophoretically separated in a 1% NuSieve agarose gel (FMC BioProducts, Rockland, Maine), stained with ethidium bromide, and visualized by UV light. To control for specificity, PCR was also performed with 100 ng of DNA from a plasmid containing AAV-2 rep and cap sequences. PCR products were purified on 1% low-melting-point agarose gels (FMC Bioproducts, Rockland, ME) and sequenced using a model 3700HT DNA sequencer (Applied Biosystems, Foster City, CA). Sequence data were analyzed with the Vector NTI, version 9.0 package (Invitrogen, Carlsbad, CA).
Construction of pHLP19-Go.1 plasmid.
The AAV-6 capsid gene and 3′ untranslated region (UTR) of pHLP19-6 (composed of the AAV-2 rep and the AAV-6 capsid and 3′ UTR [18]) were removed by digestion with SwaI and AgeI (New England Biolabs, Beverly, MA). A caprine capsid coding region tailed with an AAV-6 3′-UTR sequence was created by PCR using the primers 5′-AAATCAGGTATGTCTTTTGTTGATCACCC-3′ and 5′-ACACGAATTAACCGGTTTATTGAGGGTATGCGACATGAATGGG-3′. The PCR fragment was digested with AgeI and ligated to the digested pHLP19-6 plasmid. The complete DNA sequence for the AAV-Go.1 capsid was determined by sequencing pHLP19-Go.1.
Structural analysis of the AAV-Go.1 capsid.
Coordinates for the monomeric AAV-2 capsid protein (VP1 amino acids 217 to 735; VP2 amino acids 80 to 598) were obtained from the Protein Data Bank (http://www.rcsb.org; identification number 1LP3). The structure was analyzed using Swiss PDB viewer version 3.7 (http://www.expasy.org/spdbv/), Vector NTI 3D-Mol version 8.0 (Invitrogen, Carlsbad, CA), or Chime (MDL Information Systems, Inc., San Leandro, CA) (http://www.umass.edu/microbio/chime/getchime.htm). Multimeric structures of the AAV-2 capsid were generated using the oligomer generator program on the Virus Particle Explorer (VIPER) website (http://mmtsb.scripps.edu/viper/), using the coordinate transformation functions of Swiss PDB viewer in conjunction with matrix coordinates in the PDB (1LP3) file or downloaded from the protein quaternary structure database at the European Bioinformatics Institute (http://pqs.ebi.ac.uk/pqs-bin/macmol.pl?filename = 1lp3).
Vector production and purification.
Recombinant AAV vectors were prepared using the triple transfection procedure (23). In each case the adenovirus helper gene plasmid pLadeno5 and the appropriate AAV helper plasmid (e.g., pHLP19-Go.1)were used. The transgene plasmids were pVmLacZ to express lacZ under cytomegalovirus (CMV) promoter control, pAAV hFIX9 to express human factor IX (hFIX) under the CMV promoter control, and pAAV hFIX16 to express human factor IX under the control of an apolipoprotein E/human alpha-antitrypsin (ApoE/hAT) chimeric enhancer/promoter which is liver specific (24).
The plasmids pHLP19-Go.1, pLadeno5, and pVmLacZ were used to produce AAV-Go.1-LacZ recombinant virions. Human embryonic kidney (HEK) cells type 293 (American Type Culture Collection [ATCC]; catalog number CRL-1573) were seeded in 10-cm tissue culture-treated sterile dishes at a density of 3 × 106 cells per dish in 10 ml of cell culture medium consisting of Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and incubated in a humidified environment at 37°C in 5% CO2. After overnight incubation, 293 cells were approximately 80% confluent. The HEK 293 cells were then transfected with DNA by the calcium phosphate precipitate method. Ten micrograms of each vector (pHLP19-Go.1, pLadeno5, and pVmLacZ) were added to a 3-ml sterile, polystyrene snap-cap tube using sterile pipette tips. A 1.0-ml quantity of 300 mM CaCl2 (Sigma) was added to each tube and mixed by pipetting up and down. An equal volume of 2× HBS (274 mM NaCl, 10 mM KCl, 42 mM HEPES, 1.4 mM Na2PO4, 12 mM dextrose, pH 7.05, JRH grade) was added with a 2-ml pipette, and the solution was pipetted up and down three times. The DNA mixture was immediately added to the HEK 293 cells, one drop at a time, evenly throughout the dish. The cells were then incubated in a humidified environment at 37°C in a 5% CO2 atmosphere for 6 h. A granular precipitate was visible in the transfected cell cultures. After 6 h, the DNA mixture was removed from the cells, which were then incubated with fresh cell culture medium without fetal calf serum for an additional 72 h. After this time, the cells were lysed by three cycles of freezing on solid carbon dioxide and thawing in a 37°C water bath. Vector was purified by polyethelyene glycol precipitation followed by two rounds of CsCl density gradient centrifugation as described previously (18). The purified vector was formulated in phosphate-buffered saline-0.01% Pluronic F-68 and sterile filtered (0.22-μm pore size).
Vector genome (vg) titers were established by taking the average of three quantitative PCR determinations (20) using primers and probe specific for hFIX as previously described (33).
