Abstract
From 1991 to 2003, 24 cases of larval cyathostominosis were diagnosed at postmortem in equids, 15 (63%) from 2001 to 2003. Cases occurred from September to May, the majority from October to December. Median age was 12 mo. Diarrhea, colic, and weight loss were common clinical signs. Hypoalbuminemia and microcytosis were consistent findings.
Résumé
Cyathostominose larvaire chez des chevaux en Ontario: une maladie émergente? De 1991 à 2003, 24 cas de cyathostominose larvaire ont été diagnostiqués à l’examen post mortem chez des équidés dont 15 (63 %) entre 2001 et 2003. Les cas étaient rapportés entre septembre et mai, le pic survenant entre octobre et décembre. L’âge médian était de 12 mois. Les principaux signes cliniques consistaient en diarrhée, colique et perte de poids alors que l’hypoalbuminémie et la microcytose étaient des trouvailles constantes.
(Traduit par Docteur André Blouin)
Strongyle parasites are commonly found in the large intestine of horses and can cause disease that ranges from ill-thrift to sudden death. As a result, these parasites are the primary reason that horses at pasture should be maintained on a regular deworming program. Until approximately 20 y ago, the large strongyles (particularly Strongylus vulgaris) were considered the most important strongyles. However, subsequent to the introduction of ivermectin, large strongyles became relatively uncommon. In contrast, cyathostomes (small strongyles) are now considered the most important parasite of horses and produce almost all the strongyle eggs found in the feces of horses (1). Cyathostomes are acquired by horses within pasture and, upon ingestion, initially undergo a period of arrested development in the mucosa, submucosa, or both, of the large intestine and cecum that lasts weeks to months. Thereafter, they enlarge significantly in size, often forming an “encysted” structure, and eventually emerge into the lumen of the gut to mature. Disease is primarily due to development of the larval stages in the wall of the intestine, as this typically initiates an inflammatory protein-losing enteropathy and alterations in intestinal motility (1). Development of low numbers of cyathostome larvae does not appear to have an effect on horse health. However, synchronous emergence of large numbers (millions) of immature parasites may be associated with significant disease, termed larval cyathostominosis.
Examination of postmortem records of the Animal Health Laboratory, University of Guelph, from 1991 to 2003 inclusive, identified 24 cases of equids with larval cyathostominosis. These had been submitted for postmortem examination from the Ontario Veterinary College-Veterinary Teaching Hospital (OVC-VTH) (n = 10) and external practitioners (n = 14). One case was diagnosed from 1991 to 1995, 8 cases were diagnosed from 1996 to 2000, and 15 cases were diagnosed from 2001 to 2003. Larval cyathostome infestation was determined to be the primary cause of clinical illness in 19 of the 24 (79%) cases. In the other 5, horses had been submitted to postmortem for diseases other than larval cyathostominosis (hepatitis [n = 2], septicemia [n = 1], cecocolic intussusception [n = 1], degenerative leukoencephalopathy [n = 1]). Nonetheless, these animals had clinical signs consistent with larval cyathostominosis (diarrhea [n = 2], weight loss [n = 2], diarrhea and weight loss [n = 1]). A variety of breeds were affected, including Belgian, quarterhorse, Thoroughbred, standardbred, Clydesdale, Appaloosa, Welsh Pony, and a donkey. Cases occurred from September to May. However, 14 out of 24 (58%) occurred from October to December. The median age was 12 mo (range = 4 mo to 18 y).
Diarrhea (n = 12), colic (n = 8), and weight loss (n = 8) were common clinical signs; 1 horse was found dead without premonitory signs. Gross lesions included colonic mucosal congestion (n = 10), colonic mucosal miliary to 1-cm nodules (n = 8), colonic erosions/ulcers (n = 6), and colonic edema (n = 4). Parasites compatible with cyathostomes were observed grossly in the colonic mucosa or lumen in 6 (25%) cases (Figures 1 and 2). No macroscopic lesions were observed in 4 cases. Large numbers of cyathostome (nematode) larvae, encysted within the colonic mucosa, were consistently seen microscopically, usually accompanied by edema and infiltrates of lymphocytes, eosinophils, plasma cells, and macrophages in various combinations.
