Abstract
Recombinant human cytomegaloviruses that do not express UL97 kinase activity exhibit a distinctive plaque morphology characterized by the formation of highly refractile bodies late in infection. These structures were also observed in infected cells treated with the UL97 kinase inhibitor maribavir. Nuclear inclusions were purified to near homogeneity, and the constituent proteins were identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. This analysis demonstrated that the aggregates were formed principally of the tegument proteins pp65 and ppUL25 but also contained additional virion structural proteins including the major capsid protein. Immunoblotting experiments confirmed these results and identified a number of additional viral proteins present in the purified tegument aggregates. Interestingly, the formation of these structures appeared to be dependent on pp65, since it was not induced in cells infected with a recombinant virus with this open reading frame deleted. Morphologically similar aggregates could be reproduced in nuclei of uninfected cells by overexpressing pp65, and their formation was prevented by coexpressing the UL97 kinase. Inhibition of UL97 kinase activity with maribavir or mutation of an essential amino acid in the kinase abolished its ability to prevent aggregate formation. These data taken together suggest that the UL97 kinase impacts the aggregation of pp65 in the nuclei of infected cells. We propose that the kinase plays an important role in the acquisition of tegument during virion morphogenesis in the nucleus and that this activity represents an important step in the production of mature virus particles.
Protein kinases are encoded by diverse virus families including herpesviruses, poxviruses, and rotaviruses (5, 23, 24). All herpesviruses encode at least one conserved protein kinase that is thought to mimic aspects of cellular protein kinase, cdc2, and play a role in viral replication (11). Each of these viral enzymes contains conserved subdomains found in cellular serine/threonine (Ser/Thr) kinases and possesses both autophosphorylating and transphosphorylating activity (10). These conserved kinases likely share common functions among all the herpesviruses, since the human cytomegalovirus (HCMV) UL97 kinase can substitute to some degree for the UL13 kinase in herpes simplex virus (21).
The UL97 protein kinase shares homology with this family of conserved kinases and appears to be closely related to human Ser/Thr kinases (20). This enzyme exhibits an unusual substrate specificity in that it can activate both ganciclovir (GCV) and acyclovir through selective phosphorylation (15, 29, 30), as well as autophosphorylate and transphosphorylate protein substrates on serine and threonine residues (2, 8). Substrates identified thus far are ppUL44, histone H2B, and the carboxyl-terminal domain of the largest subunit of RNA polymerase II (1, 3). The high frequency of UL97 mutations in drug-resistant clinical isolates reflects its central role in the mechanism of action of GCV, as well as its importance in the management of CMV infections in the clinic (6). More recently, this kinase has become an antiviral target in its own right (4, 9), as maribavir (MBV, 1263W94) was shown to be a selective inhibitor of this enzyme and exhibit potent antiviral activity (4) and is being developed as a therapeutic agent for the treatment of CMV infections (9, 13). Thus, defining the function of this kinase during viral replication is vital to understanding the emergence of drug resistance in the clinic, as well as developing better antiviral therapies.
The UL97 locus contains a complex transcriptional unit that produces a number of structurally polycistronic 3′-coterminal transcripts, of which at least five contain this gene (34). Only the 4.7-kb transcript is thought to be translated, but mutations in this open reading frame (ORF) may also disrupt the transcription of other genes that are thought to be essential. UL97 kinase is expressed with early/late kinetics, localizes to the nucleus, and has an apparent migration rate of approximately 80 kDa (19). The kinase is also a constituent of virions and is posttranslationally modified by phosphorylation of serines and threonines (19). The nuclear localization signal lies in the amino-terminal domain, and this region is not required for GCV phosphorylation (20). Point mutations in the conserved kinase domains, including amino acids 340, 442, 446, and 523, result in the loss of both autophosphorylation and GCV phosphorylation activity (18).
Although an intact UL97 open reading frame is not essential for viral replication, a deficiency of the gene product reduces virus yield by more than 2 orders of magnitude (22). The first observable defect during the replication of a UL97 deletion mutant, RCΔ97, is a modest two- to sixfold decrease in the accumulation of viral DNA; however, the magnitude of this defect is insufficient to explain the poor replication characteristics of this virus (14, 35). An additional striking defect is a marked decrease in the number of mature capsids in the cytoplasm of cells infected with RCΔ97, suggesting that mature virions never exit the nucleus (14, 35). The underlying molecular defects that occur in the absence of ppUL97 remain undefined but could be related to the poorly understood events related to virion morphogenesis in the nucleus including the cleavage and packaging of DNA, the acquisition of tegument, transport to the nuclear periphery, or the process of nuclear egress.
The first report describing the phenotype of RCΔ97 noted a distinctive plaque morphology characterized by the appearance of highly refractile bodies in the nuclei of infected cells (22). We hypothesized that an investigation into the nature of these nuclear inclusions might reveal molecular defects associated with a deficiency in UL97 kinase activity. Studies presented here demonstrated that the inclusions were composed predominantly of tegument and structural proteins and that their formation may be related to the aggregation by pp65 in the absence of UL97 kinase activity. The spontaneous aggregation of pp65 was reproduced in uninfected cells and was inhibited specifically by ppUL97 and appeared to be dependent on its enzymatic activity. These results suggest that UL97 kinase activity is required for the normal function of tegument proteins in nuclei and that virion morphogenesis is defective in its absence.
MATERIALS AND METHODS
Cells and virus.
