Abstract
Purkinje cell protein-2 (Pcp2 or L7) is highly expressed in cerebellar Purkinje cells and retinal bipolar neurons and interacts with the Gαi/o family of G-proteins. Although the expression pattern of Pcp2 in the developing central nervous system suggests a role in differentiation, its function remains unknown. We established Tet-off inducible expression of Pcp2 in PC12 cells (rat pheochromocytoma cells) to determine whether Pcp2 regulates neuronal differentiation. Utilizing a polyclonal antibody, Pcp2 was localized in the cell body and throughout neurites of differentiated PC12 cells, similar to its localization in cerebellar Purkinje cells. Pcp2 expression in PC12 cells stimulated process formation (5-fold) and NGF (nerve growth factor)-stimulated neurite length (2-fold). Under basal conditions, Pcp2-PC12 cells demonstrated a 5-fold increase in Ras activation relative to non-induced PC12 cells and there was no change in extracellular-signal-regulated kinase 1/2 activity with Pcp2 expression. However, Pcp2 induction led to a >3-fold increase in basal p38 MAPK (mitogen-activated protein kinase) activity and the addition of NGF significantly stimulated both Ras and p38 MAPK in Pcp2-PC12 cells relative to the controls. Pretreatment of Pcp2-PC12 cells with the p38-specific inhibitor SB203580 blocked both the increased neurite formation and NGF-stimulated neurite growth. Pertussis toxin treatment had no effect on neurite growth in control cells, but completely blocked Pcp2-mediated increased neurite growth. Transient transfection of the β-adrenergic receptor kinase C-terminus to prevent signalling through Gβγ in Pcp2-PC12 cells also inhibited the Pcp2-induced phenotype and reduced the Pcp2-stimulated Ras activation. Taken together, these findings demonstrate that Pcp2 induces differentiation in PC12 cells, in part through Gβγ-mediated Ras and p38 MAPK activation and suggest the potential for similar signalling mechanisms in Purkinje cells.
Keywords: differentiation, G-protein regulator, mitogen-activated protein kinase (MAPK), neuron, PC12 cell, Purkinje cell protein-2 (Pcp2)
Abbreviations: AGS3, activator of G-protein signalling; β-ARK, β-adrenergic receptor kinase; Dox, doxycycline; ERK, extracellular-signal-regulated kinase; GFP, green fluorescent protein; GPR, G-protein regulatory; GST, glutathione S-transferase; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; PACAP, pituitary adenylate cyclase-activating peptide; PC12 cells, rat pheochromocytoma cells; pcd, Purkinje cell degeneration; Pcp2, Purkinje cell protein-2; PKA, protein kinase A; RBD, Ras-binding domain; RGS, regulator of G-protein signalling; TB, tissue buffer
INTRODUCTION
Purkinje cell protein-2 (Pcp2; also known as L7) is an abundant Purkinje cell protein whose function remains unknown. Pcp2 was first identified as a gene candidate [1] to account for the ataxia and spontaneous pcd (Purkinje cell degeneration) in autosomal recessive pcd mice [2]. However, subsequent studies demonstrated that Pcp2 was not the gene responsible for pcd in these animals. The expression pattern of Pcp2 is limited to specific neurons (cerebellar Purkinje cells and retinal bipolar neurons [1,3,4]) and coincides with the maturation of Purkinje cells and formation of synaptic connections. Message levels in the mouse increase from postnatal day 2 to 15 and remain relatively constant thereafter [3,5,6]. Although these findings initially suggested a role for Pcp2 in Purkinje cell development, knockout mice for Pcp2 have been characterized by two groups and there were no significant anatomic, behavioural or motor deficits identified [7,8]. In 1999, we identified an interaction of Pcp2 and the Gαo/i family of heterotrimeric G-proteins [9]. We recently confirmed this interaction in vivo by co-immunoprecipitation from mouse cerebellum and eyes, and co-localized Pcp2 with Gαo in the distal processes of cerebellar Purkinje cells including axonal endings and dendritic spines [10]. A second splice form of Pcp2 was recently identified in rodents and humans and both isoforms are differentially expressed in developing dendrites during peak synaptogenesis [11].
Pcp2 is a G-protein regulator, one of a family of proteins containing a Goloco or GPR (G-protein regulatory) motif. This family is named for the Gi/o interacting protein Loco, the Drosophila RGS 12 (regulator of G-protein signalling 12) homologue [12] and members of this family interact with Gαo/Gαi subunits to inhibit GDP release [13–17]. The functions of GPR family members are not well established and the lack of suitable cultured Purkinje cell lines has contributed to the difficulties in defining Pcp2 function(s). To begin addressing the function of Pcp2 in neurons, we developed a cell culture model of inducible expression of Pcp2 in PC12 cells (rat pheochromocytoma cells). The signalling networks for differentiation of PC12 cells are highly developed and ideally suited for testing the hypothesis that Pcp2 modulates neuronal differentiation [18]. Furthermore, PC12 cells do not express endogenous Pcp2, thereby permitting establishment of a model system to study the effect of Pcp2 on neuronal properties. We found that Pcp2 stimulates differentiation in PC12 cells by increasing the fraction of cells with neurites and stimulating NGF (nerve growth factor)-induced neurite outgrowth. Pcp2 stimulated basal Ras and p38 MAPK (mitogen-activated protein kinase) activities and further enhanced NGF-stimulated signalling through this pathway. The effects of Pcp2 were blocked by treatment with pertussis toxin, inhibiting p38 MAPK, or by interference with signalling through Gβγ subunits. These studies provide evidence for Pcp2 regulation of neuronal differentiation in PC12 cells and implicate an important role for Gβγ.
EXPERIMENTAL
Tet-off Pcp2-PC12 cell lines and anti-Pcp2 antibody
GST–Pcp2 fusion protein was purified using standard techniques as described previously [9]. The full-length fusion protein was used to create a rabbit polyclonal antibody (Research Genetics, Huntsville, AL, U.S.A.) by standard immunization procedures. Tet-off PC12 cells (BD Biosciences, San Jose, CA, U.S.A./ClonTech Laboratories, Palo Alto, CA, U.S.A.) were cultured in DMEM (Dulbecco's modified Eagle's medium) containing penicillin/streptomycin, horse serum (10%, v/v), fetal calf serum (5%, v/v; Tet-approved; ClonTech Laboratories), Dox (doxycycline; 50 ng/ml, Sigma, St. Louis, MO, U.S.A.) and G418 (100 μg/ml, Gibco BRL/Life Technology, Burlington, ON, Canada) in 10% CO2. Full-length mouse Pcp2 (gi 200249) in pCR4 Topo (Invitrogen, Carlsbad, CA, U.S.A.) was provided by Dr M. Meyer (Max-Planck-Institute of Neurobiology, Martinsried, Germany) and subcloned into EcoRI digested pTRE. Subconfluent Tet-off PC12 cells were transfected with pTRE/Pcp2 by Lipofectamine™ 2000 (Gibco BRL/Life Technology) according to the manufacturer's instructions and stable clones were selected with hygromycin B (200 μg/ml; Roche, Indianapolis, IN, U.S.A.). Clones were expanded, cultured with or without (+/−) Dox and analysed by Western blot using rabbit Pcp2 antibody. Several positive clones were identified and after confirmation of the phenotype in two independent clones, one was selected for further characterization. Confluent 100 cm2 culture dishes (∼1×107 cells) were dissociated with Cell Dissociation Solution (Sigma) and split 1:5. The medium was changed every other day (or daily, if treated with NGF) and cultures became confluent in 4–5 days.