Viral purity was assessed by SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and silver staining (Daiichi, Tokyo, Japan). Monodispersity was assessed by dynamic light scattering using a Protein Solutions DynaPro model according to the manufacturer's recommendations (Charlottesville, VA). Analysis of 1 × 1012 to 1 × 1011 particles in a volume of 12 μl showed hydrodynamic radii of 12 nm for 100% of the vectors in each preparation used for the in vivo studies (AAV-1 hFIX9, AAV-Go.1 hFIX9, AAV-Go.1 hFIX16, AAV-8 hFIX9, and AAV-8 hFIX16), indicating that the particles were monomeric and not aggregated.
Vector preparations used in vivo had low levels of endotoxin (AAV-Go.1 hFIX9, 0.221 to 0.458 endotoxin units [EU]/ml; AAV-Go.1 hFIX16, 0.356 EU/ml; AAV-8 hFIX9, 0.387 EU/ml; AAV-1 hFIX16, 0.458 EU/ml).
In vitro transduction assay.
HepG2 cells (ATCC, catalog no. HB-8065) were infected with AAV-Go.1 lacZ, AAV-2 lacZ, or AAV-8 lacZ for 1 hour with serial dilutions (5 × 106 to 1 × 1010 vg per well) of each vector in Dulbecco's modified Eagle's medium containing 0.1% bovine serum albumin (BSA). The medium was replaced with fresh medium containing 10% fetal calf serum and 20 μM etoposide (Calbiochem). After 24 h at 37°C the cells were fixed using 2% formaldehyde and 0.2% glutaraldehyde and stained using 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). After another 24 h, the numbers of blue cells in four random microscopic fields were counted and averaged for each well.
In vitro neutralization of AAV vectors by IVIG.
Human intravenous (i.v.) immune globulin (IVIG; Panglobulin; ZLB Bioplasma, Inc., Glendale, CA) was resuspended in water at a concentration of 100 mg/ml and then serially diluted in twofold increments using mouse serum (Nieffenegger Company, Woodland, CA) and heated at 56°C for 30 min. Virus was diluted to 2.5 × 109 vg/μl using Eagle's minimum essential medium (EMEM) containing 0.1% BSA (fraction V; Sigma). All neutralization reactions were performed in triplicate. Samples of EMEM-0.1% BSA and mouse serum alone were included as controls. Ten microliters of diluted AAV lacZ vector was mixed with 10 μl of serial dilutions of the IVIG and incubated at 37°C for 1 hour. During the incubation, the HepG2 cells (ATCC catalog no. HB-8065) were washed once with EMEM and then 0.5 ml of EMEM-0.1% BSA was added to each well. The amount of vector-IVIG mixture added to the cells for each vector was based on each vector's transduction efficiency; dilutions of the mixture were prepared for the vectors with higher transduction efficiency in order to have an equivalent number of blue cells when reading the plates transduced with different vectors. After 1 hour of incubation at 37°C, fetal bovine serum and etoposide were added to each well at a final concentration of 10% and 20 μM, respectively. Cells were incubated for 24 h and fixed as described above to detect β-galactosidase activity. After another 24 h, the number of blue cells in each well was counted using light microscopy. The lowest concentration of IVIG tested showing 50% or higher neutralization was determined.
In vitro neutralization antibody assay of mouse plasma samples.
Mouse plasma samples from subject mice were heated at 56°C for 30 min to inactivate complement, followed by twofold serial dilution in naïve mouse serum (Nieffenegger Company, Woodland, CA). Sixteen microliters of diluted test samples was mixed with an equal volume of 1.7 × 108 vg of AAV-2 lacZ vector. After 1 h of incubation at 37°C, 7.5 μl of this mixture was used to transduce 2.4 × 104 cells/well of HEK 293 cells seeded 24 h earlier. After overnight transduction, cells were rinsed with PBS and lysed in 60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 0.27% β-mercaptoethanol, and 0.005% SDS. The plates were frozen at −80°C and then thawed at 37°C for 15 min. After the addition of the substrate (20 μl of a 4-mg/ml stock of ONPG [o-nitrophenyl-β-d-galactopyranoside] in lysis buffer without SDS) plates were incubated at 37°C for 20 to 60 min, and the lacZ activity was measured by reading plates at an absorbance wavelength of 420 nm and analyzed with Softmax Pro software (Molecular Devices, Sunnyvale, CA). Background was defined as the absorbance in untransduced wells. Percentage of inhibition on AAV lacZ was determined as 100 − {[test sample optical density (OD) − background OD]/[naïve mouse serum OD − background OD] × 100}. Percentage of inhibition on AAV lacZ versus serum dilution was plotted and fitted using a four-parameter logistic curve fit in Sigma plot software (Systat Software Inc., Point Richmond, CA). The neutralizing titer was determined as either the 50% effective dose or the serum dilution at which 50% or higher inhibition occurred.
In vitro transduction of striatal neurons and glial cells.
Primary cultures of dissociated striatal neurons were prepared from embryonic day 18 Sprague-Dawley rat embryos as described previously (10). Cultures were incubated with AAV lacZ vectors (104 vector genomes per cell) for 5 days. Immunocytochemistry was performed using a β-galactosidase antibody (AB1211, 1:2,000; Chemicon, Temecula, CA) followed by incubation with the secondary antibody, anti-mouse immunoglobulin G (IgG) conjugated to Alexa Fluor 594, diluted 1:100 (Molecular Probes, Eugene, OR).