Figure 1.
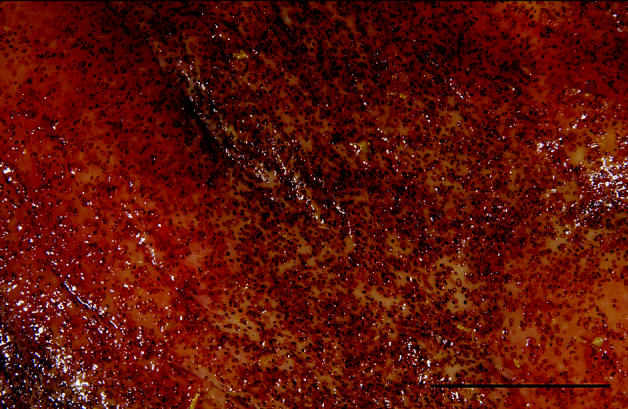
Gross appearance of the colonic mucosa of a horse with larval cyathostominosis (bar = 3 cm). Multiple brown-toblack foci contain cyathostome larvae.
Figure 2.
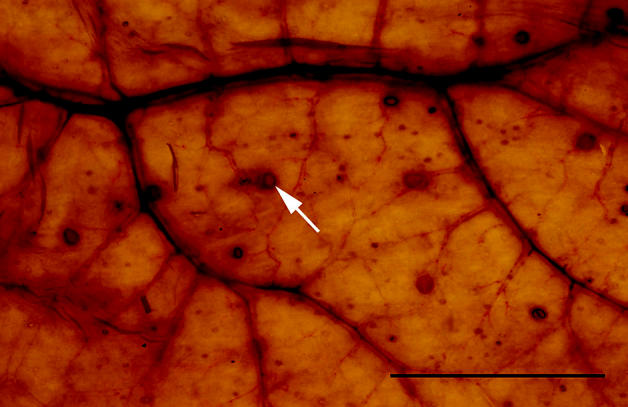
Colonic wall of a horse with larval cyathostominosis as observed by transillumination (bar = 5 cm). Arrow indicates 1 intramucosal cyathostome larva.
Larval cyathostominosis is associated with the sudden development of large numbers of cyathostome larvae in the wall of the large intestine, cecum, or both. The disease is relatively common in many parts of Europe and has a seasonal occurrence there that is similar to that of the cases reported here, except that there most cases are seen in the late winter and spring (1). The reason for the temporal difference is not known. Overall, the disease is most commonly observed in 1- to 3-year-old horses and can occur in animals despite a regular deworming program (2). Clinical signs that are typically associated with larval cyathostominosis include weight loss, weakness, acute or chronic diarrhea (not present in all cases), pyrexia, subcutaneous edema, and colic. Animals often have a normal appetite and water intake (3–5). Antemortem diagnosis of larval cyathostominosis can be challenging because of the nonspecific clinical signs. Thus, depending on the clinical presentation, salmonellosis, nonsteroidal anti-inflammatory drug-induced colitis, intestinal lymphosarcoma, inflammatory bowel disease, Lawsonia intracellularis infection (weanlings), Clostridium difficile enteritis, C. perfringens enteritis, and other causes of acute enteritis are differential diagnoses. In nondiarrheic animals, other causes of ill-thrift and hypoproteinemia (small intestinal protein losing disease, renal disease, peritonitis, pleuritis, chronic disease, and malnutrition) should be explored. Definitive diagnosis is not often possible; however, a presumptive diagnosis can be obtained based on clinical signs, signalment, and exclusion of other possible causes. In some cases, 4th stage larvae, adult cyathostomes, or both, may be found in feces, giving support to a suspicion of larval cyathostominosis in clinically affected animals. Fecal egg counts are often negative. A complete blood cell count and serum biochemical profile should be performed in all cases. All horses assessed biochemically in this study (n = 6) had severely decreased levels of serum albumin (range, 11 to 23 g/L; median, 15.5 g/L; reference range, 30 to 37 g/L) and all but 1 had decreased levels of total protein (range, 27 to 53 g/L; median, 39 g/L; reference range, 54 to 75 g/L). These changes could not be attributed to other factors in the cases that were evaluated; age would not have affected the results (6). Hematological findings typical of young horses consist of microcytosis relative to adult values, which corresponds to a period of physiological iron deficiency, and should resolve by 9 mo of age (7). Of note, all of the horses assessed hematologically in this study (n = 6) had severe microcytosis (range, 31 to 34 fL; reference range, 38 to 53 fL), despite the fact that 2 were 9 mo of age or older. Systemic inflammation was evident from increased serum fibrinogen concentrations in the 6 horses (range, 2.9 to 7.7 g/L; median, 4.6 g/L; reference range, 1.6 to 2.9 g/L), and neutrophilia in 4 of the 6 animals (range, 7.9 to 56.8 × 109/L; median, 19.5 × 109/L; reference