Cell lysates and Western blot
Cell lysates were prepared in TB (tissue buffer; 20 mM Tris, pH 7.5, 5 mm EDTA, 2 mM EGTA, 30 mM NaF, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate and 1% Triton X-100 with protease inhibitor cocktail; Roche) and membrane preparation and Western blots were performed as described previously [19].
Immunohistochemistry and microscopy
Frozen sections of mouse cerebellum were fixed in 4% (w/v) paraformaldehyde, blocked with 5% (v/v) goat serum, 5% horse serum and 0.2% Triton X-100 for 30 min followed by incubation with Pcp2 antibody or preimmune serum at 1:1000 dilution followed by biotinylated anti-rabbit serum and 0.2% streptavidin-CY3 (Zymed Laboratories, San Francisco, CA, U.S.A.). For Pcp2 staining of PC12 cells, cells were grown on poly-L-lysine coated glass coverslips. For phase contrast, cells were fixed with cacodylate buffer (2% paraformaldehyde, 2.5%, w/v, glutaraldehyde and 50 mM sodium cacodylate, pH 7.2–7.4). For immunofluorescence, cells were fixed in 4% paraformaldehyde, blocked in 5% goat serum, 5% horse serum, 1% BSA and 0.5% Triton X-100 in PBS and incubated with Pcp2 antibody at 1:500 followed by incubation with FITC-labelled goat anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR, U.S.A.) at 1:200 dilution. Long-term NGF (100 ng/ml; Sigma) stimulation (0–72 h) was done in standard media with 1% fetal bovine serum. Images were obtained using a Nikon Labophot-2 microscope and Spot Digital camera and software (Diagnostic Instruments, Sterling Heights, MI, U.S.A.; version 3.5.7) and Figures assembled using Adobe Photoshop and Illustrator (San Jose, CA, U.S.A.). Neurites were defined as processes longer than the width of the cell body. For each experiment, cells were imaged and neurite length determined with SpotSoftware. Total numbers of neurites, individual lengths, and cells were counted, and tabulated using GraphPad Prism (GraphPAD Software for Science, San Diego, CA, U.S.A.). Total neurite length was divided by the total number of cells counted to yield neurite length/cell. Independent experiments and analysis were performed at least twice for each condition with number of cells ranging from 30 to 100.
Ras activation
Ras activity was determined by precipitating Raf-1 RBD (Ras binding domain)-GST (glutathione S-transferase) from PC12 cell lysates according to the manufacturer's instructions (Upstate Biotechnology, Waltham, MA, U.S.A.). NGF was added (100 ng/ml) to media for short-term stimulation after overnight serum starvation. Cells were washed with PBS and lysates were harvested in MLB buffer (25 mM Hepes, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 1 mM EDTA and 2%, v/v, glycerol). For controls, lysates (500 μg) from +Dox cells were incubated with 10 μl of 0.5 M EDTA and GTP[S] (100 μM) (positive) or 2 mM GDP (negative control) at 30 °C for 30 min. For each condition, cell lysates (500 μg) were incubated with 20 μg of Raf-1 RBD-GST agarose at 4 °C for 45 min. Reactions were terminated by adding 32 μl of 1 M MgCl2 on ice followed by centrifugation of the beads and three washes in MLB buffer. Beads were resuspended in SDS/PAGE sample buffer, boiled and analysed by Western blot with mouse monoclonal anti-Ras antibody.
ERK1/2 (extracellular-signal-regulated kinase 1/2) and p38 MAPK activation
Basal- and NGF-stimulated activities were determined on identical amounts of PC12 cell lysates obtained from PC12 cells +/−Dox and +/−NGF as described above. Cells were washed with PBS, scraped and lysates prepared in TB as described above. Protein concentration was determined and identical amounts of total proteins were loaded on SDS/PAGE gels followed by Western-blot analysis with antibodies to total ERK1/2, phospho-ERK1/2, total p38 MAPK and phospho-p38 MAPK (all from Cell Signaling, Beverly, MA, U.S.A.).
Transient Pcp2 and β-ARK (β-adrenergic receptor kinase) expression in PC12 cells
PC12 cells (obtained from R. Diaz, Children's Hospital, Boston, MA, U.S.A.) were transiently transfected on poly-L-lysine glass coverslips in 12 mm culture plates with Pcp2 and enhanced GFP (ClonTech Laboratories; 1 μg each) using Lipofectamine™ 2000 (Gibco BRL/Life Technology) according to the manufacturer's instructions. NGF (100 ng/ml) was added 48 h after transfection in 1% serum and cultured for an additional 72 h. For β-ARK C-terminus (provided by Dr R. Lefkowitz, Duke University Medical Center, Durham, NC, U.S.A.) transfection, Pcp2-PC12 cells in +Dox were transfected with GFP and pRK5 (1 μg each) or GFP and pcDNA3 and changed to −Dox medium at 24 h. NGF was then added for an additional 72 h and coverslips fixed and imaged as described above.
Pertussis toxin treatment
PC12 cells growing on coverslips in +Dox media were incubated with pertussis toxin (List Biologicals Laboratories, Campbell, CA, U.S.A.) at 150 nM (a concentration previously shown to inhibit Gαo/i in PC cells [20]). At the time pertussis toxin was added, the cells were switched into −Dox medium and treated with NGF (100 ng/ml) for 72 h. Coverslips were then fixed, imaged and neurite length determined as described above.
Trypan Blue and cell viability
P12 cells +/−Dox and +/−NGF at various time points were dissociated, gently centrifuged and resuspended in 5 ml of Hanks balanced salt solution. An aliquot containing 0.2% Trypan Blue was incubated at room temperature (23 °C) for 15 min and blue cells counted on a haemocytometer. The percentage of Trypan Blue positive cells was averaged over five fields of 12–57 cells.
Ras activity in β-ARK-transfected Pcp2-expressing PC12 cells
PC12 cells in +Dox were transiently transfected with β-ARK C-terminus or vector control (PcDNA3) and GFP in the absence of all other antibiotics. The medium was changed at 24 h and at 48 h cultures were placed in −Dox to induce Pcp2 expression. At 96 h, cells were dissociated and resuspended in −Dox medium with 0.5% serum. For each condition, approx. 1×107 cells were obtained and GFP/β-ARK and GFP/vector positive cells were obtained by FACS separation using a EPICS, ALTRA flow cytometer (Beckman Coulter, High Wycombe, Bucks., U.K.) equipped with an argon laser emission of 488 nm. GFP was identified by using a 530 nm band-pass filter. Background fluorescence was first determined on non-transfected cells and parameters set to exclude dead cells or debris. Approximately 20% of cells were GFP+ (∼2×106) and these cells were replated in p60 culture dishes in low serum −Dox medium overnight. Cells were serum-starved overnight and stimulated the next day with NGF followed by measurement of Ras activity as described above.
