Abstract
Nonkeratinizing nasopharyngeal carcinomas (NPC) are >95% associated with the expression of the Epstein-Barr virus (EBV) LMP2A latent protein. However, the role of EBV, in particular, LMP2A, in tumor progression is not well understood. Using Affymetrix chips and a pattern-matching computational technique (neighborhood analysis), we show that the level of LMP2A expression in NPC biopsy samples correlates with that of a cellular protein, integrin-alpha-6 (ITGα6), that is associated with cellular migration in vitro and metastasis in vivo. We have recently developed a primary epithelial model from tonsil tissue to study EBV infection in epithelial cells. Here we report that LMP2A expression in primary tonsil epithelial cells causes them to become migratory and invasive, that ITGα6 RNA levels are up-regulated in epithelial cells expressing LMP2, and that ITGα6 protein levels are increased in the migrating cells. Blocking antibodies against ITGα6 abrogated LMP2-induced invasion through Matrigel by primary epithelial cells. Our results provide a link between LMP2A expression, ITGα6 expression, epithelial cell migration, and NPC metastasis and suggest that EBV infection may contribute to the high incidence of metastasis in NPC progression.
Nasopharyngeal carcinoma (NPC) is a tumor of the head and neck with a complex etiology. The incidence is rare in the western world (1 per 100,000); however, in the Guangdong region of southeast China the incidence peaks at 50 per 100,000 (56). In China and Taiwan, NPC accounts for 20% of all cancers (70). Thirty to 60 percent of patients with NPC will eventually develop distant metastasis, the key contributor to NPC mortality (27). Because of the high incidence and frequent fatal recurrence, NPC is considered a significant health problem in parts of the world where it is endemic (42).
Possible risk factors for NPC include smoking, alcohol consumption, eating salted fish, and genetic predisposition; however, the strongest association is with Epstein-Barr virus (EBV) infection. EBV was recognized as the first candidate human tumor virus, and in 1997, the International Agency for Research on Cancer officially designated EBV a class I carcinogen due to its association with several epithelial and lymphoid malignancies, including NPC, gastric carcinoma, Burkitt's lymphoma, Hodgkin's disease, and posttransplant lymphoproliferative disease. The oncogenic potential of EBV is most dramatically demonstrated by its ability to transform resting B lymphocytes in culture into continuously proliferating lymphoblasts (46).
Early serological studies demonstrated significantly elevated antibody responses to EBV antigens in NPC patients that preceded tumor development by 1 to 2 years (22). Subsequently it was shown that latent EBV was present in 100% of undifferentiated NPC tumors (5) and that three of the known latent proteins were expressed in the tumors (reviewed in reference 32). These are EBNA1, whose primary role is to enable replication of the viral episomal genome (68), LMP1, and LMP2A. Much interest has focused on LMP1 because of its known oncogenic properties in B cells (reviewed in references 26 and 61). However, in EBV-associated epithelial tumors, LMP1 either is not expressed (e.g., in gastric cancer cases) (43) or is only present in a subset of tumors (e.g., NPC). In NPC, 35 and 65% of the tumors express LMP1 at the protein and RNA level, respectively (41, 69). In contrast, LMP2A has been detected in 50% of NPC tumors at the protein level (23) while transcripts are detected in ≥95% of the tumors (8, 9). LMP2A expression is dispensable for EBV-driven B-cell transformation, but in RAG null mice LMP2A rescues B-cell development, indicating a survival function (10), and LMP2A has been reported in one study to have transforming properties in an epithelial cell line (52). Activation of the PI3K/Akt pathway plays a role in the LMP2A-mediated survival signals in both epithelial and B cells (52, 58).
Despite the oncogenic potential of EBV its exact role in tumorigenesis remains to be delineated. Specifically, for NPC it is not clear at what stage of tumor progression EBV plays a role. Recently we have presented evidence that primary cultures of epithelial cells from the nasopharyngeal region contain infected cells expressing LMP1 (44). This raises the possibility that the virus may be present at the onset of the oncogenic processes and could therefore potentially affect any stage of tumor progression.
One problem with EBV studies in epithelial cells is that the properties of EBV-encoded genes are often studied in isolation with in vitro models using established immortalized and/or transformed cell lines, often derived from tissues such as the skin, which, under normal conditions, cannot be infected with EBV. The implications of these studies are then extrapolated to the tumor setting. However, without directly testing the tumors themselves the biological significance of such studies is unclear. This is because viral genes function in vivo in a specific context defined by the patterns of expressed cellular and other viral genes (7). This is well established in B cells, where the pattern of viral latent gene expression is highly dependent on the differentiation state and site of origin of the infected cells (63); viral gene expression in many tumors is different from what is seen in in vitro-infected cells (50, 64), and the patterns of cellular gene expression in response to a particular viral latent protein are highly variable depending on the type of B cell in which the gene is expressed (47). To reduce these concerns in studying epithelial cells we decided to take the reverse approach of first identifying genetic effects associated with EBV gene expression in NPC tumors and then confirming the functional impact of these effects in vitro with epithelial cell lines and a primary tonsil epithelial cell culture system.
MATERIALS AND METHODS
Cell culture procedures.
IB4 cells were used as a positive and BJAB as a negative control for EBV transcripts. All cell lines were maintained as described previously (44) except for HaCat (ATCC), for which RPMI medium was replaced by Dulbecco's modified Eagle's medium. Primary tonsil epithelial cell cultures were generated as described previously (44).
Transient induction of LMP.
Epithelial cells were plated at semiconfluence (2 × 105 to 3 × 105 cells/well) in six-well plates (Costar). LMP2A was expressed from modified adenovectors (AdEasy; Obiogene) containing the LMP2A gene together with a reporter (green fluorescent protein [GFP]) gene, with both under the control of separate ubiquitin promoters as described previously (57). GFP provided a rapid and sensitive way for monitoring infection efficiency, which was particularly important since single-cell protein staining for LMP2A expression is unreliable and nonquantitative. An adLMP1 vector was also produced for comparative purposes, and GFP alone (adGFP) was used as a vector control. Epithelial cells were infected with adenovirus preparations at a multiplicity of infection (MOI) of ∼100 (57) in 1 ml of medium. Two volumes of fresh media were added after 1 h of incubation. At 1 day postinfection, the medium was aspirated and replaced with fresh medium. Infection efficiency was assessed by measuring GFP expression by use of a fluorescent microscope (Nikon E400) and fluorescence-activated cell sorter (FACS) analysis (FACSCalibur; BD Sciences). The MOIs used were all at least 10-fold lower than the minimum toxic dose, as assessed by staining with propidium iodide and FACS analysis. The control adGFP MOI was adjusted to the adLMP2A and AdLMP1 MOI to produce equivalent levels of GFP. At a few hours postinfection with AdLMP2A, LMP2A transcript levels increased steadily, peaking at around 1 day postinfection in HaCat cells and 3 days postinfection in the primary epithelial cells. A total of 100% of the cells in both cultures were positive for GFP expression at 1 day postinfection, implying that all cells were successfully infected and expressing LMP2A, although only those in a small subset were clearly positive for LMP2A protein expression by immunofluorescence staining (not shown). This discrepancy arises because of the lack of sensitivity of the LMP2A staining procedure demonstrated by the fact that a similar staining pattern was seen with an EBV-positive control cell line (IB4) where every cell should be LMP2A positive. For these reasons, we are confident that most if not all of the AdLMP2A-infected HaCat and primary epithelial cells express LMP2A transcripts and protein. Western blot analysis confirmed that the levels of LMP1 and LMP2a expression were equivalent to those found in B lymphoblastoid cell lines.
RT-PCR.