In vivo transduction of muscle.
Male SCID mice (cBySmn.CB17-Prkdcscid/J; The Jackson Laboratory, Maine) (15 to 25 g, 6 weeks old) were injected with AAV-Go.1 hFIX9, AAV-1 hFIX9, or AAV-8 hFIX9 vector (2 × 1011 vector genomes per mouse, five mice per group) in two sites of the quadriceps muscle (25 μl per site). Retro-orbital blood was collected at 7-day intervals after vector injection, and circulating plasma concentrations of hFIX were measured by enzyme-linked immunosorbent assay (ELISA) (FIX-EIA; Affinity Biologicals). All samples were assessed in duplicate. The limit of quantitation was 2 ng/ml hFIX (based on the average assay background × 10 standard deviations). Mice tested with IVIG were injected with 414 μl of 100-mg/ml IVIG intravenously 24 h before the vector injection. The amount of IVIG circulating in the mouse blood volume of 1.8 ml was expected to be reduced by half after the equilibration with the extravascular space 24 h later to yield a neutralizing titer of approximately 1:300 at the time of the vector injection (based on previous experiments by C. D. Scallan, T. Liu, S. Patarroyo-White, H. Jiang, J. M. Sommer, S. Zhou, L. Couto, and G. F. Pierce, submitted for publication).
In vivo transduction of liver.
Male SCID mice (15 to 25 g) were injected via the tail vein with 5 × 1011 vector genomes of AAV-Go.1 hFIX16 or AAV-8 hFIX-16 vectors (five mice per group). The AAV-2 vector data were derived from a separate experiment and were added for comparison. Retro-orbital blood was collected 1, 2, and 4 (five mice per group) and 8 (two mice per time point) weeks after injection, and circulating plasma concentrations of human factor IX were measured by ELISA (34). All samples were assessed in duplicate. Mice tested with IVIG were injected with 180 μl of 100-mg/ml IVIG via the tail vein 24 h before the vector injection. The neutralizing titer at the time of the vector injection was 150.
Mice injected with AAV-2 vector received a lower dose of IVIG (9 μl of 100-mg/ml IVIG, 5% of the dose which the other vector groups received).
Biodistribution of AAV vectors.
For biodistribution analyses, mice (two mice per group) were sacrificed and the organs were collected 4 weeks after vector injection. Organs collected included brain, testis, skeletal muscle (quadriceps), kidney, spleen, lung, heart, and liver. To measure copies of the hFIX transgene, quantitative PCR was performed on DNA samples extracted from the different tissues (18).
Nucleotide sequence accession number.
The full DNA sequence for the AAV-Go.1 capsid has been submitted to GenBank with the accession number AY724675.
RESULTS
Isolation of the caprine AAV capsid gene.
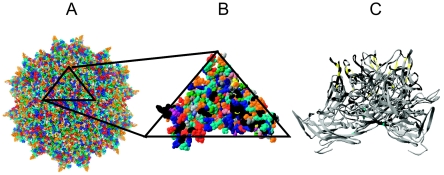
Four adenovirus preparations derived from a pool of postmortem ileum samples from four young goats concurrently afflicted with viral catarrhal enteritis and bacterial fibrinous pleuropneumonia (29) were received from the National Animal Disease Center (Ames, IA) for AAV screening. The four adenovirus preparations shared the same inoculum but were propagated using different host cells (ovine turbinate cells, primary lamb kidney cells, or Madin-Darby bovine kidney cells). Two different passages of the primary lamb kidney-derived virus were screened. Virus propagation, DNA purification, and PCR were performed in the absence of any primate-derived materials. PCR-based AAV screening employing primers homologous to the highly conserved helicase domain of rep and DNA binding domain of cap amplified a 1.5-kb fragment from all four adenovirus preparations. Sequence analysis of this PCR fragment revealed a high homology to AAV-5. Primers corresponding to the 3′ untranslated region of AAV-5 were then used to amplify and clone the full-length AAV caprine (AAV-Go.1) capsid gene. DNA sequence analysis revealed that the VP1 open reading frame of caprine AAV (GenBank accession number AY724675) was 93% identical to VP1 of AAV-5 at the nucleotide level and 94% identical when translated (Fig. 1). All of the 42 differences between AAV-Go.1 and AAV-5 capsid amino acid sequences are confined to the last half of the capsid gene. Molecular modeling based on the surface structure of the AAV-2 capsid (PDB identification no., 1LP3) predicted that 29 out of 42 amino acid differences between AAV-Go.1 and AAV-5 are located on the exterior of the goat capsid, mainly on a surface feature that crystallographers refer to as the spike (Fig. 2). Ten amino acid differences are buried just under the surface of the spike, and two differences are internal. Caprine AAV VP1 also has an insertion of two amino acids (SS) at positions 481 and 482 relative to AAV-5. The differences between AAV-Go.1 and AAV-5 capsid also are not found in any of roughly 145 other known AAV capsid sequences and thus could impart unique properties to the AAV-Go.1 capsid in terms of tropism or human preexisting humoral immunity.
FIG. 1.
Comparison of the amino acid sequence of AAV-5 and caprine AAV (AAV-Go.1) VP1. Amino acid differences are shown. The dots in the alignment represent the amino acids that are identical in the two sequences. The insertion of two amino acids (SS) in AAV-Go.1at positions 481 and 482 of VP1 is indicated by dashes in the AAV-5 sequence.