range, 2.8 to 7.7 × 109/L). The hypoalbuminemia and microcytosis were consistent with an inflammatory process involving external blood loss, such as widespread intestinal mucosal damage. Severe hypoalbuminemia in horses most commonly results from excessive intestinal or renal protein loss. Significant proteinuria in horses is rare and readily detected on urinalysis. Intestinal protein loss may be due to bleeding from mucosal ulceration associated with neoplastic infiltrates, or protein exudation associated with intestinal inflammation, most commonly due to intestinal parasitism. The latter is more common in young horses and should be considered among the differential diagnoses in horses with severe hypoalbuminemia, especially if there is concurrent microcytosis.
Treatment of larval cyathostominosis is frequently unrewarding. Only 2 drugs have efficacy against encysted cyathostomes: moxidectin at 400 μg/kg body weight (BW), PO (5), and fenbendazole at either 7.5 or 10 mg/kg BW, PO, q24h for 5 d (5,8). Supportive care including IV fluid therapy, oncotic support (plasma, synthetic colloids), and nutritional support may be required. In addition, since significant inflammation may be present in the intestinal wall, treatment with anti-inflammatory doses of corticosteroids may be useful, especially if animals are significantly hypoalbuminemic at the time of treatment; prednisolone (0.5 to 1.0 mg/kg BW, PO, q24h or q12h) or dexamethasone (0.02 to 0.05 mg/kg BW, IV, IM, or PO, q24 to 48h) can be used. Some authors have suggested that multiple anthelmintic treatments, at 10- to 30-day intervals, may be more beneficial than a single treatment regimen (9). Even so, prognosis for survival is often guarded to poor. For example, in Europe, up to 50% of animals with severe inflammatory colitis, associated with sudden onset weight loss, edema, diarrhea, or any combination, may fail to recover despite intensive treatment (1,9).
Over the last few years, there has been a marked increase in the number of cases of larval cyathostominosis diagnosed at the Animal Health Laboratory. Whether this reflects a true increase in the prevalence of the disease, potentially due to drug resistance in cyathostomes (10), is unclear and currently under investigation. The association with drug resistance appears likely, since recent work has demonstrated resistance to fenbendazole on more than 95% of examined farms in the southern USA (11). In addition, resistance to pyrantel was demonstrated on 41% of the same farms. It therefore appears that drug resistance in cyathostomes is highly prevalent in this region of North America and, by comparison with earlier work, is rapidly worsening (11). Although resistance to ivermectin has not been demonstrated in cyathostomes, and ivermectin is commonly used for deworming horses, the spread of resistance to benzimidazoles and pyrantel is perhaps not surprising, as ivermectin does not eliminate immature cyathostomes in the wall of the intestine. Thus, in light of the large movement of horses, and the parasites they harbor, between Ontario and the southern USA, it is likely that cyathostomes with a multidrug-resistance phenotype will establish in Ontario. Recent work has demonstrated the occurrence of fenbendazole- and pyrantel-resistant cyathostomes on a farm in Ontario (Peregrine, unpublished observations). However, the farm-level prevalence of such cyathostomes in the province has yet to be determined.