Low-passage-number human foreskin fibroblast (HFF) cells were routinely propagated in monolayer cultures in minimum Eagle's medium with Earle's salts supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 μg/ml penicillin G, and 25 μg/ml gentamicin. HCMV strain AD169 was obtained from the American Type Culture Collection (Manassas, VA), and virus stocks were prepared and their titers were determined as described previously (28). The UL97-null mutant (RCΔ97.08) was described previously (22), and the pp65-deficient virus (RVAd65) was obtained from Bodo Plachter (University of Mainz, Mainz, Germany); details of its construction were described previously (27). MBV, 2-bromo-5,6-dichloro-1-β-d-ribofuranosyl benzimidazole (BDCRB), and cidofovir (CDV) were obtained through the Antiviral Substances Program of the National Institute for Allergy and Infectious Diseases.
Plasmids.
Two PCRs were used to construct a K355M mutation in the UL97 ORF using UL97 forward primer 5′-CAC CAT GTC CTC CGC ACT TCG GTC T-3′ and UL97 K355M B reverse primer 5′-CTA TCG CGT GGT CAT GGT GGC GCG TAA G-3′ for the first reaction and UL97 K355M B forward primer 5′-CTT ACG CGC CAC CAT GAC CAC GCG ATA G-3′ and the UL97 reverse primer 5′-TTA CTC GGG GAA CAG TTG G-3′ for the second reaction. The fragments were amplified with Taq PCR Master Mix (QIAGEN, Valencia, CA), and the resulting PCR products were gel purified using a QIAGEN gel purification kit. PCR products were combined in an equal ratio and used as templates for the final PCR product using UL97 forward and reverse primers and PFU Turbo polymerase (Stratagene, La Jolla, CA). The resulting PCR product was TOPO cloned into pENTR (Invitrogen, Carlsbad, CA). The UL97 ORF was also amplified with the UL97 forward and UL97 reverse primers and cloned into pENTR, and the open reading frames were sequenced. Both the wild-type (wt) AD169 UL97 ORF and the K355M mutated ORF were recombined into pcDNA3.1/nV5-DEST expression vector using LR clonase (Invitrogen) to provide amino-terminal epitope tags and were designated pMP93 and pMP92, respectively. Both plasmids expressed immunoreactive proteins of the predicted molecular weight, although the K355M mutant protein was slightly smaller and less diffuse than the wt protein, consistent with its predicted underphosphorylation. The pp65-green fluorescent protein (GFP)-expressing plasmid was constructed by ligating the coding sequence of AD169 UL83 (pp65) into the enhanced GFP (EGFP) C1 plasmid (Clontech/BD, Palo Alto, CA), resulting in the expression of an EGFP fusion protein with UL83 fused to the carboxyl terminus of EGFP.
Polyacrylamide gels and blotting.
Protein samples were disrupted in 2× Laemmli buffer (Sigma, St. Louis, MO) and separated on 10% polyacrylamide gels (Bio-Rad, Hercules, CA). For Western blots, proteins were transferred to polyvinylidene difluoride membranes (Roche Applied Science, Indianapolis, IN) in a buffer containing 28 mM Tris, 39 mM glycine, 0.0375% sodium dodecyl sulfate, and 20% methanol in a semidry transfer cell (Bio-Rad). Blots were blocked in 1% blocking buffer (Roche Applied Science), incubated with primary antibodies overnight at 4°C, and washed in phosphate-buffered saline (PBS) with 0.02% Tween 20. An alkaline phosphatase-conjugated goat anti-mouse secondary antibody (Southern Biotechnology Associates, Birmingham, AL) was used to detect the primary antibody, and the blots were developed with CDP* (Roche Applied Science).
Immunofluorescence microscopy.
Immunofluorescence staining was performed in a manner similar to that previously described (26). Briefly, monolayer cultures of HFF cells were grown on 13-mm-diameter coverslips in 24-well plates. Infected coverslips were fixed for 15 min with freshly prepared 1% formaldehyde in PBS, washed two times with PBS, and permeabilized with 0.2% Triton X-100 in PBS for 15 min. Monoclonal antibodies to pp65 (28-19), pp150 (36-14), ppUL44 (28-21), and the major capsid protein (MCP) (28-4) were used as culture supernatants with goat anti-mouse secondary antibodies conjugated to fluorescein isothiocyanate or Texas Red (Southern Biotechnology).
Mass spectrometry.
Proteins were excised from polyacrylamide gels stained with Coomassie brilliant blue and analyzed at the UAB Comprehensive Cancer Center/Department of Pharmacology and Toxicology shared mass spectrometry facility. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometers (Applied Biosystems, Foster City, CA) were used to identify proteins present in the inclusions.
Isolation of nuclear and cytoplasmic tegument aggregates.
Low-passage-number HFF cells were infected with RCΔ97.08 at a multiplicity of infection of 0.01 PFU/cell in 175-cm2 flasks. Monolayers of infected cells were passaged at 7 days postinfection as plaques started to form as well as at 12 and 16 days postinfection until 100% cytopathic effect was observed. Infected cells were rinsed once in PBS, dislodged with 0.25% trypsin-EDTA (Gibco, Grand Island, NY), and resuspended in a volume of 10 ml growth medium. The cells were collected at 1,000 × g for 5 min, and the residual medium was decanted. The cell pellet was resuspended in PBS with the addition of 0.6% NP-40, and nuclei were collected by centrifugation through a cushion of Histopaque 1077 (Sigma Chemical Company) at 1,000 × g for 5 min. Nuclei were resuspended in PBS, and an equal volume of 5 M NaCl was added to lyse the nuclei. Cellular DNA was degraded by diluting the nuclear lysate fivefold in distilled water, adding 10,000 units of DNase I, and incubating the mixture for 15 min at 37°C. An equal volume of 5 M urea was added to the nuclear lysate, and the nuclear bodies were collected by centrifugation at 3,500 × g for 10 min through a Histopaque cushion. Nuclear bodies were resuspended in PBS supplemented with 0.5% NP-40 and frozen at −80°C. Aggregates were isolated from the cytoplasmic fraction by a similar strategy. Viral aggregates from the cytoplasmic fraction of the NP-40 lysate were collected by sedimentation at 3,500 × g. The sedimented material was resuspended in a PBS buffer containing 5 M urea and incubated for 5 min at ambient temperature. Viral inclusions were then isolated by sedimentation through a Histopaque cushion, resuspended in PBS with 0.5% NP-40, and frozen at −80°C.
