Abstract
Dendritic cells (DCs) are located at body surfaces such as the skin, respiratory and genital tracts, and intestine. To further analyze intestinal DCs, we adapted an epidermal sheet separation technique and obtained two intestinal layers, facing the lumen and serosa. Unexpectedly, immunolabeling of the layer toward the serosa revealed a regular, dense, planar network of cells with prominent dendritic morphology within the external muscular layer and with increasing frequency along the length of the intestine. Direct examination of the serosal-disposed layers showed a significant fraction of the DCs to express DEC-205/CD205, CD11c, Langerin/CD207, Fcγ receptor/CD16/32, CD14, and low levels of activation markers, CD25, CD80, CD86, and CD95. By more sensitive FACS analyses, cells from this layer contained two CD11c+ populations of CD45+ CD205+, CD19- leukocytes, MHC II+ and MHC II-. When ovalbumin conjugated to an anti-DEC-205 antibody was injected into mice, the conjugate targeted to these DCs, which upon isolation were able to stimulate ovalbumin-specific, CD4+ and CD8+ T cell antigen receptor-transgenic T cells. In vivo, these DCs responded to two microbial stimuli, systemic LPS and oral live bacteria, by up-regulating CD80, CD86, DEC-205, and Langerin within 12 h. This network of DCs thus represents a previously unrecognized antigen-presenting cell system in the intestine.
Keywords: DEC-205, mucosal immunology, antigen presentation
The human gastrointestinal tract has a luminal surface that is 400 m2 in area (1) and is in constant contact with antigens derived from food, commensal bacteria, parasites, and other microbial sources. In the intestine, antigens are sampled by at least two mechanisms: by the M cells in the Peyer's patches (2) and directly by dendritic cells (DCs) in the lamina propria (3, 4). Lamina propria DCs also sample dying epithelial cells and transport these self constituents to the mesenteric lymph node (5). DCs within the intestine also comprise different subsets that in turn can respond to local microbial ligands, cytokines, and presumably other stimuli, thereby turning the subsequent immune response from tolerance to different forms of immunity (6, 7).
DCs are potent and specialized antigen-presenting cells (8) and important modulators of immune responses (9). They provide a link between innate and adaptive immunity (3, 10). DCs are known to exist in two broad stages of maturation or differentiation. The immature stage is able to capture antigens by several mechanisms such as macropinocytosis, phagocytosis, and receptor-mediated uptake, especially through lectin receptors such as Langerin/CD207 (11) and DEC-205/CD205 (12). Typically, these immature cells are located in nonlymphoid organs such as skin, trachea, intestine, vagina, etc., sites that are normally in contact with environmental antigens (13, 14). In contrast, the mature stage is characterized by a high capacity for T cell stimulation and differentiation, and these cells are often located in secondary lymphoid organs (spleen, lymph nodes, and Peyer's patches). In their immature stage, DCs express many receptors for antigen uptake as well as environmental sensing, e.g., Toll-like receptors, whereas in the more mature state, DCs express high levels of activation (CD25) (15) and costimulatory (CD40 and CD86) molecules important for T cell responses (14).
In the intestine, DCs in the lamina propria and Peyer's patches are known to be arranged in a three-dimensional network close to the luminal epithelium (16, 17). Here, we applied to the intestine a technique used to separate the epidermis and dermis of the skin. We were able to separate the intestinal layers in a suitable manner for immunohistochemical analysis. Unexpectedly, we observed a strongly MHC-II+ population with clear dendritic morphology in the layer toward the serosa, which also expressed CD45, confirming their hematopoietic origin. Phenotypically, these cells expressed DC markers such as DEC-205/CD205, Langerin/CD207, and CD11c, as well as molecules such as CD16/32 and CD14, which suggest an immature state. Interestingly, this subset can process antigens from the circulation and clearly mature in vivo after stimulation with both systemic LPS and live oral bacteria. We suggest that this muscularis-associated network represents a previously unrecognized element of the DC system in the intestine.
Materials and Methods
Mice. Specific pathogen-free, adult (6-8 weeks) BALB/c and C57BL/6 (B6) mice were provided by the Centro de Investigación y de Estudios Avanzados animal facilities. For the ovalbumin (OVA)-specific T cell responses and T cell antigen receptor (TCR), transgenic OT-I and OT-II mice were purchased from Jackson ImmunoResearch. Intestines from the transgenic CD11c-EYFPhigh mice (18) were kindly provided by M. C. Nussenzweig (Howard Hughes Medical Institute, The Rockefeller University, New York).
