Abstract
SNAP-25 (25 kDa synaptosome-associated protein) is found in cells that release neurotransmitters and hormones, and plays a central role in the fusion of secretory vesicles with the plasma membrane. SNAP-25 has been shown to interact specifically with syntaxin 1, a 35 kDa membrane protein, to mediate the fusion process. Here, we investigated whether other known syntaxin isoforms found at the plasma membrane can serve as binding partners for SNAP-25 in vivo. In our analysis, we employed rat phaeochromocytoma PC12 cells that are often used as a model of neuronal functions. We now show that these cells contain large amounts of SNAP-25, which interacts not only with syntaxin 1, but also with ubiquitous syntaxins 2, 3 and 4. The plasma membrane syntaxins appear to occupy complementary domains at the plasma membrane. In defined reactions, the ubiquitous plasma membrane syntaxin isoforms, when in binary complexes with SNAP-25, readily bound vesicular synaptobrevin to form SDS-resistant SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) complexes implicated in membrane fusion. However, vesicular synaptotagmin and cytosolic complexin, both implicated in the fusion process, exhibited differential ability to interact with the SNARE complexes formed by syntaxins 1–4, suggesting that the plasma membrane syntaxins may mediate vesicle fusion events with different properties.
Keywords: chromaffin cell, PC12 cell, 25 kDa synaptosome-associated protein (SNAP-25), soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE), syntaxin, tumour
Abbreviations: CCD, charge-coupled device; GST, glutathione S-transferase; SNAP-25, etc., 25 kDa synaptosome-associated protein, etc; (t/v)-SNARE, (target membrane/vesicle-associated) soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor; VAMP, vesicle-associated membrane protein
INTRODUCTION
Numerous transport vesicles fuse with the plasma membrane to mediate many cellular processes, including cell growth, plasma membrane repair, axonal branching, recycling of plasma membrane transporters and release of soluble signalling molecules into the extracellular space. Our understanding of the molecular mechanisms operating in each process, and our ability to interfere with any given process, rely on full definition of fusion components existing at the plasma membrane. The discovery of the general principles of SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor)-mediated membrane fusion [1,2] provides a solid basis for a detailed characterization of the plasma membrane fusion machinery. The generality of the SNARE hypothesis is illustrated by the fact that many fusion events require so-called v (vesicle-associated)-SNAREs on the vesicular membrane, and t (target membrane)-SNAREs on the target membrane. The mammalian v-SNARE family consists of nine members, with synaptobrevin 2, also known as VAMP2 (vesicle-associated membrane protein 2), being the dominant isoform in the brain [2,3]. The t-SNARE machinery has a more complex composition, with two or three components being required in different membrane-trafficking pathways [3,4]. The syntaxin family of t-SNAREs consists of at least 12 gene products in animal cells that are localized to compartments throughout the secretory and endocytic pathways, indicating that these proteins mediate various membrane-trafficking pathways [3]. The three additional t-SNAREs, SNAP-25 (25 kDa synaptosome-associated protein) [5], SNAP-23 [6] and SNAP-29 [7], interact with syntaxin isoforms through combinatorial principles which are not yet fully understood.
In the present study, we sought to investigate potential syntaxin partners for the neuronal SNAP-25. SNAP-25 was initially detected as the most prominent methionine-labelled protein in rapid axonal transport, and originally was known as ‘Superprotein’ [8]. The primary structure of SNAP-25 is predominantly hydrophilic and contains no apparent membrane domains; however, in model neuroendocrine cells, this protein is almost exclusively found at the plasma membrane [5]. SNAP-25 is unstructured in solution, but adopts a highly helical structure upon binding of syntaxin 1 [9]. This syntaxin binding also stimulates SNAP-25 palmitoylation, which may help to anchor SNAP-25 firmly in the plasma membrane [10,11]. In addition to syntaxin 1, which is mainly found in the brain, syntaxins 2, -3 and 4 have been identified as plasma membrane proteins operating in many different cells [3,12]. The ability of these four syntaxins to reside at the plasma membrane is probably due to their longer transmembrane motifs compared with those of syntaxins operating in the Golgi or the endoplasmic reticulum [13].
We recently demonstrated that, in rat phaeochromocytoma PC12 cells, SNAP-25 interacts not only with syntaxin 1, but also exhibits robust binding of syntaxin 2 [14]. Since syntaxins 1–4 have been shown to form complexes with SNAP-25 and synaptobrevin 2 in in vitro reactions [15], and because PC12 cells carry syntaxin 3 and syntaxin 4 [12], it was important to establish the extent of promiscuity for SNAP-25–syntaxin interactions in this widely used neuronal model cell. We now show that the plasma membrane of PC12 cells displays a mosaic of different syntaxin isoforms, all being capable of binding the SNAP-25 molecule. These findings have important implications for recent studies of SNARE-mediated exocytosis, and shall open new avenues for investigation of multiple plasma membrane processes.