At present, there is no effective way of determining the burden of larval cyathostomes in the wall of the large intestine of horses antemortem, apart from the collection of surgical biopsies. However, surgical biopsies are impractical in most cases because of the cost and hesitancy to perform surgery on compromised, hypoproteinemic, and potentially diarrheic horses.
Since the occurrence of larval cyathostominosis appears, at least in part, to be associated with ingestion of pasture that is heavily contaminated with cyathostome larvae, preventive measures that minimize pasture burdens of cyathostomes should be adopted. If stocking density can not be reduced to approximately 1 horse per 2 acres, removal of feces from pasture at least twice weekly is an effective way to greatly reduce cyathostome burdens. In addition, fecal samples should be examined annually, ideally during July or August, to monitor the efficacy of anthelmintic regimens, by performing quantitative egg counts at the time of treatment and again 10 to 14 d later (11). Thereafter, only anthelmintics with proven efficacy should be used on a farm. Additional strategies to minimize the rate of development of anthelmintic resistance include annual rotation of the drug class used for cyathostome control (a slow rotation deworming program), decreasing the frequency of treatments, using a strategic deworming program for horses that reside permanently in Canada, and correct dosing (12).
Acknowledgments
Drs. Peter Lusis, Erin Southorn, Noel Harrington, and Gary Thomson are thanked for contributing case material to this study. CVJ
Footnotes
Reprints will not be available from the authors.
References
- 1.Love S, Murphy D, Mellor D. Pathogenicity of cyathostome infection. Vet Parasitol. 1999;85:113–122. doi: 10.1016/s0304-4017(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 2.Lyons ET, Drudge JH, Tolliver SC. Larval cyathostomiasis. Vet Clin North Am Equine Pract. 2000;16:501–513. doi: 10.1016/s0749-0739(17)30092-5. [DOI] [PubMed] [Google Scholar]
- 3.Uhlinger C. Effects of three anthelmintic schedules on the incidence of colic in horses. Equine Vet J. 1990;22:251–254. doi: 10.1111/j.2042-3306.1990.tb04263.x. [DOI] [PubMed] [Google Scholar]
- 4.Love S. Parasite-associated equine diarrhea. Compend Contin Educ Pract Vet. 1992;14:642–649. [Google Scholar]
- 5.Paul JW. Equine larval cyathostomosis. Compend Contin Educ Pract Vet. 1998;20:509–514. [Google Scholar]
- 6.Bauer JE, Asquith RL, Kivipelto J. Serum biochemical indicators of liver function in neonatal foals. Am J Vet Res. 1989;50:2037–2041. [PubMed] [Google Scholar]
- 7.Harvey JW, Asquith RL, Sussman WA, Kivipelto J. Serum ferritin, serum iron, and erythrocyte values in foals. Am J Vet Res. 1987;48:1348–1352. [PubMed] [Google Scholar]
- 8.Duncan JL, Bairden K, Abbott EM. Elimination of mucosal cyathostome larvae by five daily treatments with fenbendazole. Vet Rec. 1998;142:268–271. doi: 10.1136/vr.142.11.268. [DOI] [PubMed] [Google Scholar]
- 9.Love S, McKeand JB. Cyathostomosis: practical issues of treatment and control. Equine Vet Educ. 1997;9:253–256. [Google Scholar]
- 10.Mair TS, Cripps PJ. Benzimidazole resistance in equine strongyles: association with clinical disease. Vet Rec. 1991;128:613–614. doi: 10.1136/vr.128.26.613. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan RM, Klei TR, Lyons ET, et al. Prevalence of anthelmintic resistant cyathostomes on horse farms. J Am Vet Med Assoc. 2004;225:903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd S, Smith J, Connan RM, et al. Parasite control methods used by horse owners: factors predisposing to the development of anthelmintic resistance in nematodes. Vet Rec. 2000;146:487–492. doi: 10.1136/vr.146.17.487. [DOI] [PubMed] [Google Scholar]