Reagents. EDTA was purchased from J.T. Baker. The following monoclonal antibodies (mAb) were from BD Pharmingen: anti-CD4, anti-CD8, anti-CD19, anti-CD16/32, anti-CD14, anti-CD25, anti-CD80, anti-CD86, biotinylated anti-CD11c, anti-CD31, anti-CD95, and FITC-conjugated anti-I-A/I-E. Hybridoma supernatant anti-MHC-II (NIMR-4) was kindly provided by L. Santos-Argumedo (Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Casco de Santo Tomás, Mexico). Monoclonal antibody to mouse Langerin was produced and purified as described (19). DEC-205 antibody was produced by the NLDC-145 hybridoma. Enterotoxigenic Escherichia coli were cultured in tripticase soy broth medium, washed three times in sterile saline solution, and adjusted to be inoculated orally in a 50-μl volume. LPS from Salmonella minnesota was purchased from Sigma-Aldrich (catalog no. L6261) and reconstituted in sterile nonpyrogenic PBS.
EDTA Treatment for Intestinal Layer Separation. After dissection, portions of the intestine were placed in cold PBS to wash their contents and to eliminate the mesentery. The intestine was cut into 3-cm portions, which were then opened lengthwise and separated according to the different anatomical regions (duodenum, jejunum, ileum, and large intestine). The tissues were then incubated with 0.5 M EDTA at 37°C for 30 min for the small intestine and for 45 min for the large intestine. After EDTA incubation, the intestinal portions were washed in PBS and placed with the lumen side down. Layers were then carefully separated by traction by using fine forceps and scalpel. Two layers were thus obtained: an external one (toward lumen) and an internal one (toward serosa). Finally, the intestinal layers were placed in PBS and stretched out gently in preparation for fixation procedures.
Semithin Sections. Tissue samples of the complete intestine and also the two layers obtained after EDTA treatment were fixed in 2.5% glutaraldehyde for 1 h and postfixed with 1% osmium tetroxide for 1 h. After the samples had been washed with cacodylate buffer, pH 7.4, they were dehydrated and embedded in epoxy embedding material. The blocks were cut in an ultramicrotome (Reichert), and the 1-μm sections were stained with toluidine blue for examination.
Immunohistochemistry. Intestinal layers were fixed with cold (4°C) 100% acetone for 40 min and then washed in PBS/0.1% BSA. To block endogenous peroxidase, the samples were treated with 6% H2O2 for 40 min at room temperature (RT). Before specific Ab staining, nonspecific binding sites were blocked with BioGenex Laboratories universal blocking reagent (San Ramon, CA) for 40 min at RT. Primary unlabeled or biotinylated Abs were incubated overnight at 4°C at optimal dilutions (1 μg/ml). The reaction was developed by using a biotinylated anti-rat Ab and then streptavidin-peroxidase by using diaminobenzidine (GIBCO) as chromogen, giving a brown staining. For double labeling, samples were subsequently incubated for 30 min with rat IgG (50 μg/ml) and then fixed with 2.5% paraformaldehyde for 1 min. After blocking residual peroxidase activity as well as Fcγ receptor (6% H2O2 for 1 h and universal blocking reagent for 1 h, respectively), biotinylated anti-CD31 (second labeling) was applied overnight at 4°C. The reaction was developed by using Vector Laboratories peroxidase substrate to obtain a blue color. The samples were subsequently mounted in Immunomount (Shandon, Pittsburgh). The slides were examined and digitalized with a Zeiss Axiophot microscope. Cell frequency was evaluated by counting at least 10 different fields per sample, and the average was used to extrapolate the DC frequency per mm2. Appropriate isotype controls were negative in all experiments.
Cell Suspension Preparation and FACS Analysis. Intestinal layers were prepared as above. The muscularis layers were washed in PBS and cut into small pieces before digestion in 400 units/ml collagenase D for 45 min at 37°C. After digestion, the tissue was disrupted and filtered through a 70-μm cell strainer. The cell suspension was washed two times in cold Hanks' balanced salt solution (HBSS) (GIBCO) with 2% FCS (FBS) (GIBCO) and stained in FACS buffer (PBS/2% FBS/0.01% sodium azide). Before staining, cells were incubated with Fc Block (anti-CD16/32; BD Pharmingen) for 15 min at 4°C. Abs were added for 20 min at 4°C. Biotinylated Abs were detected by using streptavidin conjugated to allophycocyanin in a second step. Appropriate isotype controls were included in all experiments. Cells were analyzed by using a FACSCalibur cytometer and flojo software.