EXPERIMENTAL
Plasmids and antibodies
Plasmids encoding GST (glutathione S-transferase) fusion proteins with SNAP-25, syntaxin 1 (amino acids 1–261), syntaxin 2 (amino acids 1–264), syntaxin 3 (amino acids 1–261) and syntaxin 4 (amino acids 1–269) have been described previously [12,14]. Mouse anti-syntaxin-1 (clone HPC-1) and rabbit anti-syntaxin-3 antibodies were from Sigma (Gillingham, Kent, U.K.). Rabbit anti-syntaxin-2, anti-syntaxin-4, anti-complexin-1/2, anti-SNAP-23 and VAMP2 antibodies were from Synaptic Systems (Göttingen, Germany). An additional rabbit anti-SNAP-23 antibody was from Sigma, U.K. Mouse anti-SNAP-25 (clone SMI81) was from Sternberger Monoclonals (Lutherville, MD, U.S.A.). Rabbit anti-SNAP-25, anti-syntaxin-1 and anti-synaptotagmin-1 antibodies, produced in our laboratory, have been described previously [16].
Tissue culture and immunofluorescent staining
The rat phaeochromocytoma cell line, PC12, obtained from A.T.C.C. (CRL-1721), was cultured in collagen-coated Biocoat flasks (BD Biosciences, Oxford, U.K.) or on Vitrogen 100 (Cohesion Technologies, Palo Alto, CA, U.S.A.)-coated coverslips in DMEM (Dulbecco's modified Eagle's medium; Invitrogen, Paisley, Scotland, U.K.) containing 10% (v/v) horse serum (Invitrogen, Paisley, U.K.) and 5% (v/v) fetal-calf serum (PAA Laboratories, Teddington, London, U.K.) at 37 °C in a humidified atmosphere of 10% CO2. Cells were fixed with 4% (w/v) paraformaldehyde for 30 min, treated with 0.1% (v/v) Triton X-100 in PBS for 2 min, and then incubated for 30 min in PBS containing 2% BSA. The fixed cells were treated with a primary antibody for 90 min, followed by a 30 min incubation with Alexa-conjugated secondary antibodies (Molecular Probes). The bound immunofluorescence was observed on a Radiance Confocal system (Zeiss/Bio-Rad; Hemel Hempstead, Herts., U.K.) linked to a Nikon Eclipse fluorescence microscope equipped with an oil-immersion objective (100×; 1.4 numerical aperture). An argon/krypton mixed laser was used in combination with a 488 nm band pass filter (excitation) and a 585 nm long pass filter (emission) to examine Alexa 488 fluorescence. A 647 nm band pass filter (excitation) and a 660 nm long pass filter (emission) were used for Alexa 647 detection.
Protein preparation and binding reactions
Recombinant SNARE proteins were produced in BL21 Escherichia coli, and purified on glutathione–Sepharose beads (Amersham Biosciences). Proteins were washed with buffer A [20 mM Hepes/NaOH (pH 7.3)/100 mM NaCl/2 mM EDTA] and eluted either with 15 mM reduced glutathione (GSH) in buffer A or using thrombin cleavage [17]. Eluted proteins were purified further by gel filtration on a Superdex 200 column (Amersham Biosciences) equilibrated in buffer A. For binding reactions, 3 μg of GST–syntaxin 1–4 were immobilized on glutathione–Sepharose beads. Following washing in buffer A containing either 0.1% Triton X-100 or 0.8% (w/v) n-octylglucoside, the immobilized GST–syntaxin was incubated for 30 min at 24 °C with 10 μg of SNAP-25 and 5 μg of synaptobrevin 2 (amino acids 1–96) in a reaction volume of 100 μl. The beads were washed three times with buffer A in the presence of 0.1% Triton X-100 by low-speed centrifugation, and bound protein was eluted into SDS-containing sample buffer. Proteins were separated by SDS/PAGE and stained with either Sypro Orange or Coomassie staining. To analyse formation of the SDS-resistant SNARE complexes, the cytoplasmic domains of syntaxins were incubated with SNAP-25 and synaptobrevin 2 in buffer A for 30 min at 24 °C in the presence of 0.8% (w/v) n-octylglucoside. The reactions were stopped by the addition of SDS-containing sample buffer, and analysed by SDS/PAGE and Coomassie staining.
Anti-SNAP-25 affinity pull-down, MS (mass spectrometry) analysis and Western immunoblotting
PC12 cell extract was prepared as described previously [14]. Sepharose beads with covalently attached anti-SNAP-25 antibody (clone SMI81; Sternberger Monoclonals) were incubated with the cell extract for 1 h at 4 °C, and then extensively washed in buffer A containing 0.1% Triton X-100. Protein eluted into SDS-containing sample buffer was separated by SDS/PAGE and visualized by Coomassie staining. Peptides of in-gel trypsin-digested protein bands were separated by liquid chromatography on a reversed-phase C18 column (150 mm×0.075 mm internal diameter; flow rate 0.15 ml/min). The eluate was introduced directly into a Q-STAR hybrid tandem mass spectrometer (MDS Sciex, Concord, ON, Canada). The spectra were analysed against an NCBI non-redundant database with MASCOT MS/MS Ions searching (http://www.matrixscience.com).
For Western immunoblotting, cell extracts from PC12 cells, COS7 cells and chromaffin cells were prepared in buffer A containing 1% Triton X-100 and the Complete™ protease inhibitors mix (Roche, Welwyn Garden City, U.K.). Detergent extracts from other cell lines, organs and brain parts were from GenoTech (St Louis, MO, U.S.A.). Before Western immunoblotting, all samples were normalized by protein content. Protein samples (30 μg per lane) were separated on 12% Ready gels (Bio-Rad) and transferred to Immobilon-P membranes (Millipore, Billerica, MA, U.S.A.). The membranes were blocked for 30 min with 5% non-fat dried milk in PBS containing 0.2% (v/v) Tween 20, followed by incubation for 1 h with primary, and 30 min with secondary, antibodies. Chemiluminescence was developed using the West Dura kit (Pierce, Tattenhall, Cheshire, U.K.), and was imaged on the ChemiDoc XRS equipped with a 12-bit CCD (charge-coupled device) camera (Bio-Rad). Quantification of the signals was performed using the Quantity One software (Bio-Rad).
