Abstract
Stimuli for apoptotic signalling typically induce release of cyt c (cytochrome c) from mitochondria. Cyt c then initiates the formation of the apoptosome, comprising Apaf-1 (apoptotic protease-activating factor 1), caspase-9 and other cofactors. The issue of whether the redox state of the haem in cyt c affects the initiation of the apoptotic pathway is currently a subject of debate. In a cell-free reconstitution system, we found that only oxidized cyt c was capable of activating the caspase cascade. Oxidized cyt c was reduced by the physiological reductants cysteine and glutathione, after which it was unable to activate the caspase cascade. It is thus likely that cyt c with oxidized haem is in a conformation capable of interaction with Apaf-1 and forming apoptosomes. When either oxidized or reduced cyt c was treated with submillimolar concentrations of endoperoxide, which affected less than 3% of the redox state of haem, the ability of the oxidized cyt c to activate the caspase cascade was abolished. Higher amounts of singlet oxygen were required to affect the optical spectral change of haem, suggesting that the suppressed pro-apoptotic function of oxidized cyt c is a mechanism that is separate from the redox state of haem. Oxidative protein modification of cyt c by singlet oxygen was evident, on the basis of elevated contents of carbonyl compounds. Our data suggest that singlet oxygen eliminates the pro-apoptotic ability of oxidized cyt c not via the reduction of haem, but via the modification of amino acid residues that are required for apoptosome formation.
Keywords: caspase, cytochrome c, haem, redox state, singlet oxygen
Abbreviations: Apaf-1, apoptotic protease-activating factor 1; cyt c, cytochrome c; DABCO, 1,4-diazabicyclo[2,2,2]octane; DTT, dithiothreitol; MNP(E), 1-methylnaphthalene-4-propionate (endoperoxide); PARP, poly(ADP-ribose) polymerase; ROS, reactive oxygen species; TBS, Tris-buffered saline
INTRODUCTION
Several distinguishable pathways are involved in the activation of the caspase cascade during apoptotic cell death [1]. Typically, many apoptotic stimuli trigger the release of cyt c (cytochrome c) from mitochondria into the cytosol [2,3]. The released cyt c, together with dATP and Mg2+ ions, stimulates the production of protein factors, mainly Apaf-1 (apoptotic protease-activating factor 1) and procaspase-9, to form an apoptosome, resulting in the proteolytic activation of the following caspase cascade [4,5]. At a later stage, procaspase-3 is converted into the active form, which then cleaves protein components such as PARP [poly(ADP-ribose) polymerase] and ICAD (inhibitor of caspase-activated DNase). Chromatin condensation and nuclear fragmentation occur simultaneously in the general apoptotic process.
Cyt c is weakly bound to the inner-mitochondrial membrane, and plays an essential role by transferring electrons to cyt c oxidase in the mitochondrial electron-transfer system. The redox function of cyt c is performed by a haem group that is covalently bound to two cysteine residues. They are conserved among the cyt c proteins of various species, and no other cysteine residue is present. Met-80 and His-18 co-ordinate axially to the haem iron [6].
ATP levels and redox potential are intimately involved in determining the destiny of cells, whether or not they undergo apoptosis [7]. A high redox potential of cells appears to confer resistance to various apoptotic stimuli [8,9]. The anti-apoptotic function of Bcl-2 may partly be mediated by increasing the levels of the reduced form of glutathione, GSH [10,11]. Although the relationship between redox state and pro-apoptotic function of cyt c is of interest, controversial data have been reported on this issue [12]. An interesting hypothesis that concerns the control of apoptosis by redox regulation of released cyt c into the cytosol has been proposed [13].