RESULTS
Characteristic inclusions are formed in infected cells in the absence of ppUL97 activity.
The formation of refractile inclusion bodies in the nuclei of cells infected with independent isolates of RCΔ97 suggested that this event was related to the deletion of UL97 (22). In subsequent experiments, it was noted that these mutants did not induce the formation of these structures in cells that expressed ppUL97 kinase in trans, suggesting that a deficiency of this gene product results in the formation of unusual inclusions in infected cells (data not shown). The induction of nuclear inclusions that were similar in appearance was also observed by another laboratory when infected cells were treated with MBV and appeared to be related to the mechanism of action of the drug (Sunwen Chou, Oregon Health Sciences University, Portland). We also investigated the effect of this drug on infected cells to determine if the pharmacologic inhibition of the kinase might induce structures that were similar to those observed in the deletion mutant. Cells infected with RCΔ97 developed the characteristic nuclear inclusions by 72 h postinfection (hpi) as observed previously (Fig. 1A). Cells infected with AD169 and treated with 15 μM MBV also formed large refractile nuclear inclusions that were microscopically indistinguishable from those observed with the deletion mutant (Fig. 1B). The formation of inclusions was also induced in cells infected with the Towne or Toledo strain of HCMV (data not shown). Thus, it appeared that their formation might be related to the absence of UL97 kinase activity.
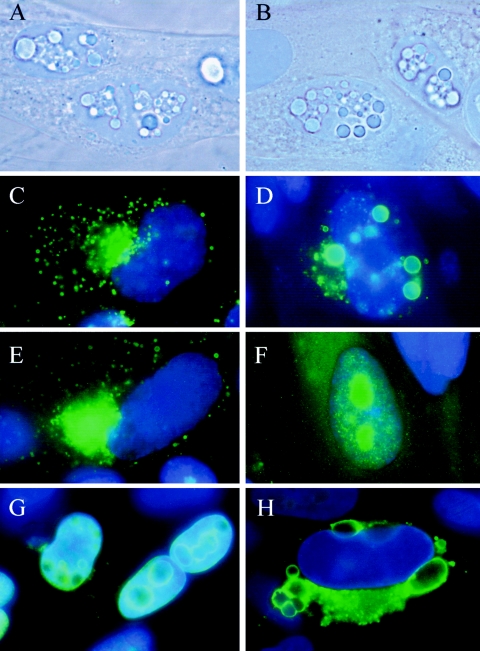
FIG. 1.
Photographs of inclusions in infected HFF cells and the effect of antiviral drugs on their formation. All images were obtained with a 100× objective and are shown merged with images of DAPI staining. Phase-contrast images of HFF cells infected with RCΔ97.08 (A) or AD169 with the addition of 15 μM MBV (B) are shown at 72 hpi. The formation of aggregates at 96 hpi was visualized with monoclonal antibody to pp65 (28-19) in AD169-infected cells without the addition of drug (C), with 15 μM MBV (D), with 15 μM BDCRB (E), or with cidofovir (F). Localization of aggregates in cells infected with RCΔ97.08 was visualized with an anti-pp65 monoclonal antibody at 48 hpi (G) and 72 hpi (H).
It is possible that these structures could simply be nonspecific aggregates that form as infected nuclei become saturated with viral proteins as a result of the block late in viral replication induced by MBV. Their formation might also reflect a more specific defect related to the absence of UL97 kinase activity. To distinguish between these interpretations of the data, cells infected with AD169 were treated with MBV, CDV, or BDCRB, a closely related analog of MBV that blocks viral replication late in infection through the inhibition of DNA packaging but does not inhibit UL97 kinase activity (31). A monoclonal antibody specific for the pp65 tegument phosphoprotein was used to specifically label these aggregates to monitor their formation in infected cells. Cells infected with AD169 exhibited punctate cytoplasmic and perinuclear staining by 96 hpi as observed previously (Fig. 1C) (26). In contrast, infected cells treated with MBV induced the development of large cytoplasmic inclusions (Fig. 1D). In the presence of BDCRB (Fig. 1E), the localization pattern of pp65 resembled that in untreated cells (Fig. 1C) and no large inclusions were observed. This observation suggested that the formation of these structures was not simply a consequence of a late block in the replication cycle but was more closely related to the specific mechanism of action of MBV, presumably its inhibition of UL97 kinase activity. An early block in viral replication by CDV greatly reduced the synthesis of pp65 and eliminated its export to the cytoplasm, as expected for an inhibitor of DNA synthesis (Fig. 1F). Thus, both the genetic data and the antiviral pharmacologic data suggested that a deficiency of UL97 kinase activity leads to the formation of these aberrant structures in infected cells.
Aggregates of structural proteins are observed both in the nucleus and in the cytoplasm.