Targeting DEC-205+ Cells in Vivo. C57BL/6 (B6) mice were injected s.c. into each of the four paws with the DEC-205 mAb (NLDC-145) and an Ig isotype control (both at 10 μg) diluted in sterile endotoxin-free PBS. Eighteen hours after injection, the intestine was removed and processed to obtain the intestinal layers. The layers were fixed as previously described and labeled with a FITC-conjugated anti-MHC-II Ab and a phycoerythrin-conjugated anti-rat Ab to detect the DEC-205 Ab.
In Vivo OVA Targeting by DEC-205 and T Cell Proliferation Assay. B6 mice were injected i.p. with anti-DEC-OVA conjugate (10 μg) prepared as described (20). Eighteen hours later, single cell suspensions from the intestinal layer and the mesenteric node (as positive control) were obtained as described in Cell Suspension Preparation and FACS Analysis. From the node (dissociated for 25 min), CD11c+ cells were purified by magnetic enrichment by using CD11c beads (Miltenyi Biotec, Auburn, CA), and CD11c- cells were also collected as negative controls. CD11c+ and CD11c- lymph node cells and cell suspensions from the intestinal layer were cocultured in a 1:9 ratio (DC:T cell) of 105 TCR transgenic OT-I CD8+ or OT-II CD4+ T cells (enriched by negative selection with anti-MHC II and anti-T cell mAbs and Dynabeads) in 96-well round-bottom plates, for 88 h. During the final 12 h, [3H]thymidine (1 μCi per well; 1 Ci = 37 GBq) was added and the cells were harvested to evaluate T cell proliferation. To identify the surface markers of the active antigen-presenting cells, we carried out depletion experiments with antibodies to CD11c, CD19, CD86, and CD205 directly conjugated to magnetic beads or used as biotinylated mAbs and streptavidin beads (Miltenyi Biotec). The procedure was performed as per manufacturer's instructions. The depleted cell suspensions were cocultured in a 1:10 ratio (DC:T cell) with 105 TCR transgenic OT-I and OT-II cells, respectively.
In Vivo Stimulation. BALB/c mice were inoculated i.v. with LPS (30 μg) and orally with live bacteria (enterotoxigenic E. coli, 3.5 × 107 bacteria). Twelve hours after inoculation, the intestine was processed to obtain intestinal layers to analyze the DC phenotype, distribution, and frequency by immunohistochemistry, as described above.
Results
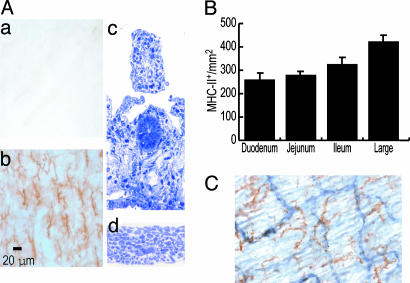
Dendritic, MHC-IIhigh Population in a Planar Distribution Toward the Intestinal Serosa. EDTA treatment was used to separate the intestine into two layers. To search for DCs, we fixed and incubated both layers with an anti-MHC-II mAb. A dense, regular, planar network of MHC-II+ cells was evident only in the layer toward the serosa, with an average density of 288 positive cells per mm2 (Fig. 1Ab). These cells had a prominent dendritic morphology. Their hematopoietic origin was confirmed by the coexpression of CD45 (data not shown). To determine the precise histological location of these cells, semithin sections of both layers were prepared. As shown in Fig. 1 A, the layer toward the lumen (c) had lost the epithelium but retained the lamina propria and submucosa (≈250 μm of thickness), whereas the layer toward the serosa (d) comprised the external muscular layer (≈80 μm of thickness) and contained the DCs. These cells were present in all intestinal regions, but their frequency increased from duodenum to large intestine (Fig. 1B). Considering that a plexus of blood and lymphatic vessels is located in the muscularis layer, the potential association of MHC-II+ cells with vessels was studied by double labeling for MHC-II and CD31. The network of dendritic MHC-II+ cells was not intimately aligned with these vessels (Fig. 1C). We were unable to detect cells expressing the lymphocyte markers CD4, CD8, and CD19 in the muscularis layer (data not shown).
Fig. 1.
A dendritic, strongly MHC-II+ population in the intestinal layer toward the serosa. (A) The intestine was incubated in 0.5 M EDTA for 30 min at 37°C, two layers were separated by traction by using fine forceps, and the layers were fixed in cold acetone. (a and b) Upon labeling, MHC-II+ cells with clear dendritic morphology and a regular pattern of distribution are observed in only one of the two intestinal layers obtained (toward the serosa). To study the precise histological location of this DC population, transverse semithin sections of each layer were obtained. The layer (c) toward the lumen comprises the lamina propria and submucosa, whereas the layer (d) toward the serosa corresponds to the external muscular layer. (B) The frequency of this MHC-II+ population was also analyzed in each of the different anatomical regions of the intestine. (C) The possible association of the MHC-II+ (brown) DC to vessels was assessed by double labeling for CD31 endothelial molecule (blue). The MHC-II+ DCs do not appear associated with CD31+ (blue) vessels (blood or lymphatics).