FIG. 2.
Predicted location of differences between AAV-5 and caprine AAV VP3, modeled on the crystal structure of AAV-2 VP3. (A) External surface amino acids are color coded by amino acid type using the RasMol software. (B) Magnified top view of an asymmetric structural unit (1/60 of the full virus). External amino acids that are different between AAV-5 and caprine AAV VP3 are indicated in black (29 of the 42 changes identified). The spike is located in the lower left and lower right corners. (C) Lateral view of a representative trimer showing all 29 external differences (in black), 10 buried differences (in yellow), and two internal differences (in cyan). Because the spikes in AAV-5 and caprine AAV are predicted to be shorter than those in AAV-2, the amino acids 315 to 320 in AAV-2 were deleted in this figure to simulate this difference in sequence and structure.
In vitro transduction by caprine AAV vectors.
One important tissue considered for gene therapy applications is the liver; therefore, in vitro transduction activities were assessed by infecting HepG2 cells. We compared caprine AAV transduction of this hepatocyte cell line with that of AAV-2 and AAV-8. We chose AAV-2 because it is the AAV prototype and was previously used in clinical trials targeting the liver and AAV-8 because it has been shown to transduce mouse liver very efficiently (16); for these same reasons these vectors were used for the in vivo experiments described below. Using the same vector genome input, transduction with AAV-Go.1 lacZ vector was 200-fold lower than that with AAV-2 lacZ vector but 20-fold higher than that with the AAV-8 lacZ vector. Taking into account the different transduction efficiencies previously observed for each vector, we calculated adjusted (normalized) ratios so as to use the same vector genome input. Thus, we determined the transduction efficiency of AAV-Go.1 lacZ vector to be 200-fold lower than that of AAV-2 lacZ vector but 20-fold higher than that of the AAV-8 lacZ vector.
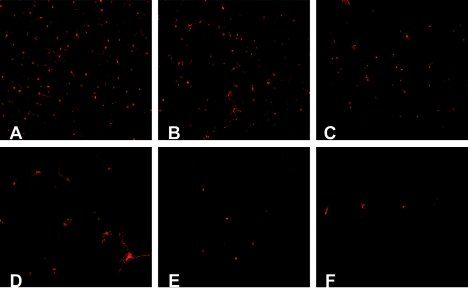
The ability of the AAV-Go.1 capsid to transduce neurons, another significant target of AAV gene therapy, was studied using primary cultures derived from rat striatum. Here we describe for the first time the transduction of these cultures by AAV. Therefore, we used a panel of different AAV capsid serotypes and compared them to the caprine AAV. Although the AAV-Go.1 lacZ vector was able to transduce rat striatal cultures, its efficiency was modest compared to that of primate-derived AAVs. AAV-6 lacZ vector transduced the cultures most efficiently, followed by the AAV-8, AAV-2, AAV-5, and AAV-4 lacZ vectors (Fig. 3). While AAV-6 lacZ vector transduced neurons exclusively, AAV-5 lacZ vector-mediated gene expression was observed exclusively in glial cells. All other vectors, including AAV-Go.1, transduced both neuronal and glial cells.
FIG. 3.
Comparative expression of AAV vectors in primary neuronal cultures. Transduction efficiency of the reporter gene lacZ in the rat striatal neurons was highest in AAV-6 lacZ (A) followed by AAV-8 lacZ (B), AAV-2 lacZ (C), AAV-5 lacZ (D), AAV-Go.1 lacZ (E), and AAV-4 lacZ (F). AAV-6 lacZ transduced neurons exclusively, whereas AAV-5 lacZ-mediated gene transfer was inefficient in neurons but significant in glial cells. All other vectors transduced both neurons and glial cells.
In vitro neutralization of caprine AAV vectors.
In order to examine the effect of preexisting human humoral immunity on the transduction by caprine AAV vectors in vitro, we preincubated AAV-Go.1 lacZ vector with increasing concentrations ofIVIG prior to transducing human HepG2 cells. IVIG ispurified human IgG derived from the pooled plasma of approximately 50,000 individuals. We also evaluated several primate-derived AAV vectors for comparison. Most of the primate-derived AAV vectors were neutralized at IVIG concentrations of 10 mg/ml (concentrations of IVIG included in the assay were 50, 10, 1, 0.1, and 0.01 mg/ml), the concentration of IgG found in human plasma, with the exception of AAV-4 and AAV-8 vectors, which were more resistant. The IVIG resistance of the AAV-Go.1 lacZ vector was similar to that of the AAV-4 and AAV-8 vectors (Table 1). Surprisingly, when AAV-Go.1 lacZ and the closely related AAV-5 lacZ vectors were compared (concentrations of IVIG included in the assay were 50, 25, 12.5, 6.25, 1, 0.5, 0.1, and 0.05 mg/ml), we found that AAV-Go.1 vectors required 500-fold-higher concentrations of IVIG for neutralization than AAV-5 did (Table 1). These results underscore the potential usefulness of recombinant caprine AAV vectors for human applications, particularly in cases where there is significant exposure of the vector to blood prior to reaching the target tissue.
TABLE 1.