RNA was isolated with TRIzol according to the manufacturer's instructions (Invitrogen). RNA (7 μl) and cDNA were prepared as described previously (44). Reverse transcription-PCR (RT-PCR) was performed by either the SYBR green or the TaqMan (ABI) method. The SYBR green method was used for LMP1, LMP2A, EBNA1(Q-K), and EBER. Amplimers and PCR conditions were as described previously (44). PCR was performed in a volume of 25 μl with an ABI PRISM 5700 sequence detection system (Perkin Elmer). For each run, 12.5 μl of Universal SYBR green master mix (PE Applied Biosystems) was prepared containing primers (100 to 450 nM) to which cDNA was added. All PCRs were evaluated for potentially nonspecific products and primer dimer formation by analyzing the dissociation curve using GeneAmp 5700 software, version 1.3. The products were analyzed by gel electrophoresis to confirm the correct product size. All TaqMan PCRs were performed with “assays on demand” (Applied Biosystems) using 10 μl primer probe master mix and 10 μl cDNA. The genes used included β-actin, ITGα6, ITGβ1, ITGβ4, and ITGβ5. The cycle run for these genes was 5 min at 95°C and 10 min at 50°C followed by 40 cycles of 60°C for 1 min and 95°C for 15 s.
Sensitivity and quantitation of the PCR assays was estimated by comparison to standard cell lines. For EBV-encoded latent genes, IB4 cells were used. Other cell lines used as standards were CLL228 (ITGα6, ITGβ4), HaCat (ITGβ1, ITGα6, keratin 6), and BJAB (c-fos, β-actin). All values were normalized to β-actin levels in procedures performed in duplicate. Typically, we were able to detect specific gene products at the single-cell level with fewer than 40 amplification cycles.
Biopsy material-chip hybridization.
Total RNA was isolated from 20 fresh nonkeratinizing nasopharyngeal carcinoma biopsy samples obtained from Taiwan. Biopsy samples were obtained with punch forceps by use of endoscopic guidance. The tumor stage was designated according to the International Union Against Cancer and American Joint Committee on Cancer staging manuals (L. H. Sobin, 5th ed., Philadelphia, Pa., 1997). NPCs were mostly of the advanced stage (IVA to IVC), and all samples were EBV positive as measured by EBER expression levels (24). Biopsy RNA was isolated by guanidinium thiocyanate extraction followed by centrifugation in CsCl (20), and RNA integrity was assessed by gel electrophoresis and optical density. We had sufficient RNA from 16 samples that also met the quality criteria for hybridization to Affymetrix microarray chips (HG-U95A). All scans were performed on Affymetrix scanners, and the expression value for each gene was calculated using Affymetrix GENECHIP software. A total of 14 samples survived the normalization and scaling criteria. For a detailed protocol, see http://www.broad.mit.edu/MPR/CNS.
Computational analysis.
The microarray data were normalized as previously described (45); details can be found at http://www.broad.mit.edu. We used a permutation test-based neighborhood analysis described previously (14, 45) to screen and select candidate cellular genes whose expression best correlated with the LMP2A expression in 14 NPC biopsy samples as determined by quantitative RT-PCR (qRT-PCR). Thus, the LMP2A level in each biopsy sample serves as an individual classifier forming a “continuum vector” based on LMP2A expression. The readout of the analysis is a Pearson correlation coefficient (r) of LMP2A versus cellular gene expression ranked from highest to lowest. Typically, 1,000 global random permutations were used to determine the 50% (median), 5%, and 1% significance levels. We also compared average gene expression levels between LMP2A high (highest five) and low (lowest nine) expressers. A simple t statistic (μclass 0 − μclass 1)/√(σ2class 0 + σ2class 1) was applied to assess significant differences between the two arbitrary subpopulations.
We validated the general authenticity of the neighborhood approach in several ways. First, we chose to test the method with a pair of cellular genes (jun and fos) whose expression is known from the literature to be biologically associated (51). Applying the neighborhood analysis with c-jun as the classifier gene, we identified three fos probes as closely related (probe numbers 3, 4 and 7) in the NPC biopsy samples; the third and fourth probes were statistically significant at the 0.05 level (see Table S1, part 2, in the supplemental material). Second, we screened the marker list from the LMP2A data set for known target genes of LMP2A and identified Jun N-terminal kinase 1 (JNK1) and JNK3 (Fig. 1, 9th and 10th probes, boxed), which had previously been shown to be regulated by LMP2A in epithelial cells (13). Taken together, these two validations demonstrate a “proof of principle” for the neighborhood analysis approach in our NPC data set as a method to identify genes whose expression levels are biologically linked.
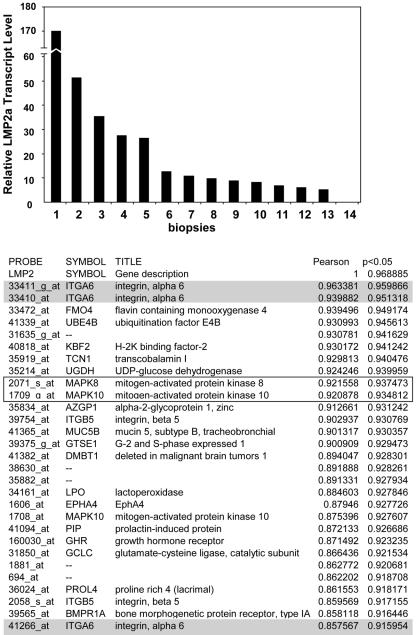
FIG. 1.
Schematic representation of the neighborhood analysis method and outcome. LMP2A transcript levels were determined in NPC biopsy samples by qRT-PCR and normalized to β-actin levels (see Materials and Methods). The biopsy samples were ranked based on LMP2A transcript expression from high to low (numbered 1 to 14, respectively) as represented in the histogram. The y axis represents the relative level of LMP2A transcripts in comparison to positive control results obtained using IB4 cells as a standard. Genes whose expression parallels that of LMP2A were identified by neighborhood analysis, a pattern-matching computational technique. The table in Fig. 1 shows the first 30 genes with the Pearson correlation coefficient and permutated score at the 0.05 level. The three hits for ITGα6 are shaded in gray. Boxed are two mitogen-activated protein kinases previously shown to be activated by LMP2A.
Scratch wound assays.
Primary tonsil epithelial cells were infected with adLMP1, adLMP2A, or adGFP as described above and grown on culture slides (Costar) to near confluence. The monolayers were wounded at 2 to 3 days postinfection with a plastic pipette tip, and cellular debris was removed by washing with medium. Bright-field and UV images (Nikon E400) were obtained at various time points.
Invasion assays.
Matrigel (BD Biosciences) was diluted 1:5 in cold serum-free medium (SFM), and 50 μl was layered on 6.5-mm Transwell chambers (Costar) of 8 um pore size and incubated at 37°C for 1 h to solidify. Adenovirus-infected and uninfected primary tonsil epithelial cells (∼7 × 104) were resuspended in 100 μl of keratinocyte SFM (Invitrogen) containing 0.1% bovine serum albumin. In some assays, the cell suspensions were preincubated for 1 h with ITGα6 (2B7, GoH3, and MP4F) or control immunoglobulin G (IgG) (Southern Biotech) antibodies (10 μg/ml) in a final volume of 100 μl of SFM. Cell suspensions were layered on Matrigel-coated Transwells. SFM containing keratinocyte growth factor (Invitrogen) was added to the bottom of the well as a chemoattractant. Cells were allowed to invade the Matrigel for 12 to 18 h. The cells that had not yet invaded were removed from the upper surface of the membranes with cotton swabs. Invaded cells, attached to the lower surface of the membranes, were stained with DAPI (4′,6′diamidino-2-phenylindole)-phosphate-buffered saline (PBS) to visualize the nuclei. Each experiment was performed in duplicate. Invasiveness was quantified by counting individual nuclei in three separate view fields of each.
Immunofluorescent staining.