RESULTS
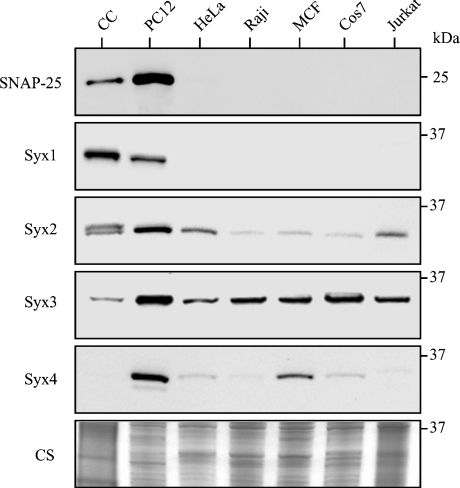
To determine the relationship between SNAP-25 and the plasma membrane syntaxins 1–4, we performed immunoblotting analysis of mature chromaffin cells from adrenal glands as well as adrenal phaeochromocytoma PC12 cells, both being widely used to study neuroendocrine exocytosis [18]. As a control, several non-neuronal cell lines (lymphoma Raji and Jurkat, cervical HeLa, breast MCF and kidney Cos7 cells) were also tested. The Western immunoblot analysis of the normalized protein samples (Figure 1) allowed us to make several conclusions. First, as expected, only PC12 cells and chromaffin cells, both secreting catecholamines in a regulated manner, contain SNAP-25. Secondly, PC12 cells carry six times more SNAP-25 than chromaffin cells, as estimated by the immunoblotting signals quantified using a cooled 12-bit CCD camera (results not shown). Thirdly, plasma membrane syntaxins 2–4 exhibited the highest expression in PC12 cells, whereas syntaxin 1 was specifically enriched in chromaffin cells. It is worth noting that mature chromaffin cells specialize in catecholamine secretion, whereas other cell lines under investigation are all of tumour origin. Interestingly, only one syntaxin isoform tested here was strongly expressed in all tumour cells: syntaxin 3. Together, the Western immunoblotting analysis indicates that PC12 cells behave, with regards to its plasma membrane syntaxins, as a hybrid of neuroendocrine and tumour cells.
Figure 1. Neuroendocrine PC12 cells display the full complement of plasma membrane syntaxins as well as enrichment of SNAP-25, in comparison with mature chromaffin cells from the adrenal gland.
A Western immunoblot using anti-SNAP-25 and anti-syntaxin (Syx) antibodies of chromaffin cells (CC), and six tumour cell lines (PC12, HeLa, Raji, MCF, COS7 and Jurkat) is shown. Normalization of total cell protein (30 μg per lane) was verified by Coomassie staining (CS). Positions of molecular-mass markers (in kDa) are shown on the right of the gels.
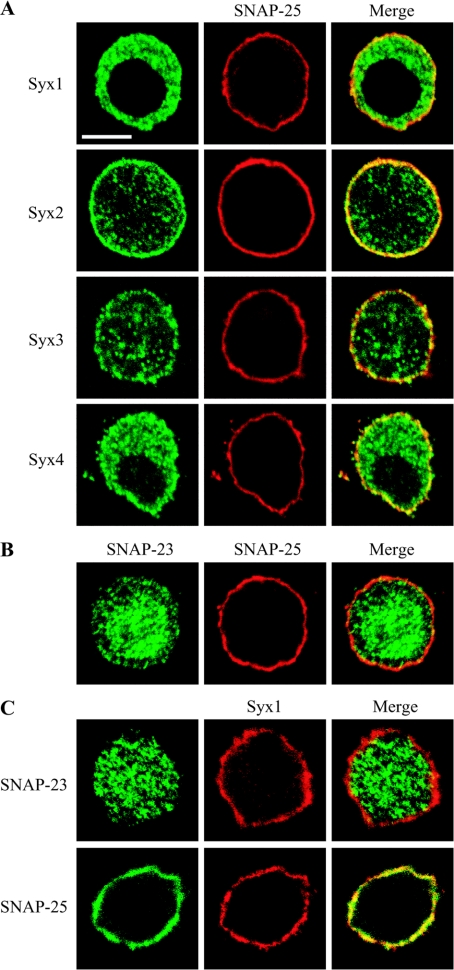
The abundance of SNAP-25 in PC12 cells was unexpected, and raised the possibility that the increased presence of SNAP-25 is linked to the higher presence of the plasma membrane syntaxin isoforms, specifically syntaxins 2–4. Therefore we tested the distribution of the t-SNAREs in cells co-immunostained with mouse anti-SNAP-25 and rabbit anti-syntaxin antibodies using confocal fluorescent microscopy (Figure 2A). The four syntaxins, at the plasma membrane in the equatorial sections, were distributed in a punctate manner, with SNAP-25 covering most of the plasma membrane. Each syntaxin isoform could also be identified within the cell interior in vesicular populations. All four syntaxin isoforms partially co-localized with SNAP-25 at the plasma membrane of PC12 cells in the merged images. Since, in non-neuronal cells, syntaxins 2–4 are known to pair with a SNAP-25 homologue, SNAP-23 [6,19,20], we determined the relative endogenous distribution of SNAP-23 and SNAP-25. Interestingly, in PC12 cells, SNAP-23 was more abundant in the cell interior, whereas SNAP-25 predominated at the plasma membrane level (Figure 2B).