The composition of these structures was investigated further to help define specific molecular defects that result from a deficiency of UL97 kinase activity. Aggregates produced in HFF monolayers infected with RCΔ97 were stained with a panel of monoclonal antibodies specific for HCMV proteins. Monoclonal antibodies to the tegument phosphoproteins pp65 and pp150 as well as ppUL44 and the MCP specifically labeled these structures, suggesting that they contained several different viral proteins. In cells infected with RCΔ97, small inclusions were observed in the nuclei starting approximately 48 hpi, while none were observed in cells infected with AD169. By 72 hpi, the nuclear aggregations had enlarged and a few cytoplasmic inclusions were observed (Fig. 1G). The aggregates acquired considerable mass by 96 hpi and were predominantly cytoplasmic, although a few remained in the nuclei (Fig. 1H). The existence of aggregates in the nucleus was confirmed by electron microscopy where the nuclear cytoplasmic membranes could be visualized clearly (Fig. 2). Immature virions were also observed immediately adjacent to the tegument aggregates, consistent with a possible accumulation of virions near these structures. The quantitative translocation of pp65 from the nuclei to the cytoplasmic juxtanuclear compartments also occurred very late in infection as described previously (Fig. 1C and H) (25). The temporal changes in the relative frequency of nuclear and cytoplasmic inclusions are consistent with formation of aggregates in nuclei and their translocation to the cytoplasm late in infection, yet it remains possible that the aggregates disaggregate and reform in the cytoplasm at a later time. We consider this unlikely given the remarkable stability of these structures. However, it is clear that aggregates formed in cells infected with the mutant virus and those induced with MBV were also observed in both the nucleus and the cytoplasm of infected cells.
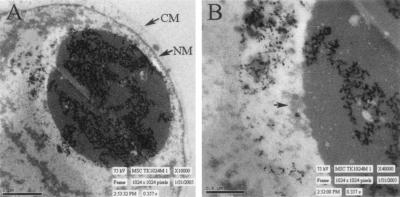
FIG. 2.
Electron micrographs of a tegument aggregate in the nucleus of a cell infected with RCΔ97. Infected cells were fixed and imaged with a Hitachi H7000 transmission electron microscope with an accelerating voltage of 75 kV. A large tegument aggregate is observed inside the nuclear membrane (NM), and the cytoplasmic membrane (CM) is shown for reference (A). A close-up of the tegument aggregate shows immature virions near the periphery of this large nuclear structure (B).
Isolation of aggregates from infected cells.
A purification scheme was developed based on the remarkable stability, density, and relatively large size (1 to 4 μm) of these structures. Briefly, nuclei from cells infected with RCΔ97 were lysed and treated with DNase I to eliminate DNA from the preparation. Nuclear aggregates were then resuspended in 2.5 M urea and harvested by sedimentation through a Ficoll cushion (data not shown). Aggregates could also be isolated from the cytoplasmic fraction using a similar procedure, and this finding is consistent with the observation that they also are present in the cytoplasm (Fig. 1D and H). Final preparations of nuclear and cellular aggregates retained their highly refractile appearance and seemed to be largely free of cellular debris. The yield of aggregates derived from nuclear and cytoplasmic fractions represented approximately 7% and 3% of the total cellular protein of infected cells, respectively. This high yield is consistent with the numerous large inclusions observed in infected cells, and the fact that these aggregates are capable of withstanding such a harsh isolation procedure suggests that they are highly stable structures.
Aggregates are formed predominantly from viral structural proteins.
Aggregates were isolated from cells infected with RCΔ97, the proteins were separated by one-dimensional and two-dimensional polyacrylamide gel electrophoresis, and the predominant species were identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (Fig. 3; Table 1). This analysis confirmed that the principal constituents of the aggregates were the tegument phosphoproteins pp65 and ppUL25 as well as the MCP encoded by UL86. Proteins with migration rates similar to those of MCP, ppUL25, and pp65 were also observed in aggregates derived from the cytoplasmic fraction (Fig. 3B). To confirm these findings, Western blot assays and immunofluorescent staining were used to detect proteins from nuclear tegument aggregates isolated from cells infected with RCΔ97 or AD169 with the addition of MBV. This analysis confirmed the presence of pp65 and MCP in these aggregates and suggested that pp150 and ppUL44 were also present in the aggregates (Table 1). The constituent proteins in the tegument aggregates described here likely represent only a subset of the viral gene products in these structures. The fact that two of the most abundant proteins in these aggregates are pp65 and ppUL25 suggests that they are largely composed of tegument proteins that are observed both in virions and in dense bodies (32).
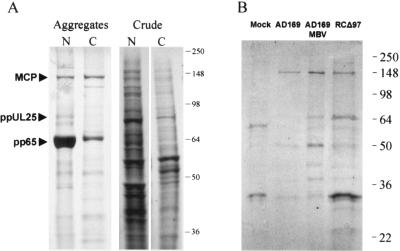
FIG. 3.
Most-abundant proteins in tegument aggregates. Aggregates were isolated from the nuclear (N) and cytoplasmic (C) fractions of HFF cells infected with RCΔ97.08 and are shown with the crude lysates from which they were derived (A). Viral proteins identified by mass spectrometry from tegument aggregates isolated from the nuclear fraction are indicated by arrowheads. The isolation procedure was used to isolate aggregates from uninfected HFF cells and HFF cells infected with RCΔ97.08 or AD169 or infected with AD169 and treated with MBV (B). Numbers to the right of each panel are molecular masses in kilodaltons.
TABLE 1.
Proteins identified in nuclear tegument aggregates isolated from cells infected with RCΔ97
| Protein | No. of peptides observed by MSa | Confirmation by:
|
|
|---|---|---|---|
| Immunoblottingb | Immunofluorescencec | ||
| MCP (pUL86) | 16 | X | X |
| pp65 (ppUL83) | 18 | X | X |
| ppUL25 | 15 | ||
| pp150 (ppUL32) | X | X | |
| ppUL44 | X | X | |
Number of peptides identified by mass spectrometry (MS) following trypsin digestion of excised protein species.
Proteins from tegument aggregates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with monoclonal antibodies.
Monolayers of HFF cells were infected with RCΔ97 and stained with monoclonal antibodies.
Aggregates isolated from cells infected with RCΔ97 or induced with MBV are similar in composition.