Integrin expression was detected by indirect immunofluorescence. CLL228 colon cancer and HaCat cells were used as positive controls. Epithelial cells grown on culture slides (Costar) were fixed and permeabilized for 30 min at room temperature in 4% paraformaldehyde followed by a 10-min incubation with 0.1% Triton X-100 in PBS at 4°C. Slides were blocked with serum (1 h at room temperature) and then incubated (30 min at room temperature) with primary monoclonal antibodies against ITGα6 (GoH3; Chemicon) (dilution, 1:200), ITGβ4 (3E1; Chemicon) (dilution, 1:500), ITGβ1 (4B73; Neomarkers) (dilution, 1:200), or the appropriate IgG control antibodies (Southern Biotech, Neomarkers) at equivalent concentrations. The S12 anti-LMP1 monoclonal antibody (dilution, 1:5,000) and rabbit anti-LMP2A serum (gift of J. Middeldorp) were used to detect LMP1 and LMP2A. The slides were washed three times in PBS and incubated for 1 h in the dark with secondary goat anti-mouse, goat anti-rat (Alexa 488 or 594), or goat anti-rabbit (fluorescein isothiocyanate) antibodies (Molecular Probes, The Netherlands) (dilution, 1:5,000). Slides were washed with PBS (3 times), mounted with Antifade (Molecular Probes), coverslipped, and examined with a fluorescence microscope (Nikon E400). Images were obtained with SPOT imaging software (Diagnostic Instruments).
RESULTS
LMP2A expression in NPC biopsy samples correlates with ITGα6.
To identify cellular targets of LMP2A in NPC biopsy samples we used a pattern-matching computational technique (neighborhood analysis) (14, 45) to mine data generated from probing Affymetrix chips with RNA extracted from NPC biopsy samples. The rationale for this approach is that the expression level of a classifier gene of interest, in this case, LMP2A, will correlate directly (LMP2A induces the expression of a gene) or inversely (LMP2A down-regulates the gene) with the levels of expression of cellular gene targets (for validation of the neighborhood analysis, see Materials and Methods). We obtained RNA from 14 EBV-positive nonkeratinizing NPCs and used it to measure the relative levels of LMP2A with a sensitive qRT-PCR and also to probe Affymetrix chips. We reproducibly detected LMP2A transcripts in 13 (∼90%) of the NPC samples, while expression results for one sample were equivocal or negative. These results are in agreement with previous findings (8, 9). The relative levels of LMP2A transcripts, normalized to the levels to β-actin, for the 14 biopsy samples are shown in Fig. 1. It is apparent that the levels of LMP2A transcripts are highly variable between NPC biopsy samples. We took advantage of this variation in LMP2A expression to apply the neighborhood analysis, the outcome of which is also depicted in Fig. 1 (the top 100 identified genes that positively correlated with LMP2A expression level are shown in Table S1, part 1, of the supplemental material). The readout from the analysis is a Pearson correlation coefficient (r) with a P value generated by permutation testing of the whole data set.
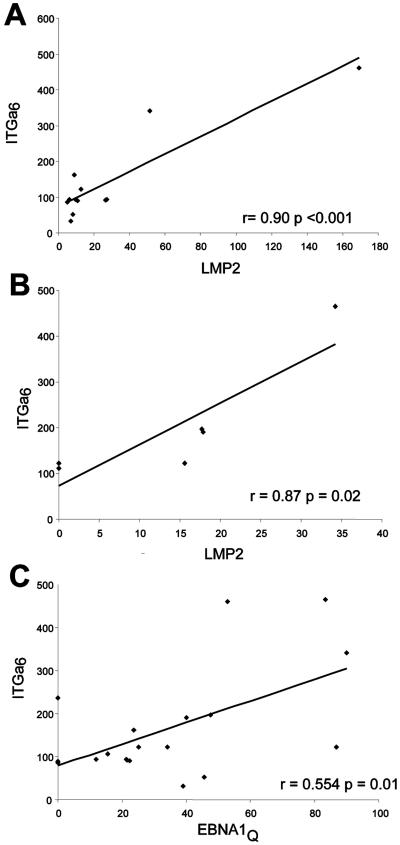
From the neighbor analysis we found that the first, second, and 30th probes code for the integrin-alpha 6 gene (ITGα6 [shaded in gray; Fig. 1]); however, only the results for the first ITGα6 probe achieved statistical significance (r = 0.96; P < 0.05). ITGα6 has been shown previously to be expressed in the epithelial tumor cells of NPC, but this is the first evidence that ITGα6 expression in NPC may be linked to the levels of LMP2A. We next performed qRT-PCR on RNA from the 14 biopsy samples to independently validate the relationship between ITGα6 and LMP2A. As in the microarray analysis, we found a good correlation between the expression of ITGα6 and LMP2A transcripts (Pearson correlation coefficient r = 0.90; P < 0.001) (Fig. 2A). By comparison, levels of transcripts for the other EBV latent protein genes expressed in NPC showed a weak [EBNA1(Q-K) r = 0.554; P = 0.01) (Fig. 2C] or no (LMP1 r = −0.32; P = 0.31) (not shown) correlation with ITGα6. We also applied this analysis to six additional LMP2A-positive NPC samples and found the same association between ITGα6 and LMP2A expression levels (r = 0.87; P = 0.02) (Fig. 2B). This independently confirms the microarray result and excludes the possibility that the initial observations were due to a fortuitous “bias” in the original data set. We concluded therefore that ITGα6 was a candidate target gene of LMP2A in NPC carcinoma cells.
FIG. 2.
ITGα6 expression is strongly associated with LMP2A expression in NPCs. (A) LMP2A mRNA expression levels were plotted against ITGα6 mRNA expression levels, determined by qRT-PCR, for the 14 NPC samples used for the neighborhood analysis shown in Fig. 1 (see Materials and Methods). The Pearson correlation coefficient and P value are represented in the plot. (B) As described for panel A but with six independent NPC biopsy samples. (C) ITGα6 transcript levels were plotted against EBNA1(Q-K) mRNA expression levels. The association is significant but weaker compared to LMP2A results.
LMP2A promotes invasion of primary epithelial cells in Matrigel.
The tumor samples we have used consist of a complex mixture of epithelial tumor and other cells, including infiltrating lymphocytes. Therefore, we can not distinguish whether the ITGα6 expression we have detected is in the tumor cells or the stroma. However, it has been shown previously that nasopharyngeal carcinoma cell lines express ITGα6 (31) and that its expression is significantly altered in many metastatic human carcinomas compared to nonmetastatic and normal tissues (36). Furthermore, ITGα6 has been implicated in cellular motility and invasiveness in vitro and in vivo (12, 36, 40, 67) and, in addition to ITGα6, the top genes in the list from the LMP2A neighborhood analysis included other proteins involved in cell migration such as integrin beta 5 (ITGβ5) (35), aPKCι, and PAR3 (37), although none achieved the level of statistical significance. These results prompted us to test the hypothesis that LMP2A alone could stimulate the invasiveness of primary epithelial cells in an ITGα6-dependent fashion. We have previously developed a technique for culturing primary epithelial cells from human tonsils (44); therefore, we expressed LMP2A in these cells and tested their invasiveness in a standard Matrigel (reconstituted basement membrane preparation) chemoinvasion assay, using keratinocyte growth factor as a chemoattractant. LMP2A was expressed using a transient adenovector expression system (AdLMP2A; for details, see Materials and Methods).
Primary epithelial cells expressing LMP2A showed a striking increase in invasiveness as early as 16 h postincubation compared to cells expressing a GFP vector control and uninfected cells (Fig. 3A). Quantification of the numbers of invaded cells demonstrated that LMP2A expression increased invasion on average 8- to 10-fold compared to control results in separate experiments with primary epithelial cells derived from three different tonsils (Fig. 3B). Invasion capacity was dependent on viral dose and LMP2A expression levels, as determined on the basis of GFP intensity (not shown). Primary epithelial cells expressing LMP1 also demonstrated increased invasion (not shown), an effect previously reported for cell lines (30) but never before for primary cells.