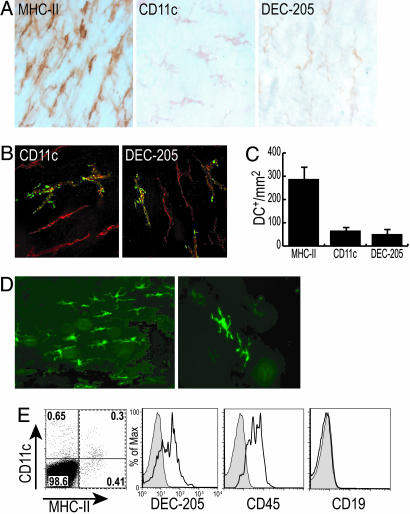
CD11c and DEC-205 Are Also Expressed in MHC-II+ Muscularis Layer DCs. To gain more information on the nature of the MHC-II+ cells in this intestinal layer, we first examined DC markers like DEC-205 and CD11c. Clearly positive dendritic cells were observed but in a lower frequency (≈70 positive cells per mm2) relative to the MHC-II+ population (Fig. 2A). We next performed double labeling by using MHC-II with DEC-205 or CD11c, and in both cases double-positive cells were noted (Fig. 2B). The CD11c+ and DEC-205+ cells represented 30% and 25%, respectively, of the total MHC-II+ population (Fig. 2C). However, when we evaluated the intestine of transgenic CD11c-EYFPhigh mice, a procedure that detects CD11c promoter function with higher sensitivity (18) (Fig. 2D), the frequency of CD11c+ DCs was higher and comparable with the MHC-II labeling. The phenotype of these presumptive DCs was confirmed by FACS analysis for CD11c+ cells, as shown in Fig. 2E. CD11c+ MHC-II positive and negative cells were noted and phenotyped by multicolor flow cytometry (Fig. 2E) and compared with CD11c+ MHC-II+ DCs in mesenteric lymph node and Peyer's patches (data not shown). The CD11c+ muscularis cells were comparable with mesenteric lymph nodes and Peyer's patch DCs in expression of CD45, MHC-II, and DEC-205 but had ≈0.5-1.0 log lower fluorescence for CD11c and CD86. CD19 was absent from all of the DCs.
Fig. 2.
CD11c and DEC-205 are also expressed in this intestinal DC population. (A) Using DC markers such as CD11c and DEC-205, we identified positive cells with dendritic morphology in this intestinal muscular layer. (B) Double labeling for MHC-II (red) and CD11c or DEC-205 (green). (C) CD11c+ and DEC-205+ cells in the layer have a lower frequency than the MHC-II+ cells. (D) Intestinal layers from the CD11c-EYFPhigh mice also show strongly EYFP+ cells with prominent dendritic morphology. FACS analysis of single-cell suspensions of the muscularis layer stained for MHC-II and CD11c show class II negative and positive subsets. (E) The indicated fractions were costained for other antigens, DEC-205, CD45, and CD19, and the expression on the MHC-II+ cells is shown.
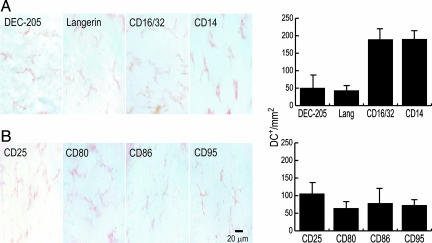
Maturation of the Intestinal DCs in Situ. We then studied the expression of several functionally significant cell surface and activation molecules. The endocytic receptors DEC-205 and Langerin/CD207 were expressed in cells with a clear dendritic morphology, with an average frequency of 60 cells per mm2 (Fig. 3A). Fc receptors (CD16/32) and CD14 (a part of the TLR4 complex for LPS responsiveness) were also expressed by cells with dendritic morphology (frequency ≈180 cells per mm2) (Fig. 3A). The CD25 activation and CD80 and CD86 costimulatory molecules were also expressed on the DCs (≈80 cells per mm2) (Fig. 3B). All these markers were distributed in a regular fashion in the network, and in all cases staining with the appropriate isotype controls was negative. These data suggest that the layer of muscularis DCs is comprised of cells at early stages of maturation.
Fig. 3.
Phenotype of the intestinal DCs in situ. The intestinal layers were fixed and processed for immunohistochemistry. (A) (Left) Several functionally significant cell surface molecules were analyzed, and in all cases positive cells with clear dendritic morphology were observed. (Right) Quantification of their frequency. (B) Activation markers were also used to characterize these DCs.