ROS (reactive oxygen species) are produced under various conditions and exert detrimental effects on cells, including apoptosis. Among these ROS, the biological function of singlet oxygen is not fully understood. Singlet oxygen is not a free radical, but is reactive to certain biological compounds, especially conjugated double bonds [14]. Proteins contain many reactive residues, such as tryptophan, tyrosine, histidine, methionine and cysteine, and consume approx. 70% of the available singlet oxygen [15]. The well-characterized physiological function of singlet oxygen, which is largely generated by UV-A irradiation, is its involvement in the photoaging of skin [16]. Cytotoxic effects include the common deletion of mitochondrial genome DNA [17]. Singlet oxygen also appears to be involved in myeloperoxidase-mediated bacterial killing in neutrophil phagosomes [18] and is utilized to exterminate viruses [19]. Singlet oxygen is produced during photochemical reactions in the presence of a photosensitizer, and plays an essential role in the cytotoxic process for tumour cells during photodynamic chemotherapy [20].
Cell death induced by singlet oxygen in T cells [21] and HL-60 cells [22] shows the characteristic profiles of apoptosis. During this process, the cleavage of Bid occurs and cyt c is released from mitochondria. Singlet oxygen appears to activate a signalling cascade, which is mediated by c-Jun N-terminal kinase, p21-activated kinase 2 and p38 mitogen-activated protein kinase [23–26]. We have recently shown that singlet oxygen induces atypical cell death [27]. DNA fragmentation and PARP cleavage occurred only to a limited extent during cell death, while cyt c release and chromosomal condensation were observed. This was mainly due to suppression of the caspase cascade prior to apoptosome formation, as well as the direct inhibition of active caspase by singlet oxygen.
In the present study, we first characterized the redox state of cyt c that is capable of activating the caspase cascade, because the issue of whether or not redox state of cyt c affects the activation of the caspase cascade is ambiguous. We then investigated the effects of singlet oxygen on cyt c in view of the haem redox state and the activation of the caspase cascade.
EXPERIMENTAL
Reagents
Horse heart cyt c was purchased from Sigma (Tokyo, Japan). Rabbit anti-caspase-9 and anti-caspase-3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Santa Cruz, CA, U.S.A.) and Cell Signaling Technology (Beverly, MA, U.S.A.) respectively. An anti-mouse cyt c monoclonal antibody was obtained from Pharmingen (San Diego, CA, U.S.A.). Substrates for caspase-9 and -3 were obtained from the Peptide Institute (Osaka, Japan). An Oxyblot kit was obtained from Intergen. All other chemicals used were purchased from Sigma (Tokyo, Japan) or Wako (Osaka, Japan).
Synthesis of endoperoxides
MNPE (1-methylnaphthalene-4-propionate endoperoxide) was synthesized as described previously [28]. The structure and purity of the endoperoxide were determined by NMR spectroscopy. The half-life of MNPE was approx. 25 min at 37 °C. The generation of singlet oxygen from MNPE was detected by EPR spectroscopy using DRD156 as a sensitive singlet-oxygen-detecting probe, which specifically reacts with singlet oxygen among the ROS [29].
Oxidation and reduction of cyt c
Cyt c was either oxidized by incubation with 10 μM K3[Fe(CN)6] for 1 h, or reduced with 0.2 mM DTT (dithiothreitol) for 1 h in PBS at room temperature (≈22 °C). Excess reagents were removed by passing the mixture through a PD10 gel-filtration column (Pharmacia LKB). To examine effects of singlet oxygen, cyt c was incubated with MNPE in PBS at 37 °C and separated from MNPE and the decomposition product on the PD10 column. In some experiments, 20 mM NaN3, 20 mM histidine, 5 mM DABCO (1,4-diazabicyclo[2,2,2]octane) or 10 μM β-carotene was included to rationalize the role of singlet oxygen. The concentration of cyt c protein was determined by the Bradford method (Bio-Rad).
Cell culture
HepG2 cells were maintained in Dulbecco's modified minimum essential medium (Sigma) containing 100 units/ml penicillin and 100 μg/ml streptomycin supplemented with 10% (v/v) fetal bovine serum (Invitrogen). Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2.