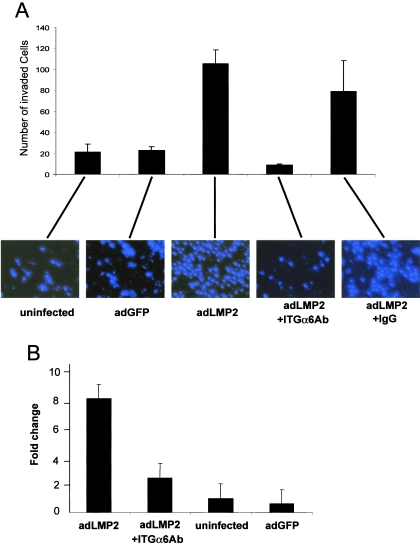
FIG. 3.
LMP2A promotes invasion by primary epithelial cells in an ITGα6-dependent fashion. (A) Primary epithelial cells were assayed for their ability to invade a reconstituted extracellular matrix composite (Matrigel). Invasion was tested for five conditions: (i) uninfected, (ii) adGFP infected, (iii) adLMP2A infected, (iv) adLMP2A infected plus preincubation for 1 h with a cocktail of ITGα6-blocking antibodies, and (v) as described for condition iv but with control antibodies. Primary epithelial cells were added to the upper chamber of a Transwell coated with 1:5-diluted Matrigel. The bottom well contained keratinocyte growth factor as the chemoattractant. After 16 h, uninvaded cells were removed and the nuclei of invaded cells were visualized under a UV microscope by DAPI staining. The histogram represents the average numbers of invaded cells in three image fields (the error bars indicate standard deviations). (B) The invasion assay was repeated on cultures of primary epithelial cells derived from three independent tonsils. Bars represent the average levels severalfold invasion (± standard deviations) compared to uninfected cell results.
ITGα6-specific antibodies block LMP2A-induced invasion.
The results reported above demonstrate that LMP2A alone can stimulate invasiveness by primary epithelial cells. To test whether this was mediated by ITGα6 we incubated adLMP2A-infected primary epithelial cells, prior to Matrigel invasion assays, with three ITGα6 blocking antibodies (2B7, MP4F, and GoH3) used separately or pooled. The single antibodies blocked invasion individually with variable efficiency, but the most efficient blocking was seen when the three antibodies were pooled. Incubation with control IgG at the same antibody concentration did not influence invasiveness (Fig. 3A). These results show that LMP2A induces invasion of primary epithelial cells in an ITGα6-dependent fashion.
LMP2A induces ITGα6 RNA in epithelial cells in vitro.
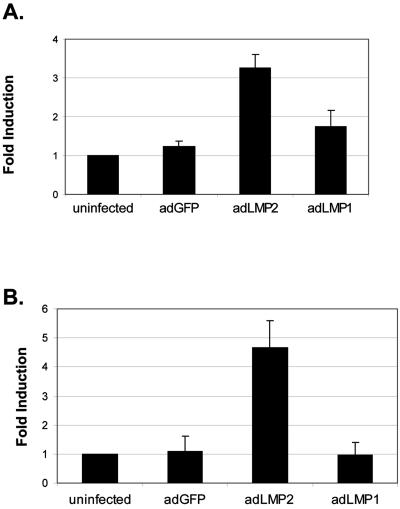
To assess whether LMP2A expression led directly to the up-regulation of ITGα6 we performed a qRT-PCR analysis of ITGα6 RNA levels on primary epithelial cells before and after expression of LMP2A. We also repeated this analysis with the HaCat immortalized human epithelial cell line. LMP2A expression led to an average ∼3.5-fold induction of ITGα6 mRNA in primary cells and a 5-fold increase in HaCat cells (Fig. 4). We did not detect any increase or decrease in transcript levels in either HaCat or primary epithelial cells for several other integrins, including the known ITGα6-associated β1 and β4 chains (not shown). Expression of LMP1 had no significant effect on ITGα6 levels in either primary epithelial cells or HaCat (Fig. 4), indicating that the observed increase in ITGα6 effect was LMP2A specific.
FIG. 4.
LMP2A induces ITGα6 RNA expression in human epithelial cells. (A) Primary epithelial cells were infected with adLMP2A, adLMP1, and adGFP. Represented are the levels of ITGα6 mRNA compared to uninfected cell results at 3 days postinfection for two independent primary cultures from different tonsils. At 1 and 2 days postinfection no significant increase in ITGα6 mRNA levels was observed (not shown). The levels of ITGα6 transcript expression were determined by qRT-PCR and normalized to β-actin levels, and the severalfold induction was calculated compared to untreated cell results (see Materials and Methods). (B) Hacat epithelial cells were infected with adLMP2A, adLMP1, and adGFP. Represented are the levels of ITGα6 mRNA compared to uninfected cell results at 2 days postinfection expressed as an average from three separate experiments. Error bars in all experiments correspond to standard deviations.
The increase in ITGα6 expression caused by LMP2A was time and dose dependent but less pronounced in primary epithelial cells than in HaCat cells, and the kinetics were delayed (not shown). These differences are likely due to differences in growth dynamics and heterogeneity between the two epithelial cell systems. The primary epithelial cultures are very heterogeneous in nature (44), in contrast to the clonal HaCat cell line. As a result, LMP2A might be exerting its fullest effect only in a subset of cells in the primary cultures which would be diluted out when observing the bulk culture. Therefore, it is conceivable that, at the single cell level, the effects of LMP2A on ITGα6 expression levels may be more pronounced in primary epithelial cells.
LMP2A induces ITGα6 protein expression in migrating cells.
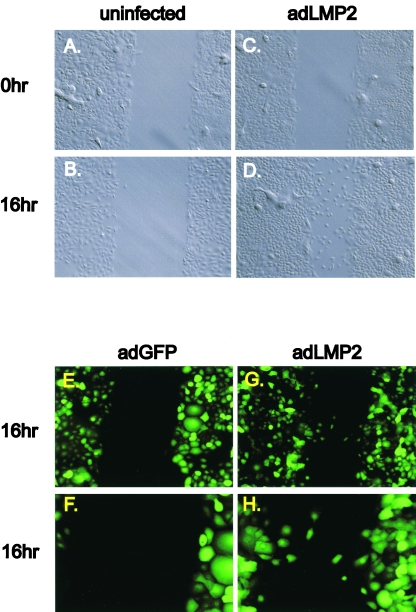
We did not observe a striking effect of LMP2A expression on the cell morphology of either primary or HaCat epithelial cells in bulk cultures. We were also unable to demonstrate an increase in ITGα6 protein expression, in confluent primary epithelial and HaCat cultures, by FACS analysis concomitant with the increase in mRNA levels caused by LMP2A expression. The resolution of this potential conflict came when we studied ITGα6 expression in cells driven to migrate by LMP2A, as assessed in a scratch migration assay (33). Primary epithelial cells tested at optimal LMP2A expression levels demonstrated potent migration in a 16-h wound assay (Fig. 5C and D) compared to uninfected control results, which showed no migration after this time (Fig. 5A and B). This independently confirmed the results seen with the invasion assays. Cells tested at suboptimal LMP2A expression levels showed reduced migration, confirming the dose-dependent effect of LMP2A on motility (not shown). Cell growth kinetics of the primary epithelial cultures with or without LMP2A expression were similar, indicating that differences in cellular growth rate did not account for the increased migration by LMP2A-expressing cells. The results from a second experiment confirmed that cells expressing GFP alone were not migratory (Fig. 5E) whereas cells expressing LMP2A-GFP were (Fig. 5G). A higher magnification photomicrograph of the same assay emphasizes the difference at the wound boundary between migrating and nonmigrating cells (Fig. 5F and H). LMP1 expression also induced migration in the primary epithelial cells (not shown).
FIG. 5.