DCs from the Muscularis Layer Efficiently Stimulate OVA-Specific T Cells. Because the ability to stimulate naive T cells is an important feature of DCs (21), we tested this ability by using TCR transgenic T cells. To deliver antigen to the muscularis DCs in situ, we first evaluated whether in vivo targeting could be achieved through anti-DEC-205 antibody as described (22). Eighteen hours after the s.c. inoculation, the cells in the intestinal layer were clearly labeled for the injected rat mAb, as shown with a phycoerythrin-conjugated anti-rat Ig in Fig. 4A. We then prepared cell suspensions and cocultured these TCR transgenic T cells, by using mice inoculated with anti-DEC-OVA or nonreactive isotype control Ig-OVA, as described (20). T cell proliferation was clearly induced by cell suspensions from the muscularis layer of mice that had been targeted with anti-DEC-205 coupled to OVA in vivo, somewhat more efficiently for OT-II T cells (Fig. 4C). We also used CD11c+ and CD11c- cells from the mesenteric lymph node as positive and negative controls, respectively (Fig. 4B), as well as unconjugated anti DEC-205 Ab as an additional negative control. Strong [3H]thymidine incorporation was induced only by the CD11c+ cells from mice targeted with anti-DEC-OVA. To establish that the active antigen-presenting cells in the muscularis suspensions were DCs, we tested if different mAbs could deplete the presenting activity. mAb to CD11c but not to CD19 depleted the stimulation of TCR transgenic T cells (Fig. 4D, representative of four experiments), as did mAb to DEC-205 (two experiments; data not shown). mAb to CD86 showed only partial depletion in contrast to the full depletion of presenting function from spleen DCs (Fig. 4D). Together these results indicate that the MHC-II+ CD11c+ DEC-205+ immature DCs are responsible for presentation of antigen to CD4+ and CD8+ T cells.
Fig. 4.
DCs from the intestinal layer stimulate proliferation of antigen-specific T cells. (A) DEC-205 targeting: 18 h after i.p. inoculation of the rat anti-mouse DEC-205 antibody and a nonreactive isotype control antibody (10 μg), the intestine was processed to obtain intestinal layers to search for bound antibody by using phycoerythrin (PE)-conjugated anti-rat Ig and indirect immunofluorescence. An FITC-conjugated anti-IA/I-E was concomitantly used to confirm that the cells targeted with DEC-205 were also MHC-II+.(B) Single-cell suspensions from the mesenteric lymph node were prepared 24 h after i.p. inoculation of DEC-OVA conjugate (10 μg). CD11c+ cells were purified by magnetic-activated cell sorting; CD11c- cells were also kept. Both suspensions were used to stimulate OT-I transgenic CD8 T cells, in a 1:3 (DC:T cell) ratio. T cell proliferation was measured by [3H]thymidine incorporation (filled bars, DEC-OVA-inoculated mice; open bars, PBS-inoculated mice). (C) Single-cell suspensions from the intestinal layer were obtained and tested for a stimulation of transgenic OT-I and OT-II T cells (1:10 ratio) 24 h after i.p. inoculation of the DEC-OVA conjugate (10 μg). (D) Single-cell suspensions from the muscularis layer were depleted with isotype control antibody (Iso) or mAbs to CD11c, CD19, and CD86 before addition to TCR transgenic T cells. Lamina propria cells were evaluated in parallel and spleen CD11c+ cells used as control. T cell proliferation was measured by [3H]thymidine incorporation (filled bars, DEC-OVA inoculated mice; open bars, Iso-OVA-inoculated mice). Representative data from four different experiments are shown.
DCs from the Intestinal Layer Mature in Vivo under Oral and Systemic Microbial Stimuli. We next evaluated the in vivo effects of a stimulus such as LPS upon the phenotype of these DCs. Twelve hours after 30 μg of LPS had been injected i.v., the expression of MHC-II decreased, whereas the expression of DEC-205, Langerin, CD80, and CD86 was up-regulated (Fig. 5A). The morphology of these cells remained clearly dendritic, and their network and planar manner of distribution were maintained. To evaluate the potential changes after an oral stimulus, considering that the DCs were found relatively distant from the intestinal lumen, we examined the DC network 12 h after live enterotoxigenic E. coli were administered orally. Again DEC-205, Langerin, CD80, and CD86 were up-regulated (Fig. 5B), and the response seemed stronger than the responses induced by i.v. LPS. These results show that even though muscularis DCs are relatively distant from the lumen, they are capable of responding to oral and systemic microbial stimulation.