Statistics and quantification
Western blots with exposures in the linear range were scanned using a desktop scanner and the images were analysed in NIH Image 1.63 (Wayne Rasband). Relative intensities of Ras, phospho-ERK1/2 and phospho p38 were normalized to the total amount of each protein in each experiment and results in arbitrary units were plotted using GraphPad Prism. All results are expressed as the means±S.D. or the range. Statistical significance was determined using two-tailed t test.
RESULTS
Establishment of Pcp2 inducible PC12 cell lines
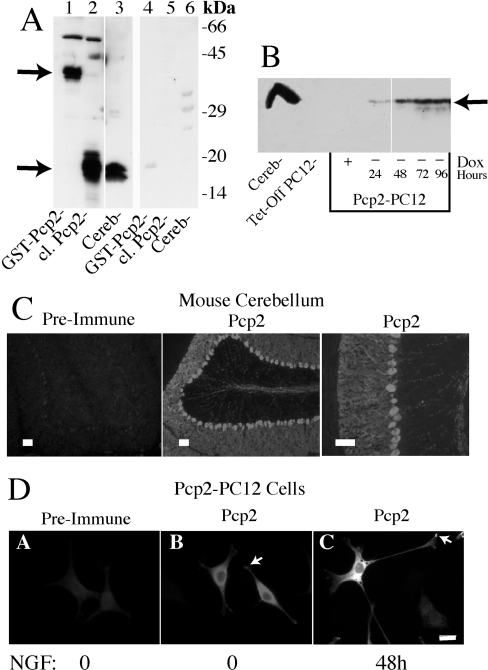
To facilitate studies of Pcp2, a rabbit polyclonal antibody to GST–Pcp2 fusion protein was generated. Figure 1(A) shows that this antibody (1:5000 dilution) detects GST–Pcp2 at approx. 40 kDa, thrombin-cleaved Pcp2 (from GST–Pcp2) at approx. 16 kDa and a similar sized protein migrating as a doublet in mouse cerebellum. Cleaved Pcp2 from GST contains five additional amino acids and migrates slightly slower than Pcp2 in cerebellum. Pcp2 is often seen as a doublet possibly reflecting detection of the two known isoforms [11]. Preincubation of the antibody with GST–Pcp2 fusion protein nearly completely inhibited the ability of the antibody to detect Pcp2 protein on Western blots (Figure 1A). Hygromycin-resistant PC12 cells were screened for Pcp2 expression by Western-blot analysis in −Dox. As shown in Figure 1(B), PC12 cells (Tet-off parental clone) did not express Pcp2. Of the eight positive clones, two were chosen for additional analysis. The time course of Pcp2 protein expression for one clone is shown in Figure 1(B). Removal of Dox induced Pcp2 protein expression within 24 h with levels that peaked and remained constant by 48–72 h. Readdition of Dox suppressed Pcp2 protein expression within 48 h (results not shown).
Figure 1. Establishment of Tet-off inducible Pcp2 expression in PC12 cells.
(A) Western blot demonstrating sensitivity and specificity of Pcp2 antibody. Lanes 1–3 show detection of Pcp2 at 1:5000 (0.4 μg/ml) dilution and lanes 4–6 are duplicate samples analysed by Western blotting after preincubation of the antibody with a 3-fold molar excess of GST–Pcp2 protein. Lanes 1 and 4, GST–Pcp2 (50 ng); lanes 2 and 5, cleaved (cl.) Pcp2 [by thrombin cleavage of GST–Pcp2 protein (100 ng)]; and lanes 3 and 6, mouse cerebellar lysate (75 μg). (B) Western blot of Pcp2 expression in Tet-off transfected PC12 cell line. Dox at 50 ng/ml (+) for 96 h was compared with parallel cultures grown in the absence of Dox for 24, 48, 72 and 96 h. Cerebellar lysate (100 μg) is shown in the first lane. 50 μg of PC12 lysate was used in each lane and Pcp2 antibody at 1:1000 dilution. (C) Pcp2 staining in mouse cerebellum. Frozen sections were fixed and stained with Pcp2 antibody at 1:1000 dilution or preimmune serum as described in the Experimental section. Scale bar=100 μM. (D) Immunolocalization of Pcp2 in PC12 cells cultured −Dox for 48 h. PC12 cells were grown on poly-lysine coated glass coverslips and stained with Pcp2 antibody or preimmune serum at 1:500 dilution as described in the Experimental section. Pcp2 localization under basal conditions (no NGF) was compared with Pcp2 expressing PC12 cells differentiated for 48 h with NGF (B, C). Images were obtained using a Nikon Labophot-2 microscope and Spot Digital camera and software (http://www.diaginc.com/SpotSoftware; version 3.5.7) and Figures assembled using Adobe Photoshop and Illustrator. Scale bar=10 μM.
Pcp2 is localized in cerebellar Purkinje cells throughout the cell body, axon and dendrites [4,10]. The utility of the Pcp2 antibody for immunolocalization was confirmed by detecting a similar staining pattern in mouse cerebellum (Figure 1C) and there was no detectable staining with preimmune serum (Figure 1C). In non-differentiated PC12 cells, short processes were visible and Pcp2 was localized in the cell body and the processes (Figure 1D, panel B). In differentiated PC12 cells expressing Pcp2, the protein distribution was similar to Purkinje cells with expression in neurites and in the cell body (Figure 1D, panel C). Pcp2 was excluded from the nucleus (confirmed by co-staining with 4,6-diamidino-2-phenylindole; results not shown) and short-term stimulation with NGF did not result in transient accumulation in the nucleus (results not shown). As expected from previous studies [4], there was co-localization of endogenous Gαo with expressed Pcp2 in neurites and cell body of Pcp2-PC12 cells (results not shown).