Figure 2. Distribution of endogenous syntaxins 1–4, SNAP-25 and SNAP-23 in PC12 cells.
(A) Cells were co-immunostained using mouse anti-SNAP-25 and rabbit anti-syntaxin-1–4 (Syx1–Syx4) (B) Co-immunostaining of PC12 cells using mouse anti-SNAP-25 and rabbit SNAP-23. (C) Cells were co-immunostained using HPC-1 monoclonal anti-syntaxin-1 antibody and either anti-SNAP-23 or anti-SNAP-25 antibodies. Scale bar, 10 μm.
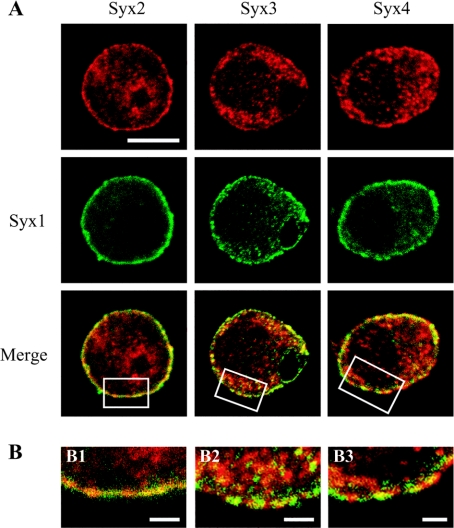
Next, we analysed distribution of t-SNAREs relative to syntaxin 1 by employing a monoclonal anti-syntaxin-1 antibody (clone HPC-1) that specifically binds its active, plasma membrane form [16]. This HPC-1 monoclonal antibody cannot recognize syntaxin 1, which is in association with a large cytosolic protein, Munc18, also known as nSec1 [21]. Co-staining of the plasma membrane form of syntaxin 1 and SNAP-23 again demonstrated the intracellular localization of the latter t-SNARE (Figure 2C). In contrast, SNAP-25 exhibited a high degree of co-localization with the plasma membrane syntaxin 1 (Figure 2C). Furthermore, co-immunostaining using the HPC-1 monoclonal antibody and the rabbit anti-syntaxin-2–4 antibodies revealed a segregation of syntaxin 1 from the ubiquitous syntaxins (Figure 3A), forming a patchwork at the plasma membrane (Figure 3B). Therefore it was possible that multiple syntaxins could account for the plasma membrane SNAP-25 staining in PC12 cells, provided that SNAP-25 could indeed interact with all four plasma membrane syntaxins (Figure 2).
Figure 3. Syntaxins 1–4 can occupy distinct patches of the plasma membrane of PC12 cells.
Cells were co-immunostained using mouse anti-syntaxin-1 (Syx1) and rabbit anti-syntaxin-2–4 (Syx2–Syx4). The rectangular areas shown in the bottom panels in (A) are magnified in the respective panels in (B) [scale bar in (A), upper left panel, 10 μm; scale bars in (B), 1 μm].
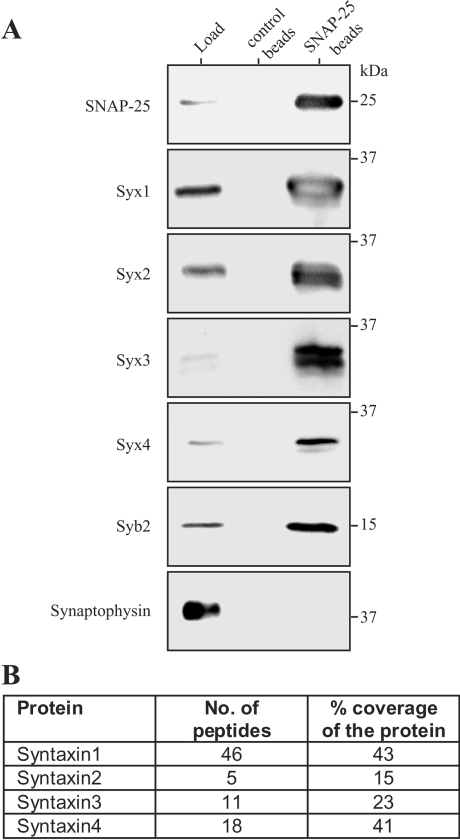
To address the physical relationship between SNAP-25 and the plasma membrane syntaxins, we immunoprecipitated SNAP-25 from PC12 cell extracts using a monoclonal antibody covalently attached to Sepharose beads [22]. Western immunoblotting of the bound material revealed that all four plasma membrane syntaxin isoforms were strongly enriched in the pull-down material (Figure 4A), indicating a direct molecular link between SNAP-25 and the plasma membrane syntaxins in these cells. The SNAP-25 antibody beads did not bring down a control protein, synaptophysin, demonstrating the specificity of the syntaxin–SNAP-25 interaction. The bound material was also analysed by liquid chromatography–tandem MS (Figure 4B and Supplementary Table 1; http://www.BiochemJ.org/bj/392/bj3920283add.htm), which further confirmed our conclusion about the ability of SNAP-25 to interact with all four plasma membrane syntaxins in neuroendocrine PC12 cells.