Fig. 5.
DCs from the intestinal layer mature in vivo. The intestine was processed to obtain intestinal layers to analyze the phenotype and frequency of these DCs 12 h after LPS inoculation (30 μg i.v.) (A) or live bacteria (3.5 × 107 bacteria orally) (B). In both cases, an up-regulation of DEC-205+ and Langerin+ DCs was observed, and the number of cells expressing activation markers such as CD80 and CD86 increased significantly (filled bars, control animals; open bars, stimulated animals).
Discussion
DCs have been characterized at several different body surfaces, such as the skin, trachea, vagina, and intestine (7, 23, 24). In these tissues, DCs are distributed without formal contacts with one another but nevertheless comprise a planar network, the best example being Langerhans cells in the suprabasal region of the epidermis. Langerhans cells represent, at most, a few percent of the nucleated cells in the skin, and epidermal sheets have been successfully used to analyze the distribution and properties of Langerhans cells in situ with much greater ease and accuracy than conventional transverse sections (25).
Different DC subsets have been described in the intestine, including the lamina propria and Peyer's patches (16, 26, 27). In general, the expression of MHC-II and the characteristic irregular morphology have been used to identify these cells in situ (16, 28). In other cases, DCs have been purified from lamina propria, without emphasis on their precise histological location (28, 29). By adapting the epithelial sheet separation to the intestine, we obtained two intestinal layers. Surprisingly, only in the muscularis layer was a planar MHC-II+ cell population with dendritic morphology and homogeneous distribution observed. Although by cross section the cells could be observed in the muscularis layer, the staining with various mAbs never delineated a clear dendritic morphology or the features of a network, and perhaps this and their peculiar location in the muscularis have precluded their previous identification.
Our present report is not the first description of DCs associated with muscle cells. DCs were described in cardiac muscle and considered immature by their phenotype in ref. 30. The DCs of the intestinal muscularis layer expressed the same markers as those reported in the cardiac muscle DCs, although here we emphasized the capacity to capture antigen in vivo and successfully process the protein into peptides presented on MHC class I and II products. DCs expressing MHC-II, DEC-205, and CD11b also have been described in mouse uterus located adjacent to the epithelium and in the myometrium, another smooth muscle like the intestine (31).
The frequency of this network of DCs increased along the intestinal anatomical regions. Most commensal and pathogenic bacteria are located in the large intestine, where the highest frequency of these DCs was found (32). This notion is also supported by the lower frequency of these DCs when we studied germ-free animals (data not shown). This finding suggests that the number of muscularis DCs could be related to exogenous environmental antigenic stimulation. We do not have data on the turnover and traffic of the network of muscularis DCs, but it is possible that under steady-state conditions, the DCs resemble cutaneous Langerhans cells, in which a small proportion undergoes continuous migration to the regional lymph nodes (33).
Besides the initial MHC-II staining, different DC markers were also assessed. CD11c and DEC-205 were detected, particularly in transgenic CD11c-EYFPhigh mice (18). This phenotype was also confirmed by FACS analysis, where the muscularis cells were compared with DCs in lymph node tissues. The muscularis DCs expressed lower levels of CD11c and CD86, but the expression of DEC-205 and MHC-II were similar to DCs in lymphoid tissues. Interestingly, the DCs also express Langerin, which has not been observed previously in intestine. Besides the skin where Langerin was originally described, this lectin has been found in lymph nodes, spleen, lung, and heart (34, 35).
In a high proportion of the muscularis layer DCs, we also observed receptors related to the recognition of bacterial antigens shared with monocytes, such as CD14, as well as immune complex receptors such as CD16/32, which are highly active in immature DCs. Activation and costimulatory markers were also expressed by DCs in this location, although in lower frequency and intensity than MHC-II. However, this phenotype was modified after the i.v. inoculation of LPS and the oral administration of live bacteria for a local, restricted stimulation. In the case of the systemic stimulation, a clear up-regulation of costimulatory molecules and also of Langerin and DEC-205 was observed. Either there was immigration of these cells, e.g., from the lamina propria after the stimulation, or the stimulus triggered an up-regulation of these molecules in situ. Interestingly, oral administration of bacteria seemed to be a more efficient stimulus for these changes in muscularis DCs relative to systemic stimulation with LPS, revealing that these cells, even in their relatively distant location from the lumen, are able to sense luminal antigens relatively quickly, within 12 h. The chemokine receptor profile of this population could help to elucidate their traffic in this organ.