Pcp2 stimulates differentiation in PC12 cells
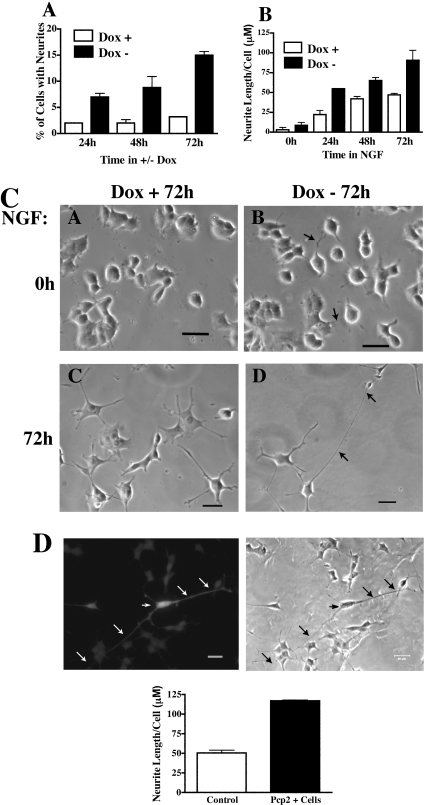
The effects of Pcp2 on differentiation in PC12 cells were determined by analysing the fraction of cells with neurites and by measuring total neurite length/cell in Pcp2-PC12 cells. In the absence of NGF, inducing Pcp2 expression led to a significant increase in the fraction of cells with neurites (Figure 2A). After switching to −Dox medium, there was an increase in the fraction of cells bearing neurites from approx. 2–3% up to 15%, whereas those cells maintained in +Dox medium (no Pcp2 expression) remained unchanged (Figure 2A). When stimulated with NGF, total neurite length increased in Pcp2 expressing cells at each time point (Figure 2B) and at 72 h was nearly double in Pcp2-PC12 cells (91±22 μM versus 47±3 μM). Pcp2 expression had no significant effect on cell viability under any condition as determined by counting detached cells with medium changes and Trypan Blue staining (results not shown). Figure 2(C) shows representative phase contrast fields of PC12 cells +/−Pcp2 expression in the presence and absence of NGF for 72 h. To determine whether the effect of Pcp2 on differentiation in PC12 cells was unique to this clone, separate analyses were performed on another Tet-off clone and an independent PC12 cell line. Similar effects of Pcp2 expression on neurite length and the fraction of cells bearing neurites were seen in another Tet-off Pcp2-PC12 cell line (results not shown). To confirm the phenotype in a PC12 cell line independently derived from the Tet-off cells, Pcp2 was co-transfected with GFP and stimulated with NGF for 72 h. Process length was determined in GFP positive cells (containing Pcp2) and compared with non-transfected cells imaged by phase contrast microscopy. Figure 2(D) (top) shows a GFP positive cell expressing Pcp2 in contrast with non-transfected cells in the same field. Quantification of neurite length in Pcp2 transfected cells is shown in Figure 2(D) and similar to the stable Tet-off cell line, there was more than 2-fold stimulation of neurite length in Pcp2 expressing cells.
Figure 2. Pcp2 stimulates differentiation in PC12 cells.
(A) Pcp2 expression increases the fraction of cells with neurites. PC12 cells were imaged as described in the Experimental section. Neurites were defined as processes greater than the width of cell body and counted in plates with similar numbers of cells in +/−Dox for designated times. The results are the means±S.D. of four independent experiments each with 80–250 cells. Differences between +Dox and −Dox treated cells were significant at all time points (P<0.01). (B) Pcp2 expression stimulates NGF-stimulated neurite growth. PC12 cells were cultured +/−Dox for 72 h followed by addition of NGF at T=0. Neurite lengths were measured using Spot Software on images of fixed cells (50–100 cells with at least 1 neurite; results are means±S.D. of 2–3 independent experiments). Neurite length in −Dox was significantly different from length in +Dox at all time points (P<0.01). (C) Representative phase contrast fields of cells +/−Dox (for 72 h) and +/−NGF at 72 h. Arrows denote neurites. Scale bar=20 μm. (D) Pcp2 transiently transfected with GFP into an independent PC12 cell line stimulates neurite growth. Pcp2 and GFP were co-transfected as described in the Experimental section. Images of Pcp2 transfected cells were analysed in comparison with phase pictures of non-transfected cells. Arrows denote processes of one Pcp2 expressing cell. Total neurite length/cell is quantified in the bar graph below (n=30–50 GFP+ cells and 100 GFP− cells). Error bars represent the range of two independent experiments.
Pcp2 expression stimulates Ras
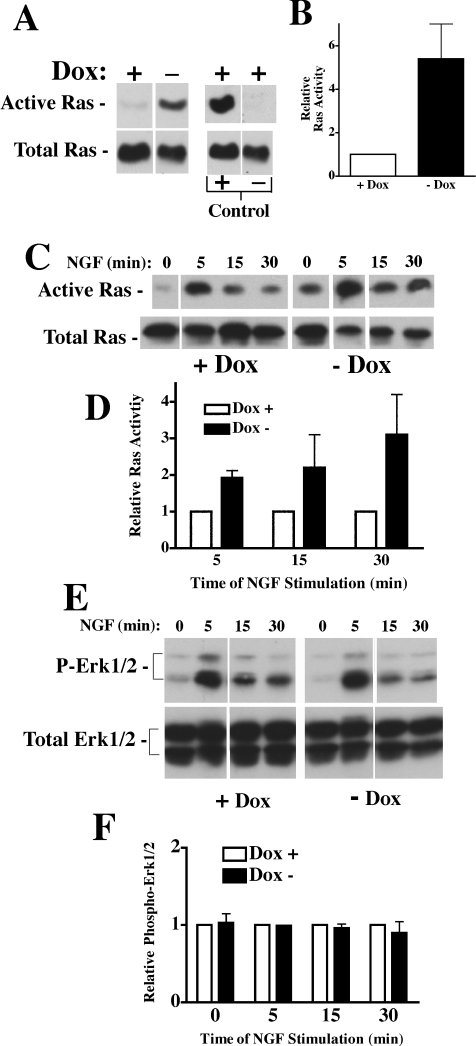
There are two main receptor-mediated pathways for differentiation in PC12 cells. One pathway utilizes a GPCR (G-protein-coupled receptor) and its ligand PACAP (pituitary adenylate cyclase-activating peptide) that stimulates adenylate cyclase and PKA (protein kinase A) through Gαs and Gαq. The other pathway is through TrkA which activates several proteins including Shc, Src and PI3K (phosphoinositide 3-kinase) (see [18]). We measured cAMP levels in PC12 cells +/−Pcp2 expression and failed to detect any difference (by ELISA; results not shown). We next measured basal Ras activity in PC12 cells +/−Pcp2 expression. Figure 3(A) shows total Ras in cellular lysates and activated Ras precipitated with the Raf-1 RBD–GST fusion protein under basal conditions. PC12 cell lysates from +Dox cells were used for controls; lysates were incubated with GDP (negative control) and GTP[S] (positive control) before pull down with Raf-1 RBD–GST fusion protein. In PC12 cells with Pcp2 expression (−Dox) there was a 5-fold increase in basal Ras activation relative to the +Dox control (Figure 3B). Based upon these findings and the fact that Pcp2 does not interact with the G-proteins in PACAP signalling system, we examined the effects of NGF on Ras activation in Pcp2 expressing PC12 cells. The addition of NGF led to a significant increase in Ras activation (2-fold) at 5 min in Pcp2-PC12 cells relative to the controls (Figures 3C and 3D) and this difference persisted through 30 min of NGF stimulation. Since Ras is a critical intermediate in the differentiation pathway of PC12 cells [18] and stimulates activation of downstream MAPKs, ERK1/2 activation was next examined. Somewhat surprisingly, there were no differences in basal or NGF-stimulated ERK1/2 activity in Pcp2-PC12 cells when compared with the control (Figures 3E and 3F).