Figure 4. SNAP-25 interacts in vivo with all four plasma membrane syntaxin isoforms.
(A) Immunoprecipitation experiments of PC12 cell extracts using either control Sepharose beads or Sepharose beads with a covalently attached monoclonal anti-SNAP-25 antibody are shown. A fraction of the load (one-hundredth) and of the pull-down material (one-tenth) was probed using rabbit antibodies against the indicated proteins. (B) MS analysis of the anti-SNAP-25 pull-down material reveals partial peptide coverage for all four plasma membrane syntaxin isoforms.
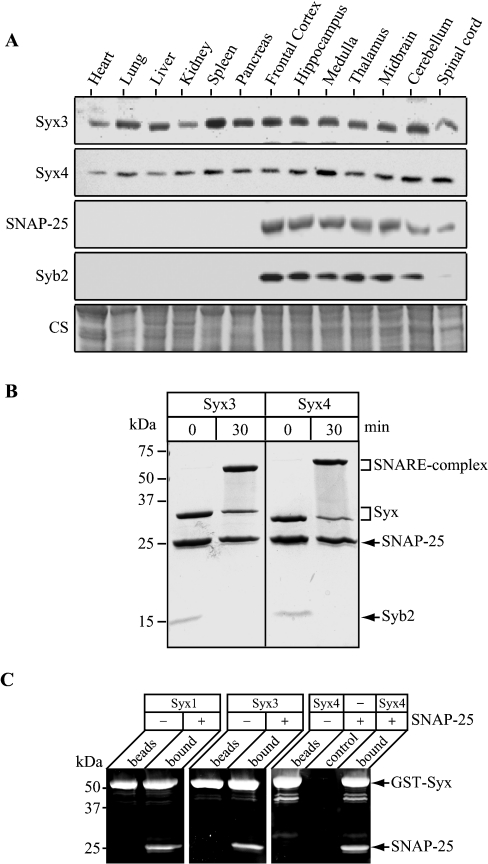
The ability of syntaxins 3 and 4 to bind SNAP-25 was surprising, and led us to analyse whether these isoforms are present in the brain. Immunoblotting of protein samples from various rat organs and parts of the brain demonstrated that the ubiquitous syntaxins are as abundant in brain tissues as in other organs, with syntaxin 4 exhibiting some enrichment in the medulla, the cerebellum and the spinal cord (Figure 5A). If SNAP-25 interacts promiscuously with the four syntaxins, can these ubiquitous syntaxins form the SDS-resistant complex implicated in fusion of synaptic vesicles [23,24]? Addition of synaptobrevin 2 to a solution of SNAP-25 containing either syntaxin 3 or syntaxin 4 resulted in the complete consumption of the v-SNARE into the SDS-resistant SNARE complexes (Figure 5B). Since synaptobrevin may have artificially enhanced pairing of syntaxins with SNAP-25, we analysed the ability of syntaxin 3 or syntaxin 4 to bind SNAP-25 in binary reactions. GST–syntaxin 3 and GST–syntaxin 4, immobilized on glutathione–Sepharose beads, were incubated with SNAP-25, and the bound material was analysed by SDS/PAGE followed by Sypro Orange staining of the gel. Both syntaxin 3 and syntaxin 4 were able to interact with SNAP-25, similar to syntaxin 1 (Figure 5C) as quantified by bound fluorescence (results not shown).
Figure 5. Syntaxins 3 and 4 are present in the brain, and are capable of in vitro interaction with both SNAP-25 and synaptobrevin 2.
(A) Western immunoblot analysis of rat internal organs and different parts of brain. Protein normalization (30 μg per lane) was verified by Coomassie staining (CS). (B) Recombinant syntaxins 3 and 4 form SDS-resistant SNARE complexes upon addition of SNAP-25 and synaptobrevin 2 (syb2) in a 30 min reaction at 24 °C. Coomassie stained gel. (C) Syntaxins 3 and 4 (Syx3 and Syx4) are as capable of direct binding to SNAP-25 as syntaxin 1. Immobilized GST fusion proteins of syntaxins were incubated with SNAP-25 for 30 min and, after extensive washing, were analysed by SDS/PAGE and Sypro Orange staining. A control reaction was performed using glutathione–Sepharose beads without GST–syntaxin, but in the presence of SNAP-25.