To compare the inclusions isolated from cells infected with RCΔ97 to those isolated from cells infected with AD169 and treated with MBV, aggregates from the nuclear fraction were isolated by methods described above, although on a smaller scale. Untreated AD169-infected cells and uninfected cells were subjected to the same isolation scheme and served as additional controls. Few proteins remained in samples derived from either uninfected cells or cells infected with wt virus (Fig. 3B). Aggregates isolated from cells infected with the UL97 deletion mutant and those induced by MBV appeared to be similar in composition and contained at least four proteins that were overrepresented or not observed in the control sample from untreated, wild-type virus-infected cells. The relative abundance of constituent proteins in MBV-induced aggregates and that of those produced by the mutant virus were similar with the exception of an unidentified 30-kDa protein that was overrepresented in aggregates produced in the mutant virus (Fig. 3B). This difference may be related either to the subtle differences in the mechanism of action of MBV on viral replication or to the lower multiplicity of infection used for the mutant virus. Nevertheless, the protein profiles are similar and support the hypothesis that the aggregates produced in the two cases are similar in composition.
A pp65-knockout virus does not form tegument aggregates.
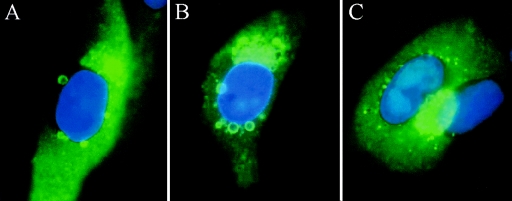
Since pp65 appeared to be a major constituent of the tegument aggregates, we thought that it might be important in the formation of these structures. To test this hypothesis, a recombinant virus that does not express pp65 was evaluated for its ability to form tegument aggregates in the presence of MBV (27). Monolayers of HFF cells were infected with RVAd65 and treated with increasing concentrations of MBV in an attempt to reproduce the inclusions observed with AD169 in the presence of this drug. For this experiment, tegument aggregates were visualized using a monoclonal antibody specific for the tegument protein pp150, since pp65 is not expressed in the knockout virus. Cells infected with either RCΔ97 or wt virus and treated with 15 μM MBV generated large numbers of aggregates by 96 hpi (Fig. 4A and B). In contrast, cells infected with RVAd65 did not produce aggregates at MBV concentrations more than 10-fold greater than those required to induce inclusions in the wt virus (60 μM) (Fig. 4C). This result suggests strongly that pp65 is required for the formation of tegument aggregates in the absence of UL97 kinase activity and is consistent with the observation that this protein is a predominant constituent of these structures and is required for their formation.
FIG. 4.
Tegument aggregates do not form in the absence of pp65. HFF cells were infected with AD169 (A) or RCΔ97.08 (B) and incubated for 96 h in the presence of 15 μM MBV. Cells infected with recombinant virus RVAd65 containing a deletion of the entire ORF (UL83) encoding pp65 were incubated for 96 h with the addition of 60 μM MBV (C). Tegument aggregates were stained with a monoclonal antibody to pp150, and images shown are merged with DAPI-stained images.
Expression of pp65 is sufficient to induce aggregates in uninfected cells, and their formation is inhibited by UL97 kinase activity.
Since pp65 is required for the formation of tegument aggregates, we hypothesized that self-aggregation might be a characteristic of this phosphoprotein and that it might be reproduced in uninfected cells. To test this hypothesis, we took advantage of an observation that pp65-EGFP fusion proteins expressed in COS7 cells formed large nuclear inclusions. This result was originally presumed to be an artifact related to the insoluble nature of the fusion protein but might also be a consequence of the normal aggregating properties of this protein in the absence of UL97 kinase. To distinguish between these two interpretations of the data, plasmids expressing EGFP-pp65 fusion proteins were transfected into COS7 cells either alone or with an epitope-tagged UL97 expression construct. Cells expressing the pp65-EGFP fusion protein exhibited nuclear inclusions that fluoresced so brightly that they were also apparent in the DAPI (4′,6′-diamidino-2-phenylindole) channel (Fig. 5A and B). Cells expressing V5-tagged ppUL97 exhibited predominantly nuclear staining, whereas the kinase-negative K355M mutant of this fusion protein was observed both in the nucleus and in the cytoplasm (Fig. 5C and D). The coexpression of the UL97 kinase with pp65-EGFP fusion protein inhibited the formation of aggregations in most cells even though both proteins were abundantly expressed in the nucleus (Fig. 5E and H). These results suggested that the expression of pp65 in uninfected cells was sufficient to form nuclear aggregates and that the coexpression of UL97 prevented this from occurring (Table 2).
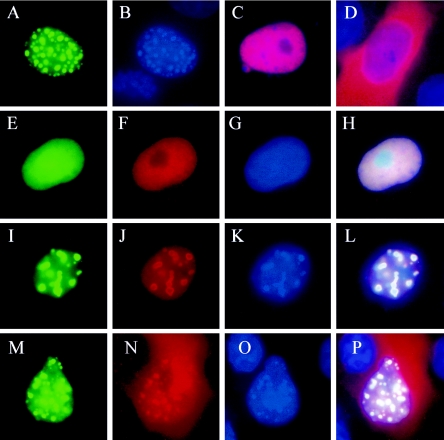
FIG. 5.