Figure 3. Pcp2 expression stimulates Ras but not ERK1/2.
(A) Basal Ras activation +/−Pcp2 expression. PC12 cells were cultured +/−Dox for 48 h and Ras activity was determined from 500 μg of cell lysates as described in Experimental section. Western blot for Ras detected after elution from Raf-1-RBD GST agarose (active Ras) and total Ras (from 50 μg cell lysate). In parallel, Dox+lysates were incubated with GTP[S] as a positive control and GDP as a negative control as described in the Experimental section. (B) Relative Ras activation in Pcp2 expressing cells (−Dox) compared with cells without Pcp2 expression (+Dox). The increase was 5.4-fold ±1.6 from four independent experiments. (C) Ras activation with NGF stimulation (0–30 min) after overnight serum starvation. Relative Ras activation in the presence of NGF is compared in (D) (n=4 for 5 min; n=2 for 15 and 30 min and are expressed ±S.D. at 5 min ± range for 15 and 30 min). Lines separate specific lanes from the same gel and exposure. (E) Western blot of basal- and NGF-stimulated total and phospho-ERK1/2 activity on +/−Dox treated cell lysates. (F) Relative phospho-ERK1/2 (means±S.D. of three experiments).
Pcp2 expression stimulates p38 MAPK
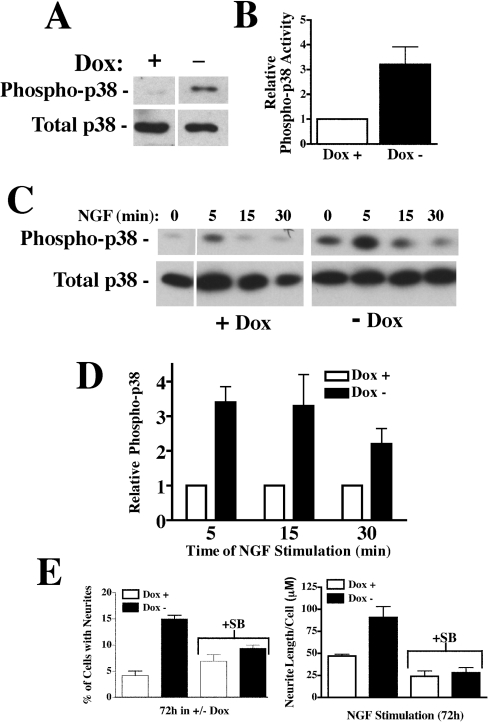
In PC12 cells, Ras activates ERK1/2 through Raf, but can also directly activate p38 MAPK. To determine if p38 MAPK was activated downstream of Ras in Pcp2 expressing cells, total and phospho-p38 levels were determined by Western-blot analysis in non-stimulated cells. Figures 4(A) and 4(B) show that under basal conditions, there was a 3–4-fold increase in phospho-p38 indicating activation in Pcp2-PC12 cells in comparison with controls (+Dox). NGF stimulation resulted in rapid increases in phospho-p38 (Figures 4C and 4D) that were significantly greater (3–4-fold) in Pcp2 expressing cells. The importance of p38 activation in stimulating differentiation with Pcp2 expression was confirmed by pretreating the cells with the p38-specific inhibitor SB203580. This inhibitor specifically blocks p38 activity [21] and is effective and specific in PC12 cells [22]. Inhibition of p38 MAPK in Pcp2-PC12 cells resulted in a similar fraction of cells with neurites, when compared with control cells (Figure 4E). Furthermore, inhibition of p38 MAPK in NGF-stimulated PC12 cells led to neurite lengths that were equivalent in PC12 cells +/−Pcp2 expression (Figure 4E). Taken together, these results reveal that Pcp2 activates Ras-p38 MAPK pathway and synergizes the effects of NGF stimulation.
Figure 4. Pcp2 stimulates p38 MAPK and inhibition with SB203580 blocks Pcp2-stimulated differentiation.
(A) Western blot to phospho-p38 and total p38 in PC12 cells +/−Dox under basal conditions. PC12 cells were cultured for 48 h in +/−Dox and 50 μg of lysates analysed by Western blot with antibodies to total p38 and phospho-p38 as described in the Experimental section. (B) Quantification of phospho-p38 activity in Pcp2 expressing cells (−Dox) relative to control (+Dox). The increase was 3.2-fold±1.6 (±S.D.) from five experiments. (C) Western blot of phospho-p38 and total p38 in +/−Dox cell lysates treated with NGF after overnight serum starvation. (D) Summary of phospho-p38 activity for Pcp2 expressing PC12 cells relative to control (+Dox). Results are the means±S.D. of four experiments. (E) Inhibition of p38 MAPK with SB203580 (10 μM) blocks Pcp2-stimulated differentiation. SB203580 (SB) was added to the media at the time of switching to −Dox media and changed every 24 h. The results are the means±range of two independent experiments and shown in comparison with the 72 h time points as described in Figure 2.
Pertussis toxin and co-expression of β-ARK inhibits Pcp2-induced phenotype and Ras activation
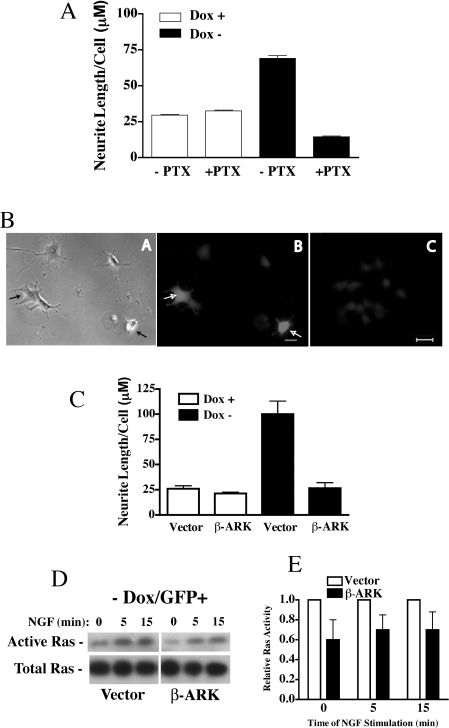
GPR proteins such as Pcp2 stabilize Gαo/i subunits in the GDP-liganded conformation and this may activate signalling pathways mediated by Gβγ [16]. To determine if inhibition of Gαo/i by pertussis toxin inhibited the Pcp2 stimulated effects on neurite growth, PC12 cells were treated with 150 nM pertussis toxin before induction of Pcp2 protein expression. Figure 5(A) shows that pertussis toxin treatment of PC12 cells without Pcp2 expression (+Dox) has no effect on neurite growth. With Pcp2 expression, neurite growth is stimulated (as shown in prior Figures), but pretreatment with pertussis toxin completely inhibited this effect. NGF-stimulated neurite growth was inhibited by pertussis toxin to lengths less than those seen in controls. These findings indicate that Pcp2 requires Gα and/or Gβγ to stimulate neurite growth. The effects of pertussis toxin on neurite growth were independent of any changes in cell viability. Trypan Blue staining and counting of detached cells at specific times +/−pertussis toxin showed no significant differences (results not shown).