The ability of the DEC-205 adsorptive endocytic receptor to mediate antigen presentation in vivo has been demonstrated for both MHC class II and MHC class I (36). The approach involved targeting of antigens to the receptor conjugated with an anti-DEC-205 mAb. To evaluate the functional properties of the muscularis DC population in vivo, we used DEC-OVA conjugates to target antigen specifically to the DEC-205+ cells, and in this way precluded the potential participation of other antigen-presenting cells. It has been shown that 6 h after s.c. inoculation of anti-DEC-205, the mAb distributes systemically to DCs in distal lymph nodes and spleen (20). We showed that anti-DEC-205 antibody also targets DCs in the intestinal layer and, when OVA was conjugated to the antibody, muscularis leukocytes were able to stimulate the proliferation of OVA-specific CD4+ and CD8+ T cells. This presentation was completely dependent on the CD11c+ DEC-205+ cells. These functional studies, coupled with the phenotyping that we performed, led to the conclusion that these MHC-II+ leukocytes in the muscularis layer are DCs.
Acknowledgments
We thank Dr. Diana Dudziak (The Rockefeller University) for her comments and help with the DEC-OVA conjugates, Dr. M. Nussenzweig (Howard Hughes Medical Institute, The Rockefeller University) for facilitating intestinal samples of CD11c-EYFPhigh mice, Dr. Arturo Gonzalez-Robles (Centro de Investigación y de Estudios Avanzados Avenida Instituto Politécnico Nacional) and Jennifer Finke (The Rockefeller University) for their excellent assistance with the photographic material, Dr. Laura Bonifaz (The Rockefeller University) for helpful discussions, and Judy Adams (The Rockefeller University) for her kind help to prepare the manuscript and the graphics. Grant support to R.M.S. was provided by National Institutes of Health Grants AI13013 and AI40478.
Author contributions: A.F.-L. and L.F.-R. designed research; A.F.-L., S.M.-P., and J.C.-A. performed research; T.E.-G., G.M., S.L., S.S., R.M.S., and L.F.-R. contributed new reagents/analytic tools; A.F.-L. and L.F.-R. analyzed data; and A.F.-L., R.M.S., and L.F.-R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: DC, dendritic cell; OVA, ovalbumin; TCR, T cell antigen receptor.
References
- 1.Mowat, A. M. & Viney, J. L. (1997) Immunol. Rev. 156, 145-166. [DOI] [PubMed] [Google Scholar]
- 2.Hussain, N., Jaitley, V. & Florence, A. T. (2001) Adv. Drug Delivery Rev. 50, 107-142. [DOI] [PubMed] [Google Scholar]
- 3.Rescigno, M., Urbano, M., Valzasina, B., Francolini, M., Rotta, G., Bonasio, R., Granucci, F., Kraehenbuhl, J. P. & Ricciardi-Castagnoli, P. (2001) Nat. Immun. 2, 361-367. [DOI] [PubMed] [Google Scholar]
- 4.Niess, J. H., Brand, S., Gu, X., Landsman, L., Jung, S., McCormick, B. A., Vyas, J. M., Boes, M., Ploegh, H. L., Fox, J. G., et al. (2005) Science 307, 254-258. [DOI] [PubMed] [Google Scholar]
- 5.Huang, F. P., Platt, N., Wykes, M., Major, J. R., Powell, T. J., Jenkins, C. D. & MacPherson, G. G. (2000) J. Exp. Med. 191, 435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macpherson, A. J. & Uhr, T. (2004) Science 303, 1662-1665. [DOI] [PubMed] [Google Scholar]
- 7.MacPherson, G. G., Jenkins, C. D., Stein, M. J. & Edwards, C. (1995) J. Immunol. 154, 1317-1322. [PubMed] [Google Scholar]
- 8.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 9.Steinman, R. M. (2003) Acta Pathol. Microbiol. Scand. Suppl. 111, 675-697. [Google Scholar]
- 10.Fujii, S. I., Liu, K., Smith, C., Bonito, A. J. & Steinman, R. M. (2004) J. Exp. Med. 199, 1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valladeau, J., Duvert-Frances, V., Pin, J. J., Dezutter-Dambuyant, C., Vincent, C., Massacrier, C., Vincent, J., Yoneda, K., Banchereau, J., Caux, C., et al. (1999) Eur. J. Immunol. 29, 2695-2704. [DOI] [PubMed] [Google Scholar]