Aggregation of pp65-GFP in transfected COS7 cells is prevented by UL97 kinase activity. Transient expression of a pp65 GFP fusion protein in COS7 cells results in the formation of large nuclear aggregates (A) 24 h posttransfection. Resulting aggregates fluoresce so brightly that they are detected with the filter used for DAPI staining (B). Transiently expressed ppUL97 with a V5 epitope tag localizes to the nucleus (C), whereas the same ppUL97-V5 fusion protein with the K355M point mutation localizes both to the nucleus and to the cytoplasm (D) as shown in the images merged with DAPI staining. Coexpression of pp65-GFP (E) and ppUL97-V5 (F) eliminates the formation of nuclear pp65-GFP aggregates and instead results in the diffuse nuclear localization of both proteins as shown in the merged image (H) with DAPI (G). The inhibition of pp65 aggregation by ppUL97 is antagonized by the addition of MBV, and pp65-GFP is formed (I to L). The inhibition of UL97 kinase activity by MBV allows both the formation of pp65-GFP aggregates (I) and the recruitment of ppUL97 to the aggregates (J) as shown in the merged image (L) with DAPI (K). Similarly, the coexpression of ppUL97-V5-K355M (N) is unable to stop the aggregation of pp65-GFP (M), and the kinase-deficient ppUL97 fusion protein is recruited to the nuclear inclusions as shown in the merged image (P) with DAPI (O).
TABLE 2.
Aggregation of transiently expressed pp65-GFP in COS7 cells
| Protein(s) expresseda | No. of cells countedb | % of cells with pp65-GFP aggregates/fieldc |
|---|---|---|
| pp65-GFP | 242 | 71 ± 6 |
| pp65-GFP, ppUL97-V5 | 260 | 8 ± 4 |
| pp65-GFP, ppUL97-V5 (K355M) | 211 | 49 ± 9 |
| pp65-GFP, ppUL97-V5 (MBV) | 206 | 64 ± 7 |
Expression of proteins was confirmed by fluorescence of GFP and staining with V5 monoclonal antibody and Texas Red secondary antibody.
Number of cells counted from eight fields that were either single positive for GFP-pp65 transfected alone or double positive for cells transfected with two plasmids.
The percentages of cells in a field exhibiting pp65-GFP nuclear aggregates were averaged and are shown with the standard deviation.
To determine if UL97 kinase activity was required to prevent aggregation, the previous experiment was repeated and transfected cells were treated with MBV to specifically inhibit UL97 kinase activity. When ppUL97 and pp65 were coexpressed in COS7 cells in the presence of MBV, nuclear aggregation of pp65 was restored (Fig. 5I and L), suggesting that UL97 kinase activity was required to inhibit aggregation. Also of interest was the localization of ppUL97 to the aggregates in the presence of MBV, suggesting that pp65 could recruit the kinase to these structures, possibly through a physical interaction. To confirm these data, the experiment was repeated using an epitope-tagged ppUL97 expression construct containing a K355M mutation that abrogates its kinase activity. This mutant was unable to prevent the formation of pp65 aggregates in cells expressing both proteins (Fig. 5M and P; Table 2), confirming that UL97 kinase activity was required to inhibit the aggregation of pp65. The kinase-negative form of ppUL97 was also recruited to the aggregates in the nucleus despite its predominantly cytoplasmic localization when expressed singly in COS7 cells (Fig. 5C and D). It is interesting that the K355M mutation affects nuclear targeting of ppUL97 given that previously mapped nuclear localization signals have been shown to reside in the amino-terminal 110 amino acids of the protein (20). It is unclear if the reduced autophosphorylation of the mutant is related to its impaired nuclear localization, particularly since it did not occur in the presence of MBV; additional experiments will be required to understand the significance of this observation. These data taken together suggested that (i) the expression of pp65 is sufficient to form aggregates in uninfected cells, (ii) coexpression of ppUL97 can prevent this aggregation, and (iii) UL97 kinase activity is specifically required to prevent the aggregation of pp65.
A recombinant virus that does not express pp65 is resistant to MBV.
Data presented thus far have suggested that ppUL97 and pp65 play a central role in the formation of tegument aggregates in the nuclei of infected cells and are both likely required for normal virion morphogenesis. If this interpretation is correct, then the deletion of either UL97 or UL83 should disrupt the normal nuclear tegumentation process and thus force the virus to rely on alternative, albeit inefficient, mechanisms to produce infectious virus. One prediction of this hypothesis is that MBV should be less effective against recombinant viruses with deletions in either of these genes since the normal pathway would be disrupted in either case. To test this hypothesis, the sensitivities of AD169, RCΔ97, and RVAd65 to the antiviral effects of MBV were determined. Each of these isogenic strains exhibited the same sensitivity to CDV and yielded 50% effective concentration values that were indistinguishable (Table 3). As reported previously, RCΔ97 was highly resistant to the antiviral effects of MBV (33). Interestingly, RVAd65 was also significantly resistant to MBV, a finding that is consistent with the notion that both these tegument proteins function in a common pathway in infected cells. Resistance to MBV has been ascribed to mutations that map to either UL97 or UL27 (7, 12, 33). The level of drug resistance observed with RVAd65 is comparable to the moderate resistance reported for UL27 deletion mutants, rather than the high-level resistance seen with the UL97-null mutant. To confirm that RVAd65 did not have mutations at these other loci, both UL27 and UL97 were sequenced, and no mutations were observed (data not shown). These data taken together suggested that both the UL97 kinase and pp65 are involved in the mechanism of action of MBV and that they may function together in a common process.
TABLE 3.
RVAd65 is resistant to the antiviral effects of maribavir
| Drug | AD169 | EC50a (fold resistanceb)
|
|
|---|---|---|---|
| RCΔ97.08 | RVAd65 | ||
| CDV | 0.22 ± 0.17 | 0.09 ± 0.04 (0.4) | 0.09 ± 0 (0.4) |
| MBV | 0.82 ± 0.8 | 33 ± 26 (40) | 3.6 ± 0.4 (4.4) |
Fifty percent effective concentration (EC50) values are the averages of three or more experiments with the standard deviations shown.
Fold resistance compared to the parent virus AD169.