LMP2A accelerates cellular migration of primary epithelial cells in a scratch wound assay. (A to D) Uninfected (A and B) and adLMP2A-infected 3 day postinfection (C and D) primary epithelial cells were grown to confluence and the monolayers wounded with a plastic pipette tip. Bright-field images were taken at 0 h (A and C) and 16 h (B and D) postwounding (×20 magnification). (E to G) GFP fluorescence images of primary epithelial cells infected with adLMP2A (which also expresses GFP; see Materials and Methods) and adGFP alone at 16 h postwounding taken at ×20 (E and G) or ×40 (F and H) magnifications. This is a different experiment from the one shown in panels A to D.
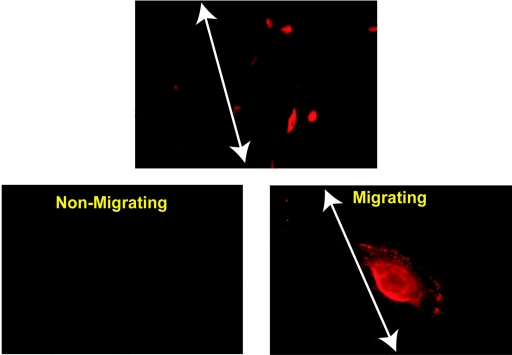
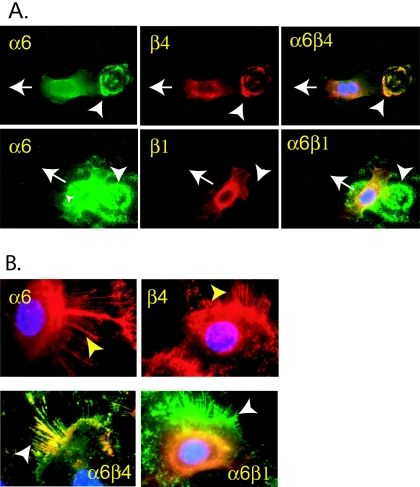
As a control for the migration assays we performed immunofluorescence staining for ITGα6 expression by cells at the scratch boundary. The results are shown in Fig. 6. Primary cells from the confluent monolayer stained weakly (lower left panel). However, when the monolayer was scratched, cells that migrated into the wound markedly up-regulated expression of ITGα6, as shown by the brightly stained cells to the right of the arrows delineating the scratch boundaries, in the upper (low magnification) and lower right (high magnification) panels of Fig. 6. Moreover, ITGα6 expression in the typical fan-shaped, fast-migrating cells is highly polarized. Cells which started to migrate left “cauliflower-like” integrin α6 “footprints” behind (Fig. 7A, white arrowheads). These “integrin footprints” are the residues of hemidesmosome structures (19). We observed clear “integrin release” of ITGα6 heterodimers from retraction fibers, as expected when this integrin plays an adhesive role in migration (Fig. 7B). ITGα6 is most commonly associated with either the ITGβ4 or β1 subunits. Double staining of the cells for coexpression of these common ITGα6-associated chains revealed that ITGα6 was predominantly associated with ITGβ4 in the motility structures of the primary epithelial cells driven to migrate through LMP2A expression (Fig. 7A and B). Note the colocalization of ITGα6 and ITGβ4 in the footprint in the upper panels (Fig. 7A) but the absence of ITGβ4 in the footprint in the lower panels (Fig. 7A, white arrowheads), whereas all three are expressed in the migrating cells (white arrows). Similarly note the presence of ITGα6 and ITGβ4 in the retraction fibers (Fig. 7B, upper panels, yellow arrowheads), their colocalization (Fig. 7B, lower left panel, white arrowhead), and the absence of ITGβ1 (Fig. 7B, lower right panel, white arrowhead). These data indicate that ITGα6 protein expression is up-regulated and relocalized in LMP2A-expressing migrating primary epithelial cells but not in stationary cells.
FIG. 6.
ITGα6 expression is up-regulated in migrating primary epithelial cells. A scratch wound assay with adLMP2A-infected primary epithelial cells was performed on culture slides as described for Fig. 5. The cells were then fixed and incubated with an ITGα6-specific antibody (GoH3), and ITGα6 expression was visualized by indirect immunofluorescence with an Alexa-594 conjugated goat anti-mouse secondary antibody (red). In the top (low magnification) and bottom right (high magnification) panels one edge of the scratch is delineated with a white arrow. The unaffected monolayer of epithelial cells is to the left of the line. The lower left panel shows a field of nonmigrating cells from an area of the culture away from the scratch to demonstrate the staining obtained with confluent cells. Note that ITGα6 staining is weak with the unaffected monolayer but that strongly positively stained cells are only observed migrating into the scratch to the right of the white arrows. The upper image was taken at ×40, and the lower two images were taken at ×100 magnification.
FIG. 7.
Possible redistribution of integrin α6β4 from hemidesmosomes to motility structures in migrating primary epithelial cells expressing LMP2A. Primary epithelial cells expressing adLMP2A were grown on culture slides at semiconfluence and stained with primary antibodies against ITGα6, ITGβ1, and ITGβ4. (A) Indirect immunofluorescence staining for ITGα6 (green) and ITGβ1 (red) or ITGβ4 (red) in migrating primary epithelial cells. The images show the cellular localization of the individual integrins. Merged images emphasize the colocalization of ITGα6-β4 as remnants of hemidesmosome structures. The white arrows indicate the direction of the migrating cells, and the arrowheads indicate deposited ITGα6β4 on the substratum. DAPI staining (blue) shows the localization of the nucleus. (B) The upper two panels show expression of ITGα6 (red) and ITGβ4 (red) in motility structures (arrowheads), most likely retraction fibers of primary epithelial cells. The lower two panels show that ITGα6 (green) colocalizes (yellow) with ITGβ4 (red, left panel) but not with ITGβ1 (red, right panel) in motility structures.
High LMP2A expression in NPC is linked to reduced survival.
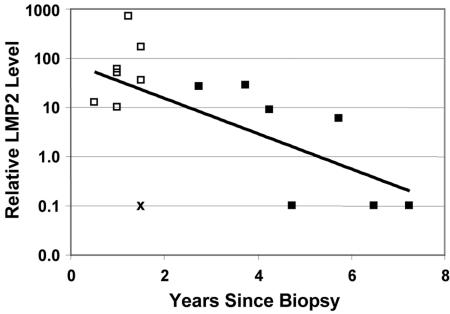
We have shown that LMP2A expression correlates with ITGα6 in NPC biopsy samples and induces ITGα6-dependent invasion and migration in primary keratinocytes. Induction of ITGα6 expression has been implicated in tumor progression and reduced survival in thyroid (54), papilloma (60), and gastric (59) and bladder (21, 29) squamous carcinoma. It seemed possible, therefore, that increased levels of LMP2A expression could be linked with poorer survival in NPC through induction of ITGα6. We had access to clinical data from 20 patients for whom we also had measured the relative LMP2A and ITGα6 expression levels in their biopsy samples. Five patients did not return after biopsy and were not available for survival analysis. For the remaining 15, although the sample size is small, there is a clearly significant correlation (Fig. 8) between LMP2A expression and poor survival (P = 0.028 in a Cox regression survival test). ITGα6 expression showed a similar trend that did not quite achieve statistical significance in the Cox survival test (P = 0.057).
FIG. 8.
LMP2A expression correlates inversely with survival. Results for 15 patients for whom clinical data were available are graphed. Filled boxes represent surviving patients; empty boxes represent deceased patients (Cox regression survival analysis; P = 0.028). X indicates one patient who died of myocardial infarction independent of NPC (Pearson correlation, P = 0.028 [including this patient] and 0.001 [excluding this patient]).
DISCUSSION
Two characteristic features of nonkeratinizing NPC are that it is always associated with EBV and that it is highly metastatic. In this report, we provide evidence of a mechanistic link between these two properties by showing that the virus-encoded LMP2A protein, which is expressed in the tumors, increases the migration and invasion of primary epithelial cells. We have demonstrated for the first time a correlation between LMP2A and ITGα6 expression both in NPC and primary epithelial cells and that LMP2A-induced invasion is ITGα6 dependent. The significance of our results is underscored by the fact that the initial observations, made with clinical material, were then verified at the molecular and cellular level in a defined primary epithelial cell culture system. We have shown previously that primary epithelial cells from tonsillar tissues are targets for EBV infection, in contrast to many epithelial cell lines, such as HaCat, which are resistant to EBV infection. This approach eliminates the possibility that any effect we see might be an artifact of immortalized or transformed epithelial cell lines. These studies in turn led to specific predictions about the effect of LMP2A expression on clinical outcome, namely, that metastasis should be more frequent and survival shorter in patients whose biopsy samples express elevated levels of LMP2A.
