Abstract
An inflammatory aural polyp was identified in a 1-year-old standardbred filly, which presented with otorrhea and head rubbing. The polyp was removed by traction-avulsion, and the filly showed no subsequent signs of otorrhea. Aural polyps have not been reported in horses, but they are commonly seen in companion animals and humans.
Résumé
Polype auditif inflammatoire chez un cheval. Un polype auditif inflammatoire a été identifié chez une pouliche Standardbred d’un an présentée pour otorrhée et frottage de tête. Le polype a été retiré par traction-avulsion et la pouliche n’a pas manifesté de signes subséquents d’otorrhée. Les polypes auditifs n’ont pas été rapportés chez les chevaux mais sont communément rencontrés chez les animaux de compagnie et chez l’homme.
(Traduit par Docteur André Blouin)
A 1-year-old standardbred filly presented to the Ontario Veterinary College, with a 6-week history of head rubbing and 3-week history of discharge from the left ear. The horse had been treated for 3 wk with trimethoprimsulfmethoxazole (Apo-sulfatrim; Apotex, Toronto, Ontario), 30 mg/kg body weight (BW), PO, q12h, and the outer portion of the left external auditory canal (EAC) had been cleaned daily with dry gauze swabs. The filly was referred for further diagnostic evaluation and treatment when there was no improvement of clinical signs.
Case description
On examination, the filly was in good body condition (body weight 405 kg) and vital parameters were within normal limits. Purulent debris around the left ear base was noticed. The ear was examined following sedation of the filly with detomidine HCl (Dormsedan; Pfizer, Kirkland, Quebec), 0.04 mg/kg BW, IV, and butorphanol tartrate (Torbugesic; Wyeth, St. Laurent, Quebec), 0.05 mg/kg BW, IV. There was a copious amount of yellow purulent discharge in the left EAC. Swabs were collected for bacterial culture, which yielded a pure growth of Streptococcus zooepidemicus. The bacterial isolate was sensitive to trimethoprim-sulfa in vitro.
After initial cleaning, a pink, firm pedunculated mass, approximately 10 mm in diameter, was visualized as arising from the medioventral wall of the left EAC, approximately 2.5 cm from the outer opening. On visualization with a video-endoscope (Olympus GIF-100; Olympus America, Melville, New York, USA), the mass was found to completely occlude the EAC, which precluded further examination of the ear (Figure 1). The right EAC and tympanic membrane appeared to be normal.
Figure 1.
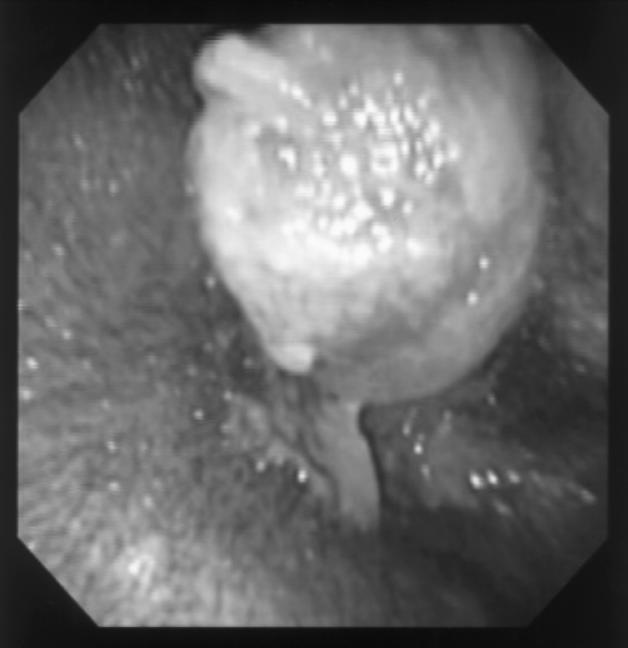
The aural mass as seen on presentation via a videoendoscope (Olympus GIF-100).
Ventrodorsal, lateromedial, and lateromedial oblique radiographs of the skull were taken. There was no evidence of abnormalities associated with the tympanic bullae or other areas of the skull. No lesions, such as dentigerous cystic lesions, were identified.
The filly was induced, IV, for general anesthesia with romifidine (Sedivet; Boehringer, Burlington, Ontario), 0.1 mg/kg BW; butorphanol tartate (Torbugesic), 0.1 mg/kg BW, guaifenesin (Guaifenesin; Rhodia, Mississauga, Ontario), 100 mg; ketamine (Ketalean; Sabex, Boucherville, Quebec), 2.0 mg/kg BW; and diazepam (Diazepam; Sabex, Boucherville, Quebec), 0.08 mg/kg BW; anesthesia was maintained using isoflurane inhalant on a circle rebreathing unit.
The left EAC was explored by using an arthroscope (Stryker 0°; Stryker Corporation, Santa Clara, California, USA), and the polyp was removed by traction-avulsion with arthroscopic rongeurs. The removed mass was placed in 10% buffered formalin and processed routinely for histologic sectioning.
The avulsion site was left to heal by 2nd intention. Mucosal bleeding from the avulsion site obscured visualization of the EAC after the procedure; therefore, the EAC was packed with gauze and scheduled for subsequent examination. Two days later, the filly was induced for general anesthesia to reexamine the surgical site. The EAC was now fully visible, and no gross abnormalities could be seen around the avulsion site. An epithelial margin was visible around the avulsion site, which was partially covered by a scab. The tympanic membrane appeared intact and without any signs of perforation or the presence of abnormal tissue.