DISCUSSION
While the biochemical activity of the UL97 kinase has been well characterized and its central role in the emergence of ganciclovir resistance in the clinic has been established, the functional role of this tegument protein during viral replication remains incompletely understood. Studies reported here suggested that tegument aggregates are produced in the absence of UL97 kinase activity and can be induced either through the deletion of UL97 or through the inhibition of its enzymatic activity by MBV. We demonstrated that pp65 was also essential for the formation of these structures in infected cells. These data taken together suggest that both pp65 and ppUL97 function during virion morphogenesis in the nucleus. Significantly, the transient expression of pp65 is sufficient to produce morphologically similar structures in uninfected cells, and its aggregation can be inhibited by the kinase activity of UL97. The apparent recruitment of ppUL97 to the aggregates by pp65 was consistent with a physical association, a finding consistent with other results suggesting a potential protein-protein interaction between these tegument proteins. Reduction of UL97 kinase activity either by treatment with MBV or by mutation of its active site largely restored the self-aggregation of pp65 (Table 2). However, it should be noted that the frequency of cells that contained pp65 aggregates remained slightly reduced compared to those expressing pp65 alone (Table 2). Thus, we cannot completely exclude the contribution of possible protein-protein interactions between these tegument proteins in the inhibition of aggregate formation.
We investigated the formation of tegument aggregates to further define the molecular defects resulting from a deletion of the viral protein kinase as well as the mechanism of action of MBV and other inhibitors of this enzyme. Results presented here have characterized a new facet of the phenotype for RCΔ97. In the absence of its enzymatic activity, the aggregation of pp65 appears to proceed unchecked and leads to the formation of large aberrant nuclear inclusions composed predominantly of pp65, ppUL25, and the MCP. These inclusions also contained other viral proteins in lesser amounts. In this regard, tegument aggregates are similar to dense bodies, but it is not clear that these structures are related in any way. The gross morphological changes in the organization of tegument and structural proteins in the nucleus likely reflect a failure of virion morphogenesis at a late stage of assembly when the initial acquisition of tegument proteins by capsids normally occurs. The sequestration of large quantities of tegument and structural proteins within these dense structures likely precludes their incorporation into mature virions. We propose that the UL97 kinase could have a critical function during virion morphogenesis and that, in the absence of this activity, capsids fail to acquire the normal complement of tegument proteins and do not mature fully. It is possible that these final maturational events are required to target the capsids for nuclear export and could account for the failure of capsids to exit the nucleus. It is also possible that the UL97 kinase may also be required for later steps in virion assembly, including the process of nuclear egress. Many functions have been ascribed to the kinase either through the characterization of RCΔ97 or through its inhibition by MBV. Defects in virion morphogenesis described here, as well as those described previously in DNA synthesis and DNA packaging, all likely contribute to the antiviral activity of MBV and the greatly reduced titers observed in the knockout virus (14, 35). Nevertheless, RCΔ97 is not completely replication deficient and a cellular kinase may complement some or all of these functions, albeit inefficiently, to facilitate the replication of this virus.
These studies also revealed an interesting and unexpected connection between the UL97 kinase and pp65. Many lines of evidence support the idea that pp65 and ppUL97 function together during viral infection. First, pp65 forms aberrant aggregates in infected cells when UL97 is deleted or when its enzymatic activity is inhibited with MBV. Indeed, ppUL97 can inhibit pp65 self-aggregation in a kinase-dependent manner, indicating that other viral proteins are not required to mediate this effect. Both ppUL97 and pp65 are important in the mechanism of action of MBV since the deletion of either protein can confer resistance to this specific inhibitor of the kinase. Phosphorylation of pp65 in infected cells and in cell extracts is specifically inhibited by MBV, suggesting that the kinase can phosphorylate this protein. (Phiroze Sethna, personal communication). Finally, pp65 recruits ppUL97 to aggregates in uninfected cells, which is consistent with a physical association and is expected given that pp65 is a substrate for this enzyme. We propose a model in which the UL97 kinase influences the physical properties of pp65 by direct phosphorylation, which is important for assembly and possibly nuclear egress of the nuclear form of the virion particle.
Results described here are intriguing in light of results from previous studies regarding the phenotype of RCΔ97 and its severely impaired ability to produce infectious virions. Previous reports regarding the phenotype of UL97 deletion mutants, as well as those describing the mechanism of action of specific inhibitors of this enzyme, have concluded that the activity of this enzyme is critical for viral replication (4, 9, 14, 16, 22, 35). However, defining precisely the critical functions of the kinase has been difficult due to the variation in the nature of the UL97 phenotype both within and among laboratories. Similarly, drugs that inhibit this kinase also induce effects that vary significantly between experiments and appear to confirm that the virus utilizes the kinase to a greater or lesser extent depending on variables likely associated with the cell substrate (Sunwen Chou, personal communication, and data not shown). Pleiotropic effects are expected when a broadly active kinase is deleted, and defining the phenotype associated with UL97 deletion mutants is complicated further by differentially expressed cellular factors that can complement the mutant virus to some degree, as evidenced by improved replication of the null mutant in dividing cells (data not shown). Nevertheless, it is clear that replication of RCΔ97 proceeds normally and that the virus expresses nearly normal levels of α, β, and γ gene products and DNA synthesis proceeds, although a modest three- to sixfold reduction in accumulated DNA is observed (14, 22, 35). In one report, defects in viral DNA synthesis were noted as well as more pronounced defects in the packaging of viral DNA and an accumulation of immature capsids in the nucleus in the absence of mature virions in the cytoplasm (35). The authors concluded that the kinase played a role both in DNA synthesis and in encapsidation of nascent genomes. A subsequent report did not describe either a defect in DNA packaging or an overabundance of immature particles in the nucleus, although mature capsids did not appear in the cytoplasm (14). The authors concluded that the virus was defective at the stage of nuclear egress, defined broadly as events following the packaging of genomic DNA but prior to exit from the nucleus of intact mature virions. This report also noted that one cell observed by electron microscopy contained an overabundance of immature capsids; thus, the differences observed between these studies may simply reflect differences in the condition of the primary cell substrate. Mechanism-of-action studies with specific inhibitors of UL97 kinase activity suggest that they exert their antiviral effects through the inhibition of viral DNA synthesis, although the polymerase is not inhibited directly (4, 16). Observed differences between these results and those obtained with the UL97-null mutant may be the result of compensatory mutations in the deletion virus, or the drugs inhibit other enzymes in addition to the kinase. It is also possible that ppUL97 has functions which are not dependent on its kinase activity and are unaffected by the small molecule inhibitors. Thus far, each of the defects attributed to either UL97 kinase inhibition or the deletion of UL97 occurs in the nucleus of infected cells, and both mature cytoplasmic virions and the production of infectious virus are severely reduced.