Figure 5. Pertussis toxin treatment and transient expression of β-ARK blocks Pcp2-stimulated differentiation in NGF-treated PC12 cells.
(A) Effects of pertussis toxin on NGF-stimulated neurite growth in PC12 cells +/−Pcp2 expression. Pertussis toxin (PTX; 150 nM) was added to culture medium at the time of media change to +/−Dox. The medium was changed daily and neurite lengths quantified at 72 h of NGF treatment. Results are the means±range of two independent experiments each with 30–60 cells for each condition. (B) Pcp2-PC12 cells were transiently transfected with GFP+β-ARK or vector (pcDNA3) as described in the Experimental section. Images of Pcp2-Pc12 cells in −Dox medium transfected with β-ARK+GFP and imaged after 72 h of NGF stimulation. Phase contrast (A) image shows five cells; two of which are expressing β-ARK (denoted with arrows; B). (C) Background fluorescence in non-transfected cells under these conditions. Scale bar=20 μM. (C) Quantified neurite length/cell in vector and β-ARK transfected Pcp2-PC12 cells (all NGF-treated for 72 h) in the presence and absence of Pcp2 expression (+/−Dox). Results are the means±S.D. of three experiments where 30–60 cells were counted in each experiment. (D) Ras activity was measured in β-ARK and vector transfected PC12 cells as described in the Experimental section. After overnight serum starvation, cells were treated with NGF for 5 and 15 min and Ras activity was determined on 150 μg of cell lysate. Total Ras from each condition (30 μg) is shown below. (E) Relative Ras activity for β-ARK and vector transfected cells (n=5 at t=0, n=3 at 5, 15 min) was observed and it is expressed as means±S.E.M.
Gβγ released via activation of Gαi-coupled receptors can directly activate Ras [23]. To test whether Gβγ released from Gαo/i after complexing with Pcp2 could account for Ras stimulation in PC12 cells with Pcp2 expression, Gβγ signalling was blocked by transient transfection with the β-ARK C-terminus that has previously been shown to inhibit Gβγ-mediated signalling [24]. PC12 cells (+Dox) were transiently transfected with β-ARK C-terminus or pcDNA3 (vector) plus GFP. One day after transfection, the cultures were switched to −Dox medium or parallel cultures kept in +Dox medium and stimulated with NGF for 72 h. Figure 5(B) shows β-ARK-transfected cells have shorter processes than non-transfected cells in the same field. Figure 5(C) quantifies total neurite length in vector- and β-ARK-transfected cells +/−Dox. β-ARK transfection into PC12 cells without Pcp2 expression (+Dox) has a small effect on neurite length/cell (white bars; Figure 5B). β-ARK transfection into PC12 cells expressing Pcp2 results in a significant reduction in total neurite length, when compared with controls (black bars; Figure 5B). The length of β-ARK-treated Pcp2 expressing cells was similar to cells treated with the p38 MAPKinhibitor SB203580 (Figure 4E).
To determine if β-ARK expression inhibited Ras activation through sequestering Gβγ, Ras activity was measured in PC12 cells transiently transfected with β-ARK or vector control along with GFP. To obtain cells expressing β-ARK in Pcp2 expressing PC12 cells (−Dox), GFP positive cells were separated by FACS. After separation, cells were replated and stimulated with short-term NGF treatment and Ras activity was measured. Figures 5(D) and 5(E) show that in Pcp2 expressing PC12 cells (−Dox), Ras activity was 30–40% inhibited by β-ARK compared with vector controls analysed under identical conditions.
DISCUSSION
The function of Pcp2 has remained elusive since its discovery as an abundant Purkinje cell protein in 1988. Previous studies have hinted at a role for Pcp2 in Purkinje cell differentiation but direct evidence has been lacking [1,10,11]. Direct study of Pcp2 in Purkinje cells is difficult due to the lack of suitable cell culture models. The PC12 model neuron recapitulates many fundamental processes important for neuronal differentiation, and permits the effects of Pcp2 expression on neuronal differentiation to begin to be addressed. Now it is shown that Pcp2 stimulates neurite formation and NGF-stimulated neurite growth through activation of Ras and p38 MAPK pathways (without ERK1/2 activation). Inhibition of Gαo/i with pertussis toxin, inhibition of p38 MAPK activity or sequestering of Gβγ with β-ARK C-terminus blocks Pcp2-stimulated differentiation. These results provide the first direct evidence that Pcp2 modulates neuronal differentiation. However, the results obtained in this study using the PC12 cell model neuron limit firm conclusions about the relevance of these findings for cerebellar Purkinje cells. The cellular environment of the Purkinje is likely to differ significantly from the PC12 cell. Nevertheless, many fundamental neuronal processes have been identified using the PC12 cell model and the insights from this study will permit future studies in Purkinje cells to validate the hypotheses generated in this model system.
The function of Pcp2 has been difficult to elucidate due, in part, to the normal phenotype observed in knockout mice and the lack of suitable models for the present study. There are several lines of evidence indicating that Pcp2 has important functions in Purkinje cells and retinal bipolar neurons. It is an abundant protein in Purkinje cells with message levels similar to those of actin and tubulin [5]. The protein is widely distributed throughout the cell body, in the nucleus and cytosol including axonal and dendritic compartments ([4] and seen in Figure 1). In addition, there are at least two highly conserved isoforms in mammals, and the proteins are developmentally regulated during times of peak synaptogenesis [1,11]. Finally, Pcp2 is a GPR family member, and it directly interacts and co-localizes with Gαo in cerebellar Purkinje cells [10]. Based upon these observations and the absence of related proteins other than GPR family members, it is likely that the lack of a phenotype in the knockout mice results from developmental compensation from another GPR protein. This raises the possibility that in addition to other cellular functions, GPR family members may regulate differentiation in a variety of specialized cells.
GPR proteins share conserved GPR or Goloco domains that are conserved 19 amino acid motifs. GPR proteins contain one or more of these domains and share the basic properties of binding to the Gαo/Gαi family of heterotrimeric G-proteins and inhibiting GDP release from Gα [12]. Pcp2 contains one or two GPR domains depending upon the isoform [11] and other GPR family members contain up to four GPR domains. Other members of the GPR family include AGS3 (activator of G-protein signalling 3), LGN (Leu-Gly-Asn), Rap1Gap, RGS 12,14 [12,25] and the recently described AGS4 [26]. AGS3 and LGN contain four GPR domains and both proteins are widely expressed in the central nervous system [27]. LGN is ubiquitously expressed in numerous tissues, whereas AGS3 is primarily enriched in the brain. Within the central nervous system, both proteins are found in all major regions including the cerebellum. Developmental analysis revealed that both proteins are expressed in the cerebellum at postnatal day 1 and levels of LGN remain constant up to day 22, while AGS3 levels are significantly reduced at day 22 [27]. Thus either AGS3 or LGN could potentially compensate in the Pcp2 null mice. Several investigators have implicated possible functions for AGS3 and LGN in the process of cytokinesis. The Drosophila homologue of AGS3, PINS (partner of inscuteable) associates with Gαi, is asymmetrically localized during mitosis, and mediates correct spindle formation in dividing cells [28,29]. LGN is redirected to the cortex of dividing cells in a Gα-dependent manner and interfering with LGN function disrupts cell-cycle progression [30]. LGN (but not AGS3) was found in the nucleus of dividing cells, including PC12 cells, and moves to the midbody, where it associates with F-actin [27]. In Caenorhabditis elegans, two GPR proteins interact with LIN-5, are co-localized to the mitotic spindle and activate G-protein signalling to affect spindle force [31,32]. Both LGN and AGS3 contain loose nuclear localization signals, but only LGN was found in the nucleus of PC12 cells [27]. Pcp2 does not contain a clear nuclear localization signal, and in PC12 cells fails to localize within the nucleus.