Neutralization of AAVs by human IVIG
| AAV | Lowest concn of IVIG (mg/ml) showing 50% neutralizationa |
|---|---|
| Expt 1 | |
| AAV-1 | 10 |
| AAV-2 | 10 |
| AAV-3B | 1 |
| AAV-4 | 50 |
| AAV-5 | 0.1 |
| AAV-6 | 10 |
| AAV-8 | 50 |
| Expt 2 | |
| AAV-5 | 0.1 |
| AAV-Go.1 | 50 |
Determined as described in Materials and Methods. A twofold difference is statistically insignificant (P > 0.05).
Mouse model for evaluating preexisting human humoral immunity.
To create a model system for evaluating the effect of human preexisting humoral immunity on vector transduction in vivo, we infused male SCID mice with IVIG, 24 h prior to vector administration (31a). This model creates in mice circulating human IgG concentrations that are similar to those found in the human population. The IgG epitope diversity in the mice is the average of the IgG epitope diversities of the approximately 50,000 plasma samples from which the IVIG was derived. Eighteen milligrams of IVIG was used per animal in experiments examining intravenous administration, and 41 mg of IVIG was used per animal in experiments examining intramuscular administration. Immediately prior to vector administration, a plasma sample was taken and assayed for IVIG content by examining the neutralizing titer against AAV-2 lacZ vectors. Mice used for transduction experiments by the i.v. and intramuscular routes had at the time of the injection 5 mg/ml and 10 mg/ml of circulating IVIG, respectively (these concentrations correspond to AAV-2 approximate neutralizing titers of 1:150 and 1:300, respectively).
The hFIX vector (named hFIX16) administered intravenously employed a liver-specific, ApoE/hAT, enhancer/proximal promoter (18), and the hFIX vectors administered intramuscularly (named hFIX9 vectors) were driven by the CMV immediate-early promoter (22). Both sets of animals are a model for the effect of preexisting human humoral immunity on vector administered via nonvascular and vascular routes.
Intramuscular administration of CMV hFIX vectors in the presence and absence of IVIG.
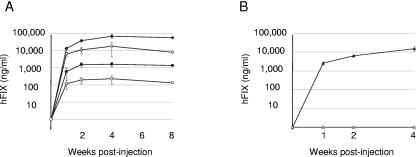
SCID mice were injected intramuscularly with 2 × 1011 vector genomes of AAV-Go.1 hFIX9, AAV-1 hFIX9, or AAV-8 hFIX9 vectors (five mice per group). AAV-1 and AAV-8 were used for comparison because it has been established that they efficiently transduce mouse skeletal muscle (15, 17). We observed only low levels of hFIX in the plasma of the mice injected with the AAV-Go.1 hFIX9 vector, suggesting that the recombinant caprine AAV vector does not efficiently transduce muscle (Fig. 4). As expected, high levels of expression were observed after administration of AAV-8 hFIX9 and AAV-1 hFIX9 vectors.
FIG. 4.
Transduction of muscle in SCID mice. Male mice were injected intramuscularly with 2 × 1011 vector genomes of rAAV-1 hFIX9, rAAV-8 hFIX9, and rAAV-Go.1 hFIX9 with (empty symbols) and without (filled symbols) previous administration of IVIG. Human factor IX concentration was measured by ELISA. Each data point corresponds to the mean of five animals. The human factor IX concentration in the control animals was considered a blank and was subtracted from human factor IX levels in the experimental animals. The amount of IVIG present in the plasma of the animal at the time of injection with the vector was calculated to be 10 mg/ml based on the AAV-2 neutralizing titer of 1:300. Squares, AAV-1; diamonds, AAV-8; circles, AAV-Go.1.
When SCID mice were injected with IVIG (41 mg/animal) prior to injection of vector, the plateau level of expression from the AAV-1 hFIX9 vector was reduced fivefold, whereas the plateau level of expression from the AAV-8 hFIX9 vector was reduced 10-fold. In contrast, expression mediated by AAV-Go.1 hFIX9 vector, though low, was unaffected by the presence of IVIG. The animals had an anti-AAV-2 neutralizing titer of 1:300, which corresponds to plasma IVIG concentrations of 10 mg/ml.
i.v. administration of ApoE/hAT (liver-specific) hFIX vectors in the presence and absence of IVIG.
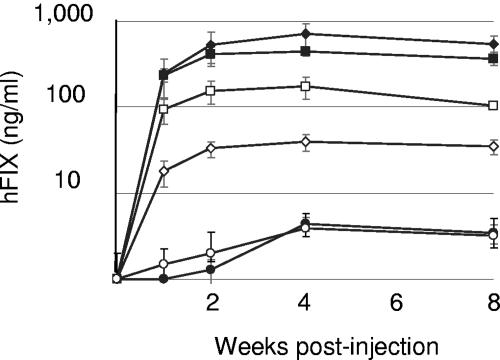
SCID mice were injected intravenously with 5 × 1011 vector genomes of AAV-Go.1 hFIX16 or AAV-8 hFIX16 vectors in the presence and absence of IVIG (Fig. 5A). Eighteen milligrams of IVIG was administered per animal, producing plasma anti-AAV-2 titers of 1:128 ± 11. AAV-2 hFIX16 vector data are shown for comparison (Fig. 5B). Animals injected with the AAV-2 vector were pretreated with 0.9 mg of IVIG per animal, and the anti-AAV-2 titer before the AAV-2 injection was 1:10. In the absence of IVIG pretreatment, the AAV-Go.1 hFIX16 vector produced plasma hFIX levels that were approximately 10-fold lower than those produced by the AAV-2 hFIX16 vector and 40-fold lower than those produced by the AAV-8 hFIX16 vector. The hFIX expression construct was driven by a well-characterized liver-specific promoter, and consequently we presume that most, if not all, of the hFIX expression is from the liver (8). In the presence of IVIG, the plasma hFIX concentrations produced by the AAV-8 hFIX16 and AAV-Go.1 hFIX16 vectors were reduced to 26% and 14% of the levels produced in the absence of IVIG, respectively. Therefore, the transduction of liver by the caprine AAV was more sensitive to the presence of IVIG than was that by AAV-8. Expression from the AAV-2 hFIX16 vector was reduced to undetectable levels (<2 ng/ml) by a far lower amount of circulating IVIG.