The assembly of mature CMV particles within the nuclei of infected cells is a highly ordered progression of concerted molecular reactions and is an event of exceptional complexity. The processes of DNA cleavage/packaging, capsid maturation, acquisition of tegument, transit to the nuclear membrane, and nuclear egress remain ill defined and incompletely understood (17). Characterizing these aspects of viral replication through continued genetic studies and investigations into the mechanism of action of novel small molecules will improve our understanding of betaherpesvirus replication and may lead to the development of improved antiviral therapies.
Acknowledgments
We thank Bodo Plachter for his gift of the pp65-deficient recombinant virus RVAd65 and Sunwen Chou for helpful discussions.
These studies were supported by Public Health Service contract NO1-AI-30049 to E.R.K. from the NIAID, NIH; grant NIH/NIAID R01 AI35602 to W.J.B.; and a grant from The Research Institute of the Alabama Children's Hospital Foundation to M.N.P.
REFERENCES
- 1.Baek, M. C., P. M. Krosky, and D. M. Coen. 2002. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J. Virol. 76:11943-11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek, M. C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 3.Baek, M. C., P. M. Krosky, A. Pearson, and D. M. Coen. 2004. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology 324:184-193. [DOI] [PubMed] [Google Scholar]
- 4.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackhall, J., A. Fuentes, K. Hansen, and G. Magnusson. 1997. Serine protein kinase activity associated with rotavirus phosphoprotein NSP5. J. Virol. 71:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou, S., A. Erice, M. C. Jordan, G. M. Vercellotti, K. R. Michels, C. L. Talarico, S. C. Stanat, and K. K. Biron. 1995. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J. Infect. Dis. 171:576-583. [DOI] [PubMed] [Google Scholar]
- 7.Chou, S., G. I. Marousek, A. E. Senters, M. G. Davis, and K. K. Biron. 2004. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J. Virol. 78:7124-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herget, T., M. Freitag, M. Morbitzer, R. Kupfer, T. Stamminger, and M. Marschall. 2004. Novel chemical class of pUL97 protein kinase-specific inhibitors with strong anticytomegaloviral activity. Antimicrob. Agents Chemother. 48:4154-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komazin, G., R. G. Ptak, B. T. Emmer, L. B. Townsend, and J. C. Drach. 2003. Resistance of human cytomegalovirus to the benzimidazole l-ribonucleoside maribavir maps to UL27. J. Virol. 77:11499-11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koszalka, G. W., N. W. Johnson, S. S. Good, L. Boyd, S. C. Chamberlain, L. B. Townsend, J. C. Drach, and K. K. Biron. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 46:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 16.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 17.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel, D., S. Kramer, S. Hohn, P. Schaarschmidt, K. Wunderlich, and T. Mertens. 1999. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J. Virol. 73:8898-8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel, D., P. Schaarschmidt, K. Wunderlich, M. Heuschmid, L. Simoncini, D. Muhlberger, A. Zimmermann, I. Pavic, and T. Mertens. 1998. Functional regions of the human cytomegalovirus protein pUL97 involved in nuclear localization and phosphorylation of ganciclovir and pUL97 itself. J. Gen. Virol. 79:2105-2112. [DOI] [PubMed] [Google Scholar]
- 21.Ng, T. I., C. Talarico, T. C. Burnette, K. Biron, and B. Roizman. 1996. Partial substitution of the functions of the herpes simplex virus 1 U(L)13 gene by the human cytomegalovirus U(L)97 gene. Virology 225:347-358. [DOI] [PubMed] [Google Scholar]
- 22.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rempel, R. E., and P. Traktman. 1992. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J. Virol. 66:4413-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmolke, S., H. F. Kern, P. Drescher, G. Jahn, and B. Plachter. 1995. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J. Virol. 69:5959-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaete, R. R., and E. S. Mocarski. 1985. Regulation of cytomegalovirus gene expression: α and β promoters are trans activated by viral functions in permissive human fibroblasts. J. Virol. 56:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 359:85. [DOI] [PubMed] [Google Scholar]
- 30.Talarico, C. L., T. C. Burnette, W. H. Miller, S. L. Smith, M. G. Davis, S. C. Stanat, T. I. Ng, Z. He, D. M. Coen, B. Roizman, and K. K. Biron. 1999. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob. Agents Chemother. 43:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams, S. L., C. B. Hartline, N. L. Kushner, E. A. Harden, D. J. Bidanset, J. C. Drach, L. B. Townsend, M. R. Underwood, K. K. Biron, and E. R. Kern. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing, B. A., and E. S. Huang. 1995. Analysis and mapping of a family of 3′-coterminal transcripts containing coding sequences for human cytomegalovirus open reading frames UL93 through UL99. J. Virol. 69:1521-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]