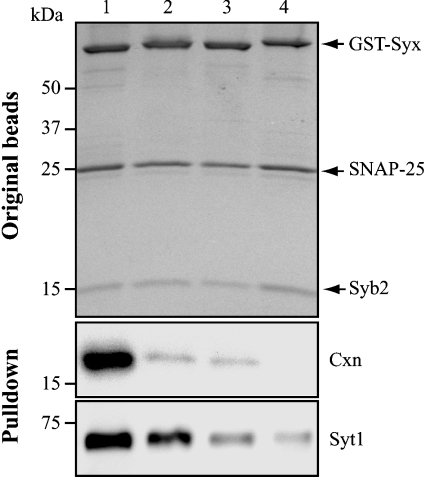
Our results so far have shown that different syntaxins together with SNAP-25 can engage the same v-SNARE protein, synaptobrevin, in a promiscuous manner. We hypothesized that the control of the SNARE fusion reactions may happen not at the level of the SNAREs themselves, but at the level of auxiliary proteins. Among several candidates, the cytosolic complexins 1 and 2 and multiple vesicular synaptotagmins bind SNAREs, and have a profound effect on the fusion reaction [2,25]. Complexins are small cytosolic proteins that bind to the ternary SNARE complex [26] to modulate the fusion reaction [27,28]. We analysed the binding of complexin from a brain detergent extract to the four different SNARE complexes formed using synaptobrevin 2, SNAP-25 and syntaxins 1–4. Native complexin bound differentially to these four SNARE complexes, with syntaxin-1-containing complex showing the strongest binding (Figure 6). Syntaxin-2- and -3-containing complexes, on the other hand, exhibited a very modest binding of the brain complexin, whereas syntaxin 4-containing complex completely failed to interact with complexin. Synaptotagmins are vesicular proteins that probably tag specific vesicles in different trafficking pathways [2,20,25], and are capable of constitutive binding to the syntaxin 1–SNAP-25 heterodimer and the ternary SNARE complex [29]. We therefore tested whether different syntaxin-containing complexes bound differently to synaptotagmin 1, which predominates in brain. Synaptotagmin 1 exhibited differential binding to the four complexes, indicating further that SNARE-associated proteins may discriminate between the four plasma membrane syntaxin isoforms during the SNARE assembly (Figure 6). Synaptophysin, a major vesicular protein, did not bind to any of the SNARE complexes (results not shown), confirming the specificity of the complexin and synaptotagmin interactions.
Figure 6. SNARE complexes formed by syntaxins 1–4, SNAP-25 and synaptobrevin 2 differentially interact with complexin and synaptotagmin 1.
SNAP-25 and synaptobrevin 2 were pre-bound to GST–syntaxin (GST-Syx) beads (upper panel; Coomassie-stained gel). The four SNARE complexes were incubated with rat brain Triton X-100 extract for 1 h at 4 °C. Following washing, the SNARE complexes were immunoblotted using anti-complexin (Cxn) or anti-synaptotagmin-1 (Syt1) antibodies (lower two panels). Syb2, synaptobrevin 2.
DISCUSSION
SNARE-mediated membrane fusion is the final step in all vesicle trafficking pathways [2,30]. Although SNAREs were originally proposed as the main determinants of the specificity of membrane fusion reactions in the cell [30], later studies resulted in the modification of such a hypothesis [4,31]. It is becoming clear that each subcellular compartment can have several SNARE isoforms, and that it is additional auxiliary proteins, such as Rab proteins, Munc18 isoforms, synaptotagmins and complexins, that play an important role in setting up the fusion of a specific vesicle with a given domain in the target membrane. Here, we found that SNAP-25 can form complexes with multiple syntaxins in a single neuroendocrine cell (Figure 4). The identification of these multiple syntaxin -SNAP-25 complexes in PC12 cells is of clear importance, considering that previous mechanistic studies of SNARE-mediated fusion relied on the assumption that syntaxin 1 is the principal interacting partner of SNAP-25 at the plasma membrane. For example, several studies of SNAP-25 function in neuronal cell lines detected SNAP-25 that was not in association with syntaxin 1, leading the authors to propose that a pool of SNAP-25 is present at the plasma in a syntaxin free form [32–34]. However, the ability of SNAP-25 to interact with other isoforms, such as syntaxins 2–4, has not been taken into account.
PC12 cells, being actively growing cells, display more diverse syntaxin content compared with chromaffin cells, which are terminally differentiated and highly specialized secretory cells of the adrenal medulla [18]. Chromaffin cells display, at the plasma membrane, a highly coincident localization of SNAP-25 and the open form of syntaxin 1, recognizable by a specific monoclonal antibody, HPC-1 [16]. In the present study we found that SNAP-25 is more abundant in PC12 cells than in chromaffin cells (Figure 1), with noticeable coverage of the PC12 cell plasma membrane (Figure 2). This correlates well with the presence of additional plasma membrane syntaxins, namely syntaxins 2, 3 and 4 in PC12 cells (Figure 1). All syntaxins, being transmembrane proteins, have to be transported in vesicular structures to their final destination. Therefore the intracellular punctuate staining for all four syntaxins detectable by polyclonal antibodies (Figures 2 and 3) probably reflects intracellular vesicular pools carrying these syntaxins. The ubiquitous syntaxins have previously been shown to interact in non-neuronal cells with SNAP-23 [6]. Our preliminary analysis of endogenous SNAP-23 distribution in PC12 cells demonstrated that this protein localizes mainly to the cell interior, in contrast with SNAP-25. Importantly, a preferential syntaxin binding has been observed previously for SNAP-25 in comparison with SNAP-23, resulting in significant differences in their palmitoylation [35]. It will be interesting to determine whether the preference of syntaxins 1–4 for SNAP-25 at the plasma membrane, in the presence of endogenous SNAP-23, is a common phenomenon, as this may have implications for both neuronal and non-neuronal cells that express SNAP-25, including oocytes [36], gastric parietal cells [37], pituitary cells [38], and pancreatic β-cells [39].