FIG. 5.
Transduction of liver in SCID mice. (A) Male SCID mice were injected via the tail vein with 5 × 1011 vector genomes of rAAV-Go.1 hFIX16 (circles) or rAAV-8 hFIX16 (diamonds) (n = 5). Retro-orbital blood was collected 1, 2, and 4 (n = 5) and 8 (n = 3) weeks after vector injection. Transduction was in the presence (empty symbols) or absence (filled symbols) of IVIG. Mice tested with IVIG were injected via the tail vein (250 μl at 100 mg/ml), 24 h before injection with the vector. In the case of AAV-Go.1 and AAV-8 the neutralizing titer was 1:120. Human factor IX was measured by ELISA. (B) In a separate study SCID mice were injected with rAAV-2 hFIX16. Empty and filled symbols, presence and absence of IVIG, respectively. Mice tested with IVIG were injected with the same total volume of 250 μl used for the injections above but now containing 9 μl at 100 mg/ml; the neutralizing titer when the virus was injected was 1:10.
Biodistribution of AAV-Go.1 in mice.
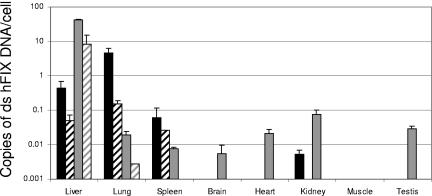
For the biodistribution analysis, mice were euthanized 4 weeks after i.v. injection of 5 × 1011 vg per mouse. Organs collected included brain, testis, skeletal muscle (quadriceps), kidney, spleen, lung, heart, and liver. To measure the copy number of the hFIX gene, quantitative PCR was performed. The biodistribution analysis of intravenously administered AAV-Go.1 hFIX16 vector in male SCID mice indicated that the caprine AAV had a pronounced lung tropism (Fig. 6). While 91% of the vector genomes were detected in the lung of AAV-Go.1-injected mice, only 0.04% of the total vector genomes were detected in the lung after AAV-8 injection (2,300-fold increase in lung tropism). AAV-Go.1 biodistribution was more restricted than that of AAV-8. No gene transfer was observed in testes, suggesting that germ line transfer with intravenously administered AAV-Go.1 is not likely to be significant. Unlike AAV-8, gene transfer to brain was not observed after intravenous administration.
FIG. 6.
Biodistribution of rAAV-Go.1 hFIX16 after intravenous administration in mice. Human factor IX double-stranded DNA was quantitated in eight tissues 4 weeks after AAV administration. Bars represent the mean numbers of copies and standard deviations after testing of two different animals. Black bars, AAV-Go.1 hFIX16; gray bars, AAV-8 hFIX16. Liver, lung, and spleen were also analyzed when vector delivery was performed in the presence of IVIG (crosshatched bars). ds, double stranded.
DISCUSSION
We identified, cloned, and characterized a novel caprine AAV capsid and analyzed its potential for use as a vector for human gene therapy. A salient feature of the amino acid sequence of this capsid is its high homology (94%) to AAV-5. These highly homologous AAV capsids have been isolated from evolutionarily divergent species. A similar case was the unanticipated homology of goose parvovirus VP1 to AAV2 VP1 (70%) (36). An important question that arises as a result of this discovery is whether AAV-5 or AAV-Go.1 directly evolved from the other, due to an interspecies transfer, or whether both evolved independently. AAV-5 is clearly of human origin, having been isolated from a human penile flat condylomatous lesion. The high AAV-5 neutralizing titer of IVIG implies that AAV-5-like viruses are probably relatively common in the human population. Similarly, the fact that two AAV-5-like viruses, the goat and cow AAVs (32), have been isolated from domesticated ruminants implies that AAV-5-like viruses may also be prevalent in this population. Humans and domestic ruminants have been living in close contact for approximately 10,000 years, and it would not be surprising if AAV-5-like viruses have evolved the ability to propagate in either host. There are several precedents for this with other viruses (6). Because AAV-Go.1 and AAV-5 share so much sequence homology, it seems most likely that one is probably a rather direct descendant of the other. Until a more comprehensive virus survey is done of both the human and ruminant populations, it will not be possible to determine the probable directionality of the interspecies jump.