- 12.Steinman, R. M., Kaplan, G., Witmer, M. D. & Cohn, Z. A. (1979) J. Exp. Med. 149, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding, C. V., Ramachandra, L. & Wick, M. J. (2003) Curr. Opin. Immunol. 15, 112-119. [DOI] [PubMed] [Google Scholar]
- 14.Flores-Romo, L. (2001) Immunology 102, 255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacPherson, G. G., Fossum, S. & Harrison, B. (1989) Immunology 68, 108-113. [PMC free article] [PubMed] [Google Scholar]
- 16.Maric, I., Holt, P. G., Perdue, M. H. & Bienenstock, J. (1996) J. Immunol. 156, 1408-1414. [PubMed] [Google Scholar]
- 17.Mayrhofer, G., Pugh, C. W. & Barclay, A. N. (1983) Eur. J. Immunol. 13, 112-122. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist, R. L., Shakhar, G., Dudziak, D., Wardemann, H., Eisenreich, T., Dustin, M. L. & Nussenzweig, M. C. (2004) Nat. Immunol. 5, 1243-1250. [DOI] [PubMed] [Google Scholar]
- 19.Valladeau, J., Clair-Moninot, V., Dezutter-Dambuyant, C., Pin, J. J., Kissenpfennig, A., Mattei, M. G., Ait-Yahia, S., Bates, E. E., Malissen, B., Koch, F., et al. (2002) J. Immunol. 168, 782-792. [DOI] [PubMed] [Google Scholar]
- 20.Bonifaz, L. C., Bonnyay, D. P., Charalambous, A., Darguste, D. I., Fujii, S. I., Soares, H., Brimnes, M. K., Moltedo, B., Moran, T. M. & Steinman, R. M. (2004) J. Exp. Med. 199, 815-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba, K., Steinman, R. M., Van Voorhis, W. C. & Muramatsu, S. (1983) Proc. Natl. Acad. Sci. USA 80, 6041-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifaz, L., Bonnyay, D., Mahnke, K., Rivera, M., Nussenzweig, M. C. & Steinman, R. M. (2002) J. Exp. Med. 196, 1627-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt, P. G. & Schon-Hegrad, M. A. (1987) Immunology 62, 349-356. [PMC free article] [PubMed] [Google Scholar]
- 24.Katz, S. I., Tamaki, K. & Sachs, D. H. (1979) Nature 282, 324-326. [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie, I. C. & Squier, C. A. (1975) Br. J. Dermatol. 92, 523-533. [DOI] [PubMed] [Google Scholar]
- 26.Pavli, P., Woodhams, C. E., Doe, W. F. & Hume, D. A. (1990) Immunology 70, 40-47. [PMC free article] [PubMed] [Google Scholar]
- 27.Pavli, P., Hume, D. A., Van De Pol, E. & Doe, W. F. (1993) Immunology 78, 132-141. [PMC free article] [PubMed] [Google Scholar]
- 28.Soesatyo, M., Biewenga, J., Kraal, G. & Sminia, T. (1990) Cell Tissue Res. 259, 587-593. [DOI] [PubMed] [Google Scholar]
- 29.MacPherson, G. G. & Liu, L. M. (1999) Curr. Top. Microbiol. Immunol. 236, 33-53. [DOI] [PubMed] [Google Scholar]
- 30.Austyn, J. M., Hankins, D. F., Larsen, C. P., Morris, P. J., Rao, A. S. & Roake, J. A. (1994) J. Immunol. 152, 2401-2410. [PubMed] [Google Scholar]
- 31.Keenihan, S. N. & Robertson, S. A. (2004) Biol. Reprod. 70, 1562-1572. [DOI] [PubMed] [Google Scholar]
- 32.Stagg, A. J., Hart, A. L., Knight, S. C. & Kamm, M. A. (2004) Baillieres Best Pract. Res. Clin. Gastroenterol. 18, 255-270. [DOI] [PubMed] [Google Scholar]
- 33.Merad, M., Manz, M. G., Karsunky, H., Wagers, A., Peters, W., Charo, I., Weissman, I. L., Cyster, J. G. & Engleman, E. G. (2002) Nat. Immunol. 3, 1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henri, S., Vremec, D., Kamath, A., Waithman, J., Williams, S., Benoist, C., Burnham, K., Saeland, S., Handman, E. & Shortman, K. (2001) J. Immunol. 167, 741-748. [DOI] [PubMed] [Google Scholar]
- 35.Valladeau, J., Ravel, O., Dezutter-Dambuyant, C., Moore, K., Kleijmeer, M., Liu, Y., Duvert-Frances, V., Vincent, C., Schmitt, D. & Davoust, J. (2000) Immunity 12, 71-81. [DOI] [PubMed] [Google Scholar]
- 36.Steinman, R. M., Hawiger, D., Liu, K., Bonifaz, L., Bonnyay, D., Mahnke, K., Iyoda, T., Ravetch, J., Dhodapkar, M., Inaba, K., et al. (2003) Ann. N.Y. Acad. Sci. 987, 15-25. [DOI] [PubMed] [Google Scholar]