What are the potential functions for multiple SNAP-25-interacting syntaxins? Our search of the literature revealed that the neurotransmitter release may rely on a combination of SNAP-25 with syntaxin 3, rather than syntaxin 1, for example, in ribbon synapses [40]. Furthermore, fast exocytosis during egg fertilization was reported to involve SNAP-25 together with syntaxin 4 [36,41], but a direct association between the native proteins has not been demonstrated previously. The SNARE motifs of syntaxins 3 and 4, but not of syntaxins 6 and 8, can inhibit exocytosis in a secretion assay in ‘cracked’ PC12 cells, suggesting a potential involvement of syntaxins 3 and 4 in catecholamine release [42]. In addition to regulated exocytosis, syntaxins with SNAP-25 have been implicated in various neuronal events, including neurite outgrowth and translocation of glucose transporter vesicles to the plasma membrane [43,44]. Overexpression of SNAP-25 has a stimulating effect on neurite outgrowth, whereas overexpression of syntaxin 1 had no effect on this process [45], indicating that other syntaxins may work together with SNAP-25 during neurite outgrowth. Botulinum toxin C, which proteolyses SNAP-25 and syntaxins 1–3 [46], causes collapse of the growth cones [47], implicating at least one of these syntaxin isoforms in the membrane expansion during neurite outgrowth.
The complete understanding of multiple trafficking pathways requires full definition of the combinatorial complement of SNARE proteins. The identification of multiple SNAP-25-interacting syntaxins at the plasma membrane of PC12 cells now provides a basis for a better assignment of multiple plasma membrane processes to each syntaxin isoform in this neuronal model. It is conceivable that the presence of a given syntaxin on a membrane microdomain contributes to a selection of a particular transport vesicle for eventual fusion. This is supported by the observation that SNARE-interacting proteins can distinguish between different syntaxin isoforms, as illustrated in our study for synaptotagmin and complexin. It will be important to investigate whether different patches of the plasma membrane in neuroendocrine cells exhibit different characteristics of membrane fusion, and whether they are targeted by distinct transport vesicles.
Online data
Acknowledgments
We thank Harvey McMahon for the syntaxins 3 and 4 plasmids and Vesa Olkkonen for the syntaxin 2 plasmid. F.D. is a recipient of an EMBO long-term fellowship.
References
- 1.Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 3.Bock J. B., Matern H. T., Peden A. A., Scheller R. H. A genomic perspective on membrane compartment organization. Nature (London) 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 4.Wendler F., Tooze S. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–611. doi: 10.1034/j.1600-0854.2001.20903.x. [DOI] [PubMed] [Google Scholar]
- 5.Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravichandran V., Chawla A., Roche P. A. Identification of a novel syntaxin and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J. Biol. Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- 7.Steegmaier M., Yang B., Yoo J. S., Huang B., Shen M., Yu S., Luo Y., Scheller R. H. Three novel proteins of the syntaxin/SNAP-25 family. J. Biol. Chem. 1998;273:34171–34179. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- 8.Loewy A., Liu W. S., Baitinger C., Willard M. B. The major 35S-methionine-labeled rapidly transported protein (superprotein) is identical to SNAP-25, a protein of synaptic terminals. J. Neurosci. 1991;11:3412–3421. doi: 10.1523/JNEUROSCI.11-11-03412.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasshauer D., Bruns D., Shen B., Jahn R., Brunger A. T. A structural change occurs upon binding of syntaxin to SNAP-25. J. Biol. Chem. 1997;272:4582–4590. doi: 10.1074/jbc.272.7.4582. [DOI] [PubMed] [Google Scholar]
- 10.Vogel K., Cabaniols J. P., Roche P. A. Targeting of SNAP-25 to membranes is mediated by its association with the target SNARE syntaxin. J. Biol. Chem. 2000;275:2959–2965. doi: 10.1074/jbc.275.4.2959. [DOI] [PubMed] [Google Scholar]
- 11.Veit M. Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem. J. 2000;345:145–151. [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett M. K., Garcia-Arraras J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 13.Watson R. T., Pessin J. E. Transmembrane domain length determines intracellular membrane compartment localization of syntaxins 3, 4, and 5. Am. J. Physiol. Cell. Physiol. 2001;281:C215–C223. doi: 10.1152/ajpcell.2001.281.1.C215. [DOI] [PubMed] [Google Scholar]
- 14.Bajohrs M., Rickman C., Binz T., Davletov B. A molecular basis underlying differences in the toxicity of botulinum serotypes A and E. EMBO Rep. 2004;5:1090–1095. doi: 10.1038/sj.embor.7400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasshauer D., Antonin W., Margittai M., Pabst S., Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 16.Rickman C., Meunier F. A., Binz T., Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J. Biol. Chem. 2004;279:644–651. doi: 10.1074/jbc.M310879200. [DOI] [PubMed] [Google Scholar]
- 17.Rickman C., Archer D. A., Meunier F. A., Craxton M., Fukuda M., Burgoyne R. D., Davletov B. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 2004;279:12574–12579. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne R. D., Morgan A. Secretory granule exocytosis. Physiol. Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 19.Galli T., Zahraoui A., Vaidyanathan V. V., Raposo G., Tian J. M., Karin M., Niemann H., Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao S. K., Huynh C., Proux-Gillardeaux V., Galli T., Andrews N. W. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 21.Rickman C., Davletov B. Arachidonic acid allows SNARE complex formation in the presence of Munc18. Chem. Biol. 2005;12:545–553. doi: 10.1016/j.chembiol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Hu K., Carroll J., Fedorovich S., Rickman C., Sukhodub A., Davletov B. Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature (London) 2002;415:646–650. doi: 10.1038/415646a. [DOI] [PubMed] [Google Scholar]