Currently, the function of LMP2A is best understood for B cells. It has been shown in transgenic mice that LMP2A can provide B cells with a potent survival signal analogous to signals provided by a functional B-cell receptor (10). LMP2A is known to be expressed in germinal center cells, and in transgenic mice LMP2A drives germinal center formation in mucosal lymphoid tissue in the absence of antigen (11). Thus, LMP2A is believed to play a crucial role in the production, through the germinal center, of latently infected memory B cells—the site of long-term persistent infection (6, 62). The movement of B cells to the follicle to form germinal centers is guided by the expression of chemokines and chemokine receptors (16). However, the processes of migration and retention are mediated by the expression of integrins (4, 34). ITGα6 is highly expressed by germinal center B cells (4) and induced by LMP2A in the background of LCL cells (47), the infected cell type that is believed to migrate to the follicle (7, 62, 64). It seems reasonable to speculate therefore that a biological role of LMP2A may be to drive the migration of latently infected B lymphocytes into the follicles of mucosal epithelium to form germinal centers. On the basis of the current study results we would anticipate that this would be mediated, at least in part, through ITGα6. This process, which is benign in B-cell development, may be co-opted in the tumor to cause the epithelial cells to develop a more invasive and metastatic phenotype.
Our findings are consistent with previous reports (13, 18, 52) that LMP2A expression affects epithelial cell migration. In one study, LMP2A coexpression with LMP1 in epithelial cell lines led to small invasive pockets in organotypic epithelial raft cultures accompanied by changes in ITGα6β4 expression (18). However, the relative contributions of LMP1, LMP2A, and ITGα6 to this phenomenon were not assessed. In other studies LMP2A caused increased invasion by cell lines (13), and in a recent study, published while this article was in preparation, stable LMP2A expression was shown to enhance cell spreading (2) by epithelial cell lines. To our knowledge this is the first report showing that transient expression of LMP2A drives chemotactic invasion and migration of primary epithelial cells in an ITGα6-dependent fashion. LMP1 has been shown previously to induce migration and invasion by an NPC cell line in parallel with increased expression of ITGα6 RNA (30). In the present study we also found that LMP1 increases the motility of primary epithelial cells, but we could not detect changes in expression of ITGα6 RNA. Therefore, both LMP1 and LMP2A might contribute to the metastatic activity of NPC. The argument could be made that LMP2A may be more important, because metastasis is a late event in tumor development and LMP1 is only expressed in a fraction of NPC tumors whereas LMP2A is ubiquitous. The fact that LMP2A expression has such strong effects on motility in primary epithelial cells suggests that the migratory function of LMP2A in NPC is independent of the transformation process.
LMP2A has been reported to activate potentially oncogenic signaling pathways in epithelial cell lines involving β-catenin, PI3-K, extracellular signal-regulated kinase, and JNK (13, 38, 39, 53). We did not find evidence from the neighbor analysis for transcriptional regulation of these pathways by LMP2A in NPC. This may be because the neighborhood analysis is too conservative or too insensitive to detect these changes or because these signaling pathways are activated in cell lines but not in the tumors. However, two members of the JNK pathway were high in the list generated by the neighbor analysis (Fig. 1). In a less rigorous approach we identified additional potential LMP2A targets when we arbitrarily grouped the NPC samples into groups of highest and lowest LMP2A expressers. This screening method yielded, besides ITGα6, its partner ITGβ4 (2-fold induced; P < 0.01), c-jun (∼2-fold induced; P < 0.05), and CD9 (∼1.7-fold induced; P < 0.006); the latter has been reported to be regulated by LMP2A in B cells (13, 47). We also found induction of the Id1 (three probes) (fourfold induced; P < 0.002) and Id3 (twofold induced; P < 0.03) proteins previously reported to be up-regulated in epithelial cells by LMP1 (28) (see Table S1, part 3, in the supplemental material). Although this method is less rigorous, it provides evidence complementary to the cell line studies implicating these pathways in NPC pathogenesis.
We have presented evidence that ITGα6 is regulated transcriptionally by LMP2A in both NPC and primary epithelial cells. Yet we have been unable to detect marked increases in ITGα6 protein expression by flow cytometry in bulk cultures of primary epithelial cells expressing LMP2A, indicating that LMP2A does not cause an immediate increase in ITGα6 protein expression. Instead we found that ITGα6 protein expression became markedly up-regulated when the LMP2A-expressing cells began migrating. Our interpretation of this finding is that LMP2A does not increase the level of ITGα6 protein expression per se but increases the motility of epithelial cells, leading to increased ITGα6 protein expression when they start migrating. This might suggest that LMP2A signaling does not work directly on ITGα6 expression but indirectly through the stimulation of motility. Evidence that ITGα6 protein expression was critical for LMP2A-induced migration was provided by the studies indicating that specific ITGα6-blocking antibodies completely blocked LMP2A-induced invasion through a Matrigel matrix. A role for ITGα6 is also consistent with the major component of Matrigel matrix being laminin I, since this is a known substrate for ITGα6-mediated cell migration (49).
ITGα6 has been shown previously to play an important role in a wide range of carcinomas, including head and neck (66), bladder (29), lung (15), skin (17, 60), and colon (12, 48) cancers. Recent studies indicate that ITGα6, particularly in association with the β4 chain, is important and sometimes crucial for the invasive behavior of tumor cells (12, 17, 55). Despite these advances, it is currently unknown what the exact role of ITGα6 is in epithelial cell migration since its adhesive and signaling functions are highly context (substrate, expression level, localization) dependent.
ITGα6 forms heterodimers exclusively with integrin β1 or β4 chains (25). Besides mediating attachment to the extracellular matrix in hemidesmosomes, the heterodimer ITGα6β4 also has signaling and physical properties that are involved in regulating cell migration (55, 65). Our observations are consistent with the known properties of ITGα6 when associated with the β4 chain, namely, that it may become redistributed from hemidesmosomes to motility structures during the transition from a nonmigrating to a migrating cell type (19). We found that cells which start to migrate leave integrin α6β4 “footprints” behind. These “footprints” are the residues of hemidesmosome structures (19). In addition, we observed clear “integrin release” of ITGα6β4 heterodimers from retraction fibers and hemidesmosomal structures, as expected when these integrins play an adhesive role in migration. Neither of these effects was observed for ITGα6β1. Future studies are necessary to determine the exact role ITGα6 and its partners play in LMP2A-induced motility and the possible contribution(s) of other integrins such as ITGα3β1.
It is commonly accepted that the most dangerous form of cancer is disseminated disease and that most high-grade NPC tumors are highly invasive (3). In one study 5,037 NPC cases were analyzed, and only 9% were found to be confined to the nasopharynx without clinically involved nodes (27). Moreover, in autopsy studies 38 to 87% of NPC patients suffered from distant metastasis (1). When we ranked the biopsy samples on the basis of LMP2A expression levels we found a negative correlation between the level of LMP2A transcript expression and survival. In addition, distant metastasis, local invasion, and recurrence were common in the tumors highly expressing LMP2A but virtually absent for the low expressers. Although the cohort we studied is too small for drawing definitive conclusions these studies are consistent with the idea that LMP2A decreases survival through its effects on the motility and invasiveness of the tumors. Thus, it will now be important to conduct a long-term survival study of a large cohort of NPC patients to test whether the poor survival we have found associated with LMP2A and ITGα6 expression can be confirmed and directly attributed to faster and or more-extensive metastatic behavior by the tumor cells. It will also be of interest to test whether high-level expression of ITGα6 in premalignant nasopharyngeal lesions is prognostic for more rapid tumor development and poor long-term survival.