The results of the present study reveal that Pcp2 stimulates differentiation in PC12 cells and synergistically increases neurite growth with NGF stimulation. Although there are important differences between Pcp2 expressed in PC12 cells and in cerebellar Purkinje cells, these findings are the first demonstration that Pcp2 can stimulate neuronal differentiation. Pcp2 expression leads to robust Ras activation under basal conditions and preferentially activates p38 MAPK but not ERK1/2. This selective MAPK activation could occur, if the Pcp2–Ras complex was restricted to specific cellular domains without access to ERK1/2. Activation of p38 MAPK is sufficient for stimulating differentiation in PC12 cells [22] and inhibiting p38 MAPK with SB203580 indicates that p38 is critical for Pcp2-stimulated differentiation. Pertussis toxin does not significantly affect the differentiation pathway in PC12 cells [18] and this is confirmed in the present study (Figure 5A). A potential role for pertussis toxin G-proteins in NGF-stimulated differentiation, however, is suggested by the finding that pertussis toxin blocked NGF-stimulated ERK1/2 activation [33] and pertussis toxin-sensitive G-proteins were shown to regulate NGF-induced phosphorylation of tuberin, an important regulator of NGF-stimulated neuronal surivival in PC12 cells [34]. Furthermore, NGF treatment increases pertussis toxin G-protein levels [35]. Pretreatment of Pcp2 expressing PC12 cells with pertussis toxin suppressed NGF-stimulated neurite growth to below control levels (Figure 5A). Ribosylation of endogenous Gαo/i is likely to prevent interaction with Pcp2 suggesting that the inhibited neurite outgrowth may result from signalling via unbound or free Pcp2. Taken together, these observations suggest that pertussis toxin-sensitive G-proteins may have important roles in regulating neuronal differentiation in specific cell types, and raises the possibility that Pcp2 can signal independently of Gαo/i.
The binding of Pcp2 to Gαo/i could release Gβγ leading to Ras activation. The observation that Pcp2 expressing PC12 cells fail to show enhanced differentiation, when Gβγ signalling is blocked with β-ARK expression is consistent with this mechanism. The partially inhibited Ras activation in Pcp2-PC12 cells expressing β-ARK supports Gβγ-mediated Ras activation in this model. However, this does not exclude other potential mechanisms and direct evidence for a signalling role of Gαo/i-Pcp2 remains to be found. The inhibition of neurite length by pertussis toxin to levels below the controls could be consistent with an additive signal from Gαo/i-Pcp2. A recent study of the effects of Pcp2 (L7) on P-type calcium channels in oocytes found that Pcp2 inhibited the channel [36]. There was no effect without receptor activation indicating that dissociation of Gαo/iβγ is necessary; a finding consistent with our results. However, there was complex regulation seen when either Gβγ or Gαi/o was overexpressed. Additional mechanisms are possible in the Pcp2-PC12 cell model system. For example, p38 MAPK can be activated by upstream adenylate cyclase-PKA stimulation, but this would not account for the enhanced Ras activity observed with Pcp2 expression. In addition, the effects of Gβγ on adenylate cyclase depend upon the isoform, and the absence of any significant difference in cAMP levels with Pcp2 expression suggests that Gβγ released via this mechanism is not affecting the net balance of adenylate cyclase activity. Finally, we have not excluded the possibility that Pcp2 alone or in complex with a Gα subunit contributes to Ras activation through other mechanisms.
The results of the present study provide the first evidence for a role of Pcp2 in regulating neuronal differentiation and reveal new insights into the signalling mechanisms. However, the findings in the present study using PC12 cells need to be corroborated in a Purkinje cell system. Although a highly abundant protein in cerebellar Purkinje cells, the function(s) of Pcp2 have been difficult to elucidate. These findings indicate that Pcp2 stimulates differentiation in the PC12 model neuron through Gβγ, Ras and p38 activation. This will permit future studies addressing these mechanisms directly in Purkinje cells and is likely to provide additional insights into novel functions for GPR family members.
Acknowledgments
Supported by National Institutes of Health grant RO1 GM55223 to BMD. The authors would like to thank Dr U. Berger (Brigham and Women's Hospital) for help with staining mouse cerebellum and Dr U. Mende (Brigham and Women's Hospital) and Dr R. Diaz for their helpful suggestions.