- 23.Leveque C., Boudier J. A., Takahashi M., Seagar M. Calcium-dependent dissociation of synaptotagmin from synaptic SNARE complexes. J. Neurochem. 2000;74:367–374. doi: 10.1046/j.1471-4159.2000.0740367.x. [DOI] [PubMed] [Google Scholar]
- 24.Tolar L. A., Pallanck L. NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J. Neurosci. 1998;18:10250–10256. doi: 10.1523/JNEUROSCI.18-24-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marqueze B., Berton F., Seagar M. Synaptotagmins in membrane traffic: which vesicles do the tagmins tag? Biochimie. 2000;82:409–420. doi: 10.1016/s0300-9084(00)00220-0. [DOI] [PubMed] [Google Scholar]
- 26.McMahon H. T., Missler M., Li C., Sudhof T. C. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 27.Reim K., Mansour M., Varoqueaux F., McMahon H. T., Sudhof T. C., Brose N., Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 28.Archer D. A., Graham M. E., Burgoyne R. D. Complexin regulates the closure of the fusion pore during regulated vesicle exocytosis. J. Biol. Chem. 2002;277:18249–18252. doi: 10.1074/jbc.C200166200. [DOI] [PubMed] [Google Scholar]
- 29.Rickman C., Davletov B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 2003;278:5501–5504. doi: 10.1074/jbc.C200692200. [DOI] [PubMed] [Google Scholar]
- 30.Rothman J. E. Intracellular membrane fusion. Adv. Second Messenger Phosphoprotein Res. 1994;29:81–96. doi: 10.1016/s1040-7952(06)80008-x. [DOI] [PubMed] [Google Scholar]
- 31.Sannerud R., Saraste J., Goud B. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr. Opin. Cell Biol. 2003;15:438–445. doi: 10.1016/s0955-0674(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 32.Xiao J., Xia Z., Pradhan A., Zhou Q., Liu Y. An immunohistochemical method that distinguishes free from complexed SNAP-25. J. Neurosci. Res. 2004;75:143–151. doi: 10.1002/jnr.10840. [DOI] [PubMed] [Google Scholar]
- 33.Lang T., Margittai M., Holzler H., Jahn R. SNAREs in native plasma membranes are active and readily form core complexes with endogenous and exogenous SNAREs. J. Cell Biol. 2002;158:751–760. doi: 10.1083/jcb.200203088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loranger S. S., Linder M. E. SNAP-25 traffics to the plasma membrane by a syntaxin independent mechanism. J. Biol. Chem. 2002;277:34303–34309. doi: 10.1074/jbc.M202125200. [DOI] [PubMed] [Google Scholar]
- 35.Vogel K., Roche P. A. SNAP-23 and SNAP-25 are palmitoylated in vivo. Biochem. Biophys. Res. Commun. 1999;258:407–410. doi: 10.1006/bbrc.1999.0652. [DOI] [PubMed] [Google Scholar]
- 36.Ikebuchi Y., Masumoto N., Matsuoka T., Yokoi T., Tahara M., Tasaka K., Miyake A., Murata Y. SNAP-25 is essential for cortical granule exocytosis in mouse eggs. Am. J. Physiol. 1998;274:C1496–C1500. doi: 10.1152/ajpcell.1998.274.6.C1496. [DOI] [PubMed] [Google Scholar]
- 37.Peng X. R., Yao X., Chow D. C., Forte J. G., Bennett M. K. Association of syntaxin 3 and vesicle-associated membrane protein (VAMP) with H+/K+-ATPase-containing tubulovesicles in gastric parietal cells. Mol. Biol. Cell. 1997;8:399–407. doi: 10.1091/mbc.8.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsson G., Meister B. Molecular components of the exocytotic machinery in the rat pituitary gland. Endocrinology. 1996;137:5344–5356. doi: 10.1210/endo.137.12.8940356. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler M. B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U., Beaudoin A. R., Bennett M. K., Trimble W. S., Gaisano H. Y. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137:1340–1348. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]
- 40.Morgans C. W., Brandstatter J. H., Kellerman J., Betz H., Wassle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J. Neurosci. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwahashi K., Kuji N., Fujiwara T., Tanaka H., Takahashi J., Inagaki N., Komatsu S., Yamamoto A., Yoshimura Y., Akagawa K. Expression of the exocytotic protein syntaxin in mouse oocytes. Reproduction. 2003;126:73–81. doi: 10.1530/rep.0.1260073. [DOI] [PubMed] [Google Scholar]
- 42.Scales S. J., Chen Y. A., Yoo B. Y., Patel S. M., Doung Y. C., Scheller R. H. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 43.Osen-Sand A., Catsicas M., Staple J. K., Jones K. A., Ayala G., Knowles J., Grenningloh G., Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature (London) 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- 44.Greenlee M., Uemura E., Carpenter S. L., Doyle R. T., Buss J. E. Glucose uptake in PC12 cells: GLUT3 vesicle trafficking and fusion as revealed with a novel GLUT3-GFP fusion protein. J. Neurosci. Res. 2003;73:518–525. doi: 10.1002/jnr.10684. [DOI] [PubMed] [Google Scholar]
- 45.Kimura K., Mizoguchi A., Ide C. Regulation of growth cone extension by SNARE proteins. J. Histochem. Cytochem. 2003;51:429–433. doi: 10.1177/002215540305100404. [DOI] [PubMed] [Google Scholar]
- 46.Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi M., Kozaki S., Terakawa S., Kawano S., Ide C., Komiya Y. Growth cone collapse and inhibition of neurite growth by Botulinum neurotoxin C1: a t-SNARE is involved in axonal growth. J. Cell Biol. 1996;134:205–215. doi: 10.1083/jcb.134.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.