Microscopic examination of the prepared sections (stained with hematoxylin and eosin) of the polyp revealed edematous fibrous tissue with abundant angiogenesis, covered by stratified squamous epithelium. The epithelium was extensively ulcerated, with associated hemorrhage, necrosis, fibrin exudation, and active suppurative inflammation. There was no evidence of neoplasia in the sections examined.
A diagnosis of inflammatory aural polyp was made, based on the clinical and histological findings described above.
The otorrhea and head rubbing resolved after polypectomy. However, the horse was treated with antibiotics; trimethoprim sulfmethoxazole (Apo-sulfatrim), 30 mg/kg BW, PO, q12h for another 3 wk after the procedure. The referring veterinarian observed no further signs of otorrhea over the next 2 mo.
Discussion
Any lesion of the EAC may present as a polypoid mass, which may obscure the lumen, protrude through the tympanic membrane, or both (1). Histologic evaluation is necessary to differentiate simple inflammatory polyps, as seen in this case, from other underlying disease processes or neoplasia. Inflammatory aural polyps are one of the most common EAC masses in cats (2), whereas EAC malignancies are rare in domestic animals (3,4). To the authors’ knowledge, there are no previous reports of either EAC malignancies or aural polyps in the horse. Nasopharyngeal polyps are described, however, and are thought to arise secondarily to chronic inflammation and infection (5,6).
The etiology of inflammatory aural polyps is unclear. In cats, ascending infection from the nasopharynx has been suggested (2), as concurrent bacterial infection is frequently observed. It is still uncertain, however, whether the concurrent infection is secondary to the polyp or a primary factor. Congenital origin, chronic upper airway inflammation, and chronic otitis media have also been proposed as possible causes for feline aural polyps (2).
Clinical signs with aural polyps depend on the structures affected. In cats, respiratory stridor, nasal discharge, sneezing, dyspnoea, and dysphasia are commonly seen with involvement of the nasopharynx, whereas Horner’s syndrome, head tilt, nystagmus, and ataxia may be present with polyps originating from the middle ear (2). When only the EAC is affected, otorrhea and otitis externa dominate as presenting signs (2). In this case, there were no clinical or radiographic signs suggestive of involvement of either the middle ear or the nasopharynx.
Radiographic evaluation of the equine tympanic bullae can be challenging because of location. A ventrodorsal view is recommended, since it will allow each bulla to be seen as a separate structure and minimize superimposition of the skull. Evidence of middle ear disease may be seen as increased radio-opacity, enlargement of the bullae, or both (7). In the presence of a dentigerous cyst, dental elements (dentin, cement, or enamel) may be identified radiographically. However, radiographs can be inconclusive, and a contrast fistulogram may be necessary. As no clinical signs supported a dentigerous cyst in this case, further radiographic evaluation was not attempted.
The characteristic histological appearance of an inflammatory polyp is that of a fibrovascular stroma infiltrated with inflammatory cells and covered with ulcerated squamous epithelium (2). Presence of ciliated columnar epithelium suggests middle ear origin (8). Based on the histologic findings in this case, the diagnosis of an inflammatory aural polyp, most likely of external auditory canal origin was made.
In cats, simple polypectomy by traction-avulsion through the ear canal is proposed as a first line therapy when there is no evidence of middle ear involvement (9). The prognosis is excellent with complete removal of the polyp (2). Long-term follow-up is not currently available in this case; however, based on reports in other species, recurrence is a potential complication. CVJ
References
- 1.Friedmann I. Pathological lesions of the external auditory meatus: a review. J R Soc Med. 1990;83:34–37. doi: 10.1177/014107689008300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DM, Robinson RK, White RAS. Management of inflammatory polyps in 37 cats. Vet Rec. 2000;147:684–687. [PubMed] [Google Scholar]
- 3.Fan TM, Lorimier LP. Inflammatory polyps and aural neoplasia. Vet Clin North Am Small Anim Pract. 2004;34:489–509. doi: 10.1016/j.cvsm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 4.London CA, Dubilzeg RR, Vail DM, et al. Evaluation of dogs and cats with tumors of the ear canal: 145 cases (1978–1992) J Am Vet Med Assoc. 1996;208:1413–1418. [PubMed] [Google Scholar]
- 5.Head KW, Dixon PM. Equine nasal and paranasal sinus tumours. Part 1: Review of the literature and tumour classification. Vet J. 1999;157:261–278. doi: 10.1053/tvjl.1998.0370. [DOI] [PubMed] [Google Scholar]
- 6.Tremaine WH, Clarke CJ, Dixon PM. Histopathological findings in equine sinonasal disorders. Equine Vet J. 1999;31:296–303. doi: 10.1111/j.2042-3306.1999.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 7.Power H, Wahrens B, de Lahunta A. Facial and vestibulocochlear nerve disease in 6 horses. J Am Vet Med Assoc. 1983;183:1076–1080. [PubMed] [Google Scholar]
- 8.van der Gaag I. The pathology of the external ear canal in dogs and cats. Vet Q. 1986;8:307–317. doi: 10.1080/01652176.1986.9694061. [DOI] [PubMed] [Google Scholar]
- 9.Veir JK, Lappin MR, Foley JE, Getzy DM. Feline inflammatory poyps: historical, clinical, and PCR findings for feline calici virus and feline herpes virus-1 in 28 cases. J Feline Med Surg. 2002;4:195–199. doi: 10.1053/jfms.2002.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]