Structural modeling of the AAV-5 and goat capsids, based on the crystal structure of AAV-2, predicts that most of the amino acid differences between AAV-5 and caprine AAV lie on or under the spike of the capsid. The fact that all but two of the amino acid differences between AAV-5 and AAV-Go.1 are on the exterior or exterior-proximal regions of the capsids suggests that their evolutionary divergence is being driven principally by selective pressures that affect the exterior of the capsid. These may include humoral immunity and changes in receptor usage. The fact that few amino acid differences occur in regions other than the surface and surface-proximal regions suggests that T-cell immunity is not a principal driving force in the divergence of AAV-5 and AAV-Go.1.
Amino acid differences on the surfaces of AAV-Go-1 and AAV-5 have resulted in tropism differences that may indicate that the two viruses differ in receptor usage or other entry functions. When used to transduce the mixed cell populations that comprise rat striatal primary cultures, AAV-5 lacZ vectors transduced exclusively glial cells, while AAV-Go.1 lacZ vectors transduced neurons and glial cells. Furthermore, sialic acid, an inhibitor of AAV-5 transduction, does not inhibit transduction by AAV-Go.1.
AAV-Go.1 vectors are similar to AAV-4 and AAV-8 vectors in terms of resistance to neutralization by IVIG, in vitro, and substantially more resistant than AAV-1, AAV-2, AAV-3B, AAV-5, and AAV-6 vectors. It is remarkable that AAV-Go.1 and AAV-5 share 94% of their amino acids and yet differ in resistance to IVIG neutralization by 500-fold. In our in vivo model system for human preexisting humoral immunity, AAV-Go.1 vectors were more resistant to neutralization by IVIG than AAV-1 or -8 vectors when administered intramuscularly and were similar to AAV-8 vectors but far superior to AAV-2 vectors when administered by the i.v. route. Why IVIG resistance of the goat vector is better than that of the AAV-8 vector using one route of administration but similar using another is unclear but may relate to more rapid binding and internalization of AAV-Go.1 within skeletal muscle (despite less overall transduction).
Though far from exhaustive, we have begun to define the tropism of AAV-Go.1 vectors. AAV-Go.1 vectors transduce human cells (HepG2) in vitro, and transduction is not inhibited by heparin or sialic acid at concentrations inhibitory to AAV-2 and AAV-5 vectors, respectively. In SCID mice, AAV-Go.1 vectors transduce skeletal muscle about 2 logs more poorly than AAV-1 or -8 vectors. Biodistribution studies in SCID mice revealed that AAV-Go.1 vectors have a pronounced tropism for lung; 91% of the vector administered intravenously was recovered in the lung. Because of the robust lung tropism, we cannot determine how efficiently tissues downstream from the lungs are transduced when vector is administered by tail vein. Small amounts of vector were recovered from the liver, kidney, and spleen.
In summary, we have isolated a novel AAV capsid from goats that raises questions with regard to the origin and evolution of human AAV-5. During the isolation of the caprine AAV no human cell cultures or products were employed. The unique tropism and substantial resistance to preexisting humoral immunity of AAV-Go.1 vectors may make them useful for gene delivery to the lungs in humans.
Acknowledgments
This work was supported by Avigen.
We thank Gibrail Haniff for his technical assistance with the mouse surgical procedures. We also thank Dawn McGuire for critical reading of the manuscript.
REFERENCES
- 1.Bankiewicz, K. S., J. L. Eberling, M. Kohutnicka, W. Jagust, P. Pivirotto, J. Bringas, J. Cunningham, T. F. Budinger, and J. Harvey-White. 2000. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 164:2-14. [DOI] [PubMed] [Google Scholar]
- 2.Bossis, I., and J. A. Chiorini. 2003. Cloning of an avian adeno-associated virus (AAAV) and generation of recombinant AAAV particles. J. Virol. 77:6799-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. [DOI] [PubMed] [Google Scholar]
- 4.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiorini, J. A., L. Yang, Y. Liu, B. Safer, and R. M. Kotin. 1997. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J. Virol. 71:6823-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claas, E. C., J. C. de Jong, R. van Beek, G. F. Rimmelzwaan, and A. D. Osterhaus. 1998. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine 16:977-978. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, J. K., J. B. McFerran, E. R. McKillop, and W. L. Curran. 1979. Isolation of an adeno-associated virus from sheep. Brief report. Arch. Virol. 60:171-176. [DOI] [PubMed] [Google Scholar]
- 8.Couto, L. B. 2004. Preclinical gene therapy studies for hemophilia using adeno-associated virus (AAV) vectors. Semin. Thromb. Hemost. 30:161-171. [DOI] [PubMed] [Google Scholar]
- 9.del Monte, F., E. Kizana, A. Tabchy, and R. J. Hajjar. 2004. Targeted gene transfer in heart failure: implications for novel gene identification. Curr. Opin. Mol. Ther. 6:381-394. [PubMed] [Google Scholar]