References
- 1.Nordquist D. T., Kozak C. A., Orr H. T. cDNA cloning and characterization of three genes uniquely expressed in cerebellum by Purkinje neurons. J. Neurosci. 1988;8:4780–4789. doi: 10.1523/JNEUROSCI.08-12-04780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen R. J., Eicher E. M., Sidman R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. U.S.A. 1976;73:208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandaele S., Nordquist D. T., Feddersen R. M., Tretjakoff I., Peterson A. C., Orr H. T. Purkinje cell protein-2 regulatory regions and transgene expression in cerebellar compartments. Genes Dev. 1991;5:1136–1148. doi: 10.1101/gad.5.7.1136. [DOI] [PubMed] [Google Scholar]
- 4.Berrebi A. S., Mugnaini E. Characteristics of labeling of the cerebellar Purkinje neuron by L7 antiserum. J. Chem. Neuroanat. 1992;5:235–243. doi: 10.1016/0891-0618(92)90048-u. [DOI] [PubMed] [Google Scholar]
- 5.Oberdick J., Levinthal F., Levinthal C. A Purkinje cell differentiation marker shows a partial DNA sequence homology to the cellular sis/PDGF2 gene. Neuron. 1988;1:367–376. doi: 10.1016/0896-6273(88)90186-9. [DOI] [PubMed] [Google Scholar]
- 6.Wanner I., Baader S. L., Oberdick J., Schilling K. Changing subcellular distribution and activity-dependent utilization of a dendritically localized mRNA in developing Purkinje cells. Mol. Cell. Neurosci. 2000;15:275–287. doi: 10.1006/mcne.1999.0824. [DOI] [PubMed] [Google Scholar]
- 7.Mohn A. R., Feddersen R. M., Nguyen M. S., Koller B. H. Phenotypic analysis of mice lacking the highly abundant Purkinje cell- and bipolar neuron-specific PCP2 protein. Mol. Cell. Neurosci. 1997;9:63–76. doi: 10.1006/mcne.1997.0606. [DOI] [PubMed] [Google Scholar]
- 8.Vassileva G., Smeyne R. J., Morgan J. I. Absence of neuroanatomical and behavioral deficits in L7/pcp-2-null mice. Brain Res. Mol. Brain Res. 1997;46:333–337. doi: 10.1016/s0169-328x(97)00081-8. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y., Denker B. M. Interaction of heterotrimeric G protein Gαo with Purkinje cell protein-2. Evidence for a novel nucleotide exchange factor. J. Biol. Chem. 1999;274:10685–10688. doi: 10.1074/jbc.274.16.10685. [DOI] [PubMed] [Google Scholar]
- 10.Redd K. J., Oberdick J., McCoy J., Denker B. M., Luo Y. Association and colocalization of G protein α subunits and Purkinje cell protein 2 (Pcp2) in mammalian cerebellum. J. Neurosci. Res. 2002;70:631–637. doi: 10.1002/jnr.10460. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Zhang H., Oberdick J. Conservation of the developmentally regulated dendritic localization of a Purkinje cell-specific mRNA that encodes a G-protein modulator: comparison of rodent and human Pcp2(L7) gene structure and expression. Brain Res. Mol. Brain Res. 2002;105:1–10. doi: 10.1016/s0169-328x(02)00379-0. [DOI] [PubMed] [Google Scholar]
- 12.Siderovski D. P., Diverse-Pierluissi M., De Vries L. The GoLoco motif: a Gαi/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem. Sci. 1999;24:340–341. doi: 10.1016/s0968-0004(99)01441-3. [DOI] [PubMed] [Google Scholar]
- 13.Natochin M., Gasimov K. G., Artemyev N. O. Inhibition of GDP/GTP exchange on G α subunits by proteins containing G-protein regulatory motifs. Biochemistry. 2001;40:5322–5328. doi: 10.1021/bi015505w. [DOI] [PubMed] [Google Scholar]
- 14.Natochin M., Lester B., Peterson Y. K., Bernard M. L., Lanier S. M., Artemyev N. O. AGS3 inhibits GDP dissociation from Gα subunits of the Gi family and rhodopsin-dependent activation of transducin. J. Biol. Chem. 2000;275:40981–40985. doi: 10.1074/jbc.M006478200. [DOI] [PubMed] [Google Scholar]
- 15.De Vries L., Fischer T., Tronchere H., Brothers G. M., Strockbine B., Siderovski D. P., Farquhar M. G. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Gαi subunits. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14364–14369. doi: 10.1073/pnas.97.26.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard M. L., Peterson Y. K., Chung P., Jourdan J., Lanier S. M. Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem. 2001;276:1585–1593. doi: 10.1074/jbc.M005291200. [DOI] [PubMed] [Google Scholar]
- 17.Peterson Y. K., Bernard M. L., Ma H., Hazard S., III, Graber S. G., Lanier S. M. Stabilization of the GDP-bound conformation of gialpha by a peptide derived from the G-protein regulatory motif of AGS3. J. Biol. Chem. 2000;275:33193–33196. doi: 10.1074/jbc.C000509200. [DOI] [PubMed] [Google Scholar]
- 18.Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 19.Busconi L., Denker B. M. Analysis of the N-terminal binding domain of Go α. Biochem. J. 1997;328:23–31. doi: 10.1042/bj3280023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusse O., Neer E. J. Localization of Gαo to growth cones in PC12 cells: role of Gαo association with receptors and Gβγ. J. Cell Sci. 1996;109:221–228. doi: 10.1242/jcs.109.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Young P. R., McLaughlin M. M., Kumar S., Kassis S., Doyle M. L., McNulty D., Gallagher T. F., Fisher S., McDonnell P. C., Carr S. A., et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki S., Iguchi M., Watanabe K., Hoshino R., Tsujimoto M., Kohno M. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 1999;274:26503–26510. doi: 10.1074/jbc.274.37.26503. [DOI] [PubMed] [Google Scholar]
- 23.Crespo P., Xu N., Simonds W. F., Gutkind J. S. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 24.Koch W. J., Hawes B. E., Inglese J., Luttrell L. M., Lefkowitz R. J. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J. Biol. Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 25.Takesono A., Cismowski M. J., Ribas C., Bernard M., Chung P., Hazard S., Duzic E., Lanier S. M. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J. Biol. Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 26.Cao X., Cismowski M. J., Sato M., Blumer J. B., Lanier S. M. Identification and characterization of AGS4: a protein containing three G-protein regulatory motifs that regulate the activation state of Gαi. J. Biol. Chem. 2004;279:27567–27574. doi: 10.1074/jbc.M312786200. [DOI] [PubMed] [Google Scholar]
- 27.Blumer J. B., Chandler L. J., Lanier S. M. Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J. Biol. Chem. 2002;277:15897–15903. doi: 10.1074/jbc.M112185200. [DOI] [PubMed] [Google Scholar]
- 28.Yu F., Morin X., Cai Y., Yang X., Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell (Cambridge, Mass.) 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M., Petronczki M., Dorner D., Forte M., Knoblich J. A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell (Cambridge, Mass.) 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik R., Yu F., Chia W., Yang X., Bahri S. Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol. Biol. Cell. 2003;14:3144–3155. doi: 10.1091/mbc.E03-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan D. G., Fisk R. M., Xu H., van den Heuvel S. A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C. elegans. Genes Dev. 2003;17:1225–1239. doi: 10.1101/gad.1081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshar K., Willard F. S., Colombo K., Johnston C. A., McCudden C. R., Siderovski D. P., Gonczy P. RIC-8 is required for GPR-1/2-dependent Gα function during asymmetric division of C. elegans embryos. Cell (Cambridge, Mass.) 2004;119:219–230. doi: 10.1016/j.cell.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Rakhit S., Pyne S., Pyne N. J. Nerve growth factor stimulation of p42/p44 mitogen-activated protein kinase in PC12 cells: role of G(i/o), G protein-coupled receptor kinase 2, β-arrestin I, and endocytic processing. Mol. Pharmacol. 2001;60:63–70. doi: 10.1124/mol.60.1.63. [DOI] [PubMed] [Google Scholar]
- 34.Wu E. H., Wong Y. H. Pertussis toxin-sensitive G(i/o) proteins are involved in nerve growth factor-induced pro-survival Akt signaling cascade in PC12 cells. Cell. Signal. 2005;17:881–890. doi: 10.1016/j.cellsig.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Zubiaur M., Neer E. J. Nerve growth factor changes G protein levels and localization in PC12 cells. J. Neurosci. Res. 1993;35:207–217. doi: 10.1002/jnr.490350212. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita-Kawada M., Oberdick J., Xi Zhu M. A Purkinje cell specific GoLoco domain protein, L7/Pcp-2, modulates receptor-mediated inhibition of Cav2.1 Ca2+ channels in a dose-dependent manner. Brain Res. Mol. Brain Res. 2004;132:73–86. doi: 10.1016/j.molbrainres.2004.09.007. [DOI] [PubMed] [Google Scholar]