In conclusion, we show for the first time a link between LMP2A and ITGα6 expression in NPC biopsy samples and hypothesize that LMP2A might play a critical role in NPC metastasis on the basis of its ability to drive invasion of primary nasopharyngeal epithelial cells in an ITGα6-dependent manner. This behavior may reflect the biological function of LMP2A in modulating B-cell migration during the process of germinal center formation. The data presented in this report and our earlier findings, which suggest that EBV is present in healthy nasopharyngeal epithelial cells, strongly support the evolving concept that EBV functions as a tumor virus in NPC progression.
Supplementary Material
Acknowledgments
We thank Pablo Tamayo for critical reading of the manuscript and Cheryl Green (Massachusetts General Hospital, Boston, Mass.) for providing the tonsils.
This work was supported by Public Health Service grants AI 18757 and CA 65883 to D.T.-L.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org.
REFERENCES
- 1.Ahmad, A., and S. Stefani. 1986. Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J. Surg. Oncol. 33:194-197. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. D., L. S. Young, and C. W. Dawson. 2005. The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J. Virol. 79:1789-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altun, M., A. Fandi, O. Dupuis, E. Cvitkovic, Z. Krajina, and F. Eschwege. 1995. Undifferentiated nasopharyngeal cancer (UCNT): current diagnostic and therapeutic aspects. Int. J. Radiat. Oncol. Biol. Phys. 32:859-877. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose, H. E., and S. D. Wagner. 2004. Alpha6-integrin is expressed on germinal centre B cells and modifies growth of a B-cell line. Immunology 111:400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson-Anvret, M., N. Forsby, G. Klein, and W. Henle. 1977. Relationship between the Epstein-Barr virus and undifferentiated nasopharyngeal carcinoma: correlated nucleic acid hybridization and histopathological examination. Int. J. Cancer 20:486-494. [DOI] [PubMed] [Google Scholar]
- 6.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 7.Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. [DOI] [PubMed] [Google Scholar]
- 8.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busson, P., R. McCoy, R. Sadler, K. Gilligan, T. Tursz, and N. Raab-Traub. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 11.Casola, S., K. L. Otipoby, M. Alimzhanov, S. Humme, N. Uyttersprot, J. L. Kutok, M. C. Carroll, and K. Rajewsky. 2004. B cell receptor signal strength determines B cell fate. Nat. Immunol. 5:317-327. [DOI] [PubMed] [Google Scholar]
- 12.Chao, C., M. M. Lotz, A. C. Clarke, and A. M. Mercurio. 1996. A function for the integrin alpha6beta4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 56:4811-4819. [PubMed] [Google Scholar]
- 13.Chen, S.-Y., J. Lu, Y.-C. Shih, and C.-H. Tsai. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coller, H. A., C. Grandori, P. Tamayo, T. Colbert, E. S. Lander, R. N. Eisenman, and T. R. Golub. 2000. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc. Natl. Acad. Sci. USA 97:3260-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costantini, R. M., R. Falcioni, P. Battista, G. Zupi, S. J. Kennel, A. Colasante, I. Venturo, C. G. Curio, and A. Sacchi. 1990. Integrin (alpha 6/beta 4) expression in human lung cancer as monitored by specific monoclonal antibodies. Cancer Res. 50:6107-6112. [PubMed] [Google Scholar]
- 16.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 17.Dajee, M., M. Lazarov, J. Y. Zhang, T. Cai, C. L. Green, A. J. Russell, M. P. Marinkovich, S. Tao, Q. Lin, Y. Kubo, and P. A. Khavari. 2003. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421:639-643. [DOI] [PubMed] [Google Scholar]
- 18.Farwell, D. G., J. K. McDougall, and M. D. Coltrera. 1999. Expression of Epstein-Barr virus latent membrane proteins leads to changes in keratinocyte cell adhesion. Ann. Otol. Rhinol. Laryngol. 108:851-859. [DOI] [PubMed] [Google Scholar]
- 19.Geuijen, C. A., and A. Sonnenberg. 2002. Dynamics of the alpha6beta4 integrin in keratinocytes. Mol. Biol. Cell 13:3845-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glisin, V., R. Crkvenjakov, and C. Byus. 1974. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry 13:2633-2637. [DOI] [PubMed] [Google Scholar]
- 21.Grossman, H. B., C. Lee, J. Bromberg, and M. Liebert. 2000. Expression of the alpha6beta4 integrin provides prognostic information in bladder cancer. Oncol. Rep. 7:13-16. [PubMed] [Google Scholar]
- 22.Henle, W., and G. Henle. 1979. Seroepidemiology of the virus, p. 61-78. In M. A. Epstein and B. G. Achong (ed.), The Epstein-Barr virus. Springer-Verlag, Berlin, Germany.
- 23.Heussinger, N., M. Buttner, G. Ott, E. Brachtel, B. Z. Pilch, E. Kremmer, and G. Niedobitek. 2004. Expression of the Epstein-Barr virus (EBV)-encoded latent membrane protein 2A (LMP2A) in EBV-associated nasopharyngeal carcinoma. J. Pathol. 203:696-699. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg, D., T. Souza, M. Catalina, J. L. Sullivan, K. Luzuriaga, and D. A. Thorley-Lawson. 2004. Acute infection with Epstein-Bar virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 78:5194-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 26.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, New York, N.Y. [Google Scholar]
- 27.Lee, A. W., Y. F. Poon, W. Foo, S. C. Law, F. K. Cheung, D. K. Chan, S. Y. Tung, M. Thaw, and J. H. Ho. 1992. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int. J. Radiat. Oncol. Biol. Phys. 23:261-270. [DOI] [PubMed] [Google Scholar]
- 28.Li, H. M., Z. H. Zhuang, Q. Wang, J. C. Pang, X. H. Wang, H. L. Wong, H. C. Feng, D. Y. Jin, M. T. Ling, Y. C. Wong, A. G. Eliopoulos, L. S. Young, D. P. Huang, and S. W. Tsao. 2004. Epstein-Barr virus latent membrane protein 1 (LMP1) upregulates Id1 expression in nasopharyngeal epithelial cells. Oncogene 23:4488-4494. [DOI] [PubMed] [Google Scholar]
- 29.Liebert, M., G. Wedemeyer, J. A. Stein, R. W. Washington, Jr., C. Van Waes, T. E. Carey, and H. B. Grossman. 1993. The monoclonal antibody BQ16 identifies the alpha 6 beta 4 integrin on bladder cancer. Hybridoma 12:67-80. [DOI] [PubMed] [Google Scholar]
- 30.Lo, A. K., Y. Liu, X. H. Wang, D. P. Huang, P. W. Yuen, Y. C. Wong, and G. S. Tsao. 2003. Alterations of biologic properties and gene expression in nasopharyngeal epithelial cells by the Epstein-Barr virus-encoded latent membrane protein 1. Lab. Investig. 83:697-709. [DOI] [PubMed] [Google Scholar]
- 31.Lo, A. K., P. W. Yuen, Y. Liu, X. H. Wang, A. L. Cheung, Y. C. Wong, and S. W. Tsao. 2001. Downregulation of hemidesmosomal proteins in nasopharyngeal carcinoma cells. Cancer Lett. 163:117-123. [DOI] [PubMed] [Google Scholar]
- 32.Lo, K. W., K. F. To, and D. P. Huang. 2004. Focus on nasopharyngeal carcinoma. Cancer Cell 5:423-428. [DOI] [PubMed] [Google Scholar]
- 33.Lu, J., H. H. Chua, S. Y. Chen, J. Y. Chen, and C. H. Tsai. 2003. Regulation of matrix metalloproteinase-1 by Epstein-Barr virus proteins. Cancer Res. 63:256-262. [PubMed] [Google Scholar]