- 10.Doroudchi, M. M., J. Liauw, K. Heaton, Z. Zhen, and J. R. Forsayeth. 2005. Adeno-associated virus-mediated gene transfer of human aromatic l-amino acid decarboxylase protects mixed striatal primary cultures from l-DOPA toxicity. J. Neurochem. 93:634-640. [DOI] [PubMed] [Google Scholar]
- 11.Dutta, S. K. 1975. Isolation and characterization of an adenovirus and isolation of its adenovirus-associated virus in cell culture from foals with respiratory tract disease. Am. J. Vet. Res. 36:247-250. [PubMed] [Google Scholar]
- 12.Estevez, C., and P. Villegas. 2004. Sequence analysis, viral rescue from infectious clones and generation of recombinant virions of the avian adeno-associated virus. Virus Res. 105:195-208. [DOI] [PubMed] [Google Scholar]
- 13.Farkas, S. L., Z. Zadori, M. Benko, S. Essbauer, B. Harrach, and P. Tijssen. 2004. A parvovirus isolated from royal python (Python regius) is a member of the genus Dependovirus. J. Gen. Virol. 85:555-561. [DOI] [PubMed] [Google Scholar]
- 14.Flotte, T. R., and B. L. Laube. 2001. Gene therapy in cystic fibrosis. Chest 120:124S-131S. [DOI] [PubMed] [Google Scholar]
- 15.Gao, G., L. H. Vandenberghe, M. R. Alvira, Y. Lu, R. Calcedo, X. Zhou, and J. M. Wilson. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm, D., and M. A. Kay. 2003. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr. Gene Ther. 3:281-304. [DOI] [PubMed] [Google Scholar]
- 18.Grimm, D., S. Zhou, H. Nakai, C. E. Thomas, T. A. Storm, S. Fuess, T. Matsushita, J. Allen, R. Surosky, M. Lochrie, L. Meuse, A. McClelland, P. Colosi, and M. A. Kay. 2003. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood 102:2412-2419. [DOI] [PubMed] [Google Scholar]
- 19.Hadaczek, P., H. Mirek, J. Bringas, J. Cunningham, and K. Bankiewicz. 2004. Basic fibroblast growth factor enhances transduction, distribution, and axonal transport of adeno-associated virus type 2 vector in rat brain. Hum. Gene Ther. 15:469-479. [DOI] [PubMed] [Google Scholar]
- 20.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 21.Hoshijima, M., Y. Ikeda, Y. Iwanaga, S. Minamisawa, M. O. Date, Y. Gu, M. Iwatate, M. Li, L. Wang, J. M. Wilson, Y. Wang, J. Ross, Jr., and K. R. Chien. 2002. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 8:864-871. [DOI] [PubMed] [Google Scholar]
- 22.Manno, C. S., A. J. Chew, S. Hutchison, P. J. Larson, R. W. Herzog, V. R. Arruda, S. J. Tai, M. V. Ragni, A. Thompson, M. Ozelo, L. B. Couto, D. G. Leonard, F. A. Johnson, A. McClelland, C. Scallan, E. Skarsgard, A. W. Flake, M. A. Kay, K. A. High, and B. Glader. 2003. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101:2963-2972. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita, T., S. Elliger, C. Elliger, G. Podsakoff, L. Villarreal, G. J. Kurtzman, Y. Iwaki, and P. Colosi. 1998. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 5:938-945. [DOI] [PubMed] [Google Scholar]
- 24.Miao, C. H., X. Ye, and A. R. Thompson. 2003. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum. Gene Ther. 14:1297-1305. [DOI] [PubMed] [Google Scholar]
- 25.Mori, S., L. Wang, T. Takeuchi, and T. Kanda. 2004. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 330:375-383. [DOI] [PubMed] [Google Scholar]
- 26.Muramatsu, S., H. Mizukami, N. S. Young, and K. E. Brown. 1996. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 221:208-217. [DOI] [PubMed] [Google Scholar]
- 27.Muzyczka, N. 1992. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158:97-129. [DOI] [PubMed] [Google Scholar]
- 28.Muzyczka, N., and K. I. Berns. 2001. Parvoviridae: the viruses and their replication, p. 2327-2359. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 29.Olson, E. J., S. R. Haskell, R. K. Frank, H. D. Lehmkuhl, L. A. Hobbs, J. V. Warg, J. G. Landgraf, and A. Wunschmann. 2004. Isolation of an adenovirus and an adeno-associated virus from goat kids with enteritis. J. Vet. Diagn. Investig. 16:461-464. [DOI] [PubMed] [Google Scholar]
- 30.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanftner, L. M., B. M. Suzuki, M. M. Doroudchi, L. Feng, A. McClelland, J. R. Forsayeth, and J. Cunningham. 2004. Striatal delivery of rAAV-hAADCto rats with preexisting immunity to AAV. Mol. Ther. 9:403-409. [DOI] [PubMed] [Google Scholar]
- 31a.Scallan, C. D., H. Jiang, T. Liu, S. Patarroyo-White, J. M. Sommer, S. Zhou, L. B. Couto, and G. F. Pierce. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood, in press. [DOI] [PubMed]
- 32.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 78:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommer, J. M., P. H. Smith, S. Parthasarathy, J. Isaacs, S. Vijay, J. Kieran, S. K. Powell, A. McClelland, and J. F. Wright. 2003. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 7:122-128. [DOI] [PubMed] [Google Scholar]
- 34.Walter, J., Q. You, J. N. Hagstrom, M. Sands, and K. A. High. 1996. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc. Natl. Acad. Sci. USA 93:3056-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao, W., N. Chirmule, S. C. Berta, B. McCullough, G. Gao, and J. M. Wilson. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 73:3994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zadori, Z., R. Stefancsik, T. Rauch, and J. Kisary. 1995. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2. Virology 212:562-573. [DOI] [PubMed] [Google Scholar]