- 34.Lu, T. T., and J. G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297:409-412. [DOI] [PubMed] [Google Scholar]
- 35.Maschler, S., G. Wirl, H. Spring, D. V. Bredow, I. Sordat, H. Beug, and E. Reichmann. 2005. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 24:2032-2041. [DOI] [PubMed] [Google Scholar]
- 36.Mercurio, A. M., and I. Rabinovitz. 2001. Towards a mechanistic understanding of tumor invasion—lessons from the alpha6beta 4 integrin. Semin. Cancer Biol. 11:129-141. [DOI] [PubMed] [Google Scholar]
- 37.Mishima, A., A. Suzuki, M. Enaka, T. Hirose, K. Mizuno, T. Ohnishi, H. Mohri, Y. Ishigatsubo, and S. Ohno. 2002. Over-expression of PAR-3 suppresses contact-mediated inhibition of cell migration in MDCK cells. Genes Cells 7:581-596. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, J. A., M. L. Gulley, R. Pathmanathan, and N. Raab-Traub. 2004. Differential signaling pathways are activated in the Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res. 64:5251-5260. [DOI] [PubMed] [Google Scholar]
- 39.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 77:12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukhopadhyay, R., R. L. Theriault, and J. E. Price. 1999. Increased levels of alpha6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin. Exp. Metastasis 17:325-332. [DOI] [PubMed] [Google Scholar]
- 41.Niedobitek, G., L. S. Young, C. K. Sam, L. Brooks, U. Prasad, and A. B. Rickinson. 1992. Expression of Epstein-Barr virus genes and of lymphocyte activation molecules in undifferentiated nasopharyngeal carcinomas. Am. J. Pathol. 140:879-887. [PMC free article] [PubMed] [Google Scholar]
- 42.Ong, Y. K., D. M. Heng, B. Chung, S. S. Leong, J. Wee, K. W. Fong, T. Tan, and E. H. Tan. 2003. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur. J. Cancer 39:1535-1541. [DOI] [PubMed] [Google Scholar]
- 43.Osato, T., and S. Imai. 1996. Epstein-Barr virus and gastric carcinoma. Semin. Cancer Biol. 7:175-182. [DOI] [PubMed] [Google Scholar]
- 44.Pegtel, D. M., J. Middeldorp, and D. A. Thorley-Lawson. 2004. Epstein-Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers. J. Virol. 78:12613-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pomeroy, S. L., P. Tamayo, M. Gaasenbeek, L. M. Sturla, M. Angelo, M. E. McLaughlin, J. Y. Kim, L. C. Goumnerova, P. M. Black, C. Lau, J. C. Allen, D. Zagzag, J. M. Olson, T. Curran, C. Wetmore, J. A. Biegel, T. Poggio, S. Mukherjee, R. Rifkin, A. Califano, G. Stolovitzky, D. N. Louis, J. P. Mesirov, E. S. Lander, and T. R. Golub. 2002. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436-442. [DOI] [PubMed] [Google Scholar]
- 46.Pope, J. H., M. K. Horne, and W. Scott. 1968. Transformation of foetal human leukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int. J. Cancer 3:857-866. [DOI] [PubMed] [Google Scholar]
- 47.Portis, T., P. Dyck, and R. Longnecker. 2003. Epstein-Barr virus (EBV) LMP2A induces alterations in gene transcription similar to those observed in Reed-Sternberg cells of Hodgkin lymphoma. Blood 102:4166-4178. [DOI] [PubMed] [Google Scholar]
- 48.Pouliot, N., E. C. Nice, and A. W. Burgess. 2001. Laminin-10 mediates basal and EGF-stimulated motility of human colon carcinoma cells via alpha(3)beta(1) and alpha(6)beta(4) integrins. Exp. Cell Res. 266:1-10. [DOI] [PubMed] [Google Scholar]
- 49.Rabinovitz, I., and A. M. Mercurio. 1997. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 139:1873-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, New York, N.Y. [Google Scholar]
- 51.Sassone-Corsi, P., L. J. Ransone, W. W. Lamph, and I. M. Verma. 1988. Direct interaction between fos and jun nuclear oncoproteins: role of the ‘leucine zipper’ domain. Nature 336:692-695. [DOI] [PubMed] [Google Scholar]
- 52.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scholle, F., R. Longnecker, and N. Raab-Traub. 1999. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J. Virol. 73:4767-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serini, G., L. Trusolino, E. Saggiorato, O. Cremona, M. De Rossi, A. Angeli, F. Orlandi, and P. C. Marchisio. 1996. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J. Natl. Cancer Inst. 88:442-449. [DOI] [PubMed] [Google Scholar]
- 55.Shaw, L. M., I. Rabinovitz, H. H. Wang, A. Toker, and A. M. Mercurio. 1997. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 91:949-960. [DOI] [PubMed] [Google Scholar]
- 56.Spano, J. P., P. Busson, D. Atlan, J. Bourhis, J. P. Pignon, C. Esteban, and J. P. Armand. 2003. Nasopharyngeal carcinomas: an update. Eur. J. Cancer 39:2121-2135. [DOI] [PubMed] [Google Scholar]
- 57.Sutkowski, N., G. Chen, G. Calderon, and B. T. Huber. 2004. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J. Virol. 78:7852-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tani, T., T. Karttunen, T. Kiviluoto, E. Kivilaakso, R. E. Burgeson, P. Sipponen, and I. Virtanen. 1996. Alpha 6 beta 4 integrin and newly deposited laminin-1 and laminin-5 form the adhesion mechanism of gastric carcinoma. Continuous expression of laminins but not that of collagen VII is preserved in invasive parts of the carcinomas: implications for acquisition of the invading phenotype. Am. J. Pathol. 149:781-793.8780383 [Google Scholar]
- 60.Tennenbaum, T., A. K. Weiner, A. J. Belanger, A. B. Glick, H. Hennings, and S. H. Yuspa. 1993. The suprabasal expression of alpha 6 beta 4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res. 53:4803-4810. [PubMed] [Google Scholar]
- 61.Thorley-Lawson, D. A. 2001. Epstein-Barr virus, p. 970-985. In K. F. Austen, M. M. Frank, J. P. Atkinson, and H. Cantor (ed.), Sampter's immunologic diseases, 6th ed., vol. 2. Williams and Wilkins, New York, N.Y. [Google Scholar]
- 62.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75-82. [DOI] [PubMed] [Google Scholar]
- 63.Thorley-Lawson, D. A., and G. J. Babcock. 1999. A model for persistent infection with Epstein-Barr virus: the stealth virus of human B cells. Life Sci. 65:1433-1453. [DOI] [PubMed] [Google Scholar]
- 64.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 65.Trusolino, L., A. Bertotti, and P. M. Comoglio. 2001. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107:643-654. [DOI] [PubMed] [Google Scholar]
- 66.Van Waes, C., and T. E. Carey. 1992. Overexpression of the A9 antigen/alpha 6 beta 4 integrin in head and neck cancer. Otolaryngol. Clin. N. Am. 25:1117-1139. [PubMed] [Google Scholar]
- 67.Yamamoto, H., A. Irie, Y. Fukushima, T. Ohnishi, N. Arita, T. Hayakawa, and K. Sekiguchi. 1996. Abrogation of lung metastasis of human fibrosarcoma cells by ribozyme-mediated suppression of integrin alpha6 subunit expression. Int. J. Cancer 65:519-524. [DOI] [PubMed] [Google Scholar]
- 68.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]
- 69.Young, L. S., C. W. Dawson, D. Clark, H. Rupani, P. Busson, T. Tursz, A. Johnson, and A. B. Rickinson. 1988. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J. Gen. Virol. 69:1051-1065. [DOI] [PubMed] [Google Scholar]
- 70.Yu, M. C., and J. M. Yuan. 2002. Epidemiology of nasopharyngeal carcinoma. Semin. Cancer Biol. 12:421-429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.