Evaluation of caspase activation in a cell-free system
Caspase activation in a cell-free system was achieved essentially according to a method described previously [27]. Briefly, a cell lysate was prepared from HepG2 cells in 4 vol. of buffer containing 0.32 M sucrose, 3 mM MgCl2, 20 mM Tris/HCl, pH 7.5, 2 μg/ml aprotinin, 2 μg/ml pepstatin A and 50 μM (p-amidinophenyl)methanesulphonyl fluoride hydrochloride by homogenizing the cells with 40 strokes using a pestle in a Dounce homogenizer. The homogenates were centrifuged at 8000 g for 10 min, and the resulting supernatant was centrifuged at 100000 g for 1 h. The ability of cyt c to activate the caspase cascade was assessed by incubating the supernatant with cyt c (6.25 μM for the standard experiment), 2 mM dATP and 2 mM MgCl2 at 37 °C for 1 h.
Caspase assay
Caspase activity was determined essentially as described in a previous report [27]. Acetyl-Leu-Glu-His-Asp-4-methylcoumaryl-7-amide and acetyl-Asp-Glu-Val-Asp-4-methylcoumaryl-7-amide were used for the measurement of caspase-9 and caspase-3 activity respectively [30]. The reaction mixture contained 25 mM Pipes/KOH, pH 7.2, 5 mM MgCl2, 10 mM DTT, 0.1% CHAPS, 10% sucrose and 0.05 mM substrate in 200 μl. The initial rates of enzymatic hydrolysis were determined by measuring the release of 4-methylcoumaryl-7-amide from the substrate as the emission at 460 nm upon excitation at 380 nm using a BioLumin 960 Fluorometer (Molecular Dynamics, Tokyo, Japan) equipped with a thermostat-controlled plate reader. Since caspase-9 activity was lower than that of caspase-3, the baseline level was set slightly higher.
Spectrophotometry
Absorbance change in cyt c was measured using a spectrophotometer (Hitachi, Tokyo, Japan). To assess the effects of singlet oxygen, 200 μM of either oxidized or reduced cyt c was treated with various concentrations of MNPE at 37 °C for 1 h.
EPR spectroscopy
EPR spectra of cyt c (4 mM) were recorded at 77 K with a spectrometer (TE-200; Jeol, Tokyo, Japan). The instrument settings were: sweep time, 8 min; time constant, 0.3 s; modulation amplitude, 0.5 mT; modulation frequency, 100 K; microwave power, 8 mW; and microwave frequency, ≈9.2 GHz. The samples were introduced into an EPR quartz tube (outer diameter 5 mm) and placed in the microwave cavity under liquid nitrogen conditions.
SDS/PAGE and immunoblot analysis
Protein samples were subjected to SDS/PAGE (10–15% gels), and then transferred to a Hypond-P membrane (Amersham Biosciences, Bucks., U.K.) under semi-dry conditions with the use of a Transfer-blot SD Semi-dry transfer cell (Bio-Rad). The membrane was then blocked by incubation with 5% skimmed milk in TBS (Tris-buffered saline; 150 mM NaCl/20 mM Tris/HCl, pH 7.6) for 2 h at room temperature. The membranes were then incubated with rabbit anti-caspase-9, anti-caspase-3, or anti-mouse cyt c monoclonal antibody (1:1000 dilution) for 16 h at 4 °C. After washing with TBS containing 0.1% (v/v) Tween 20, the membrane was incubated with horseradish-peroxidase-conjugated goat anti-mouse IgG (1:1000 dilution; Santa Cruz Biotechnology) for 1 h at room temperature. Detection of protein carbonyls was performed by using an Oxyblot kit (Intergen) according to the manufacturer's instructions. After washing, the peroxidase activity on the membranes was detected by a chemiluminescence method using an ECL® Plus kit (Amersham Biosciences) and exposed to X-ray films (Kodak, Rochester, NY, U.S.A.). The amounts of cyt c protein were quantified by densitometric scanning of X-ray films by Densitography (Atto, Tokyo, Japan).
Statistics
Data are presented as the means±S.D. for triplicate experiments. The Student's t test was used to compare the significance of the differences between data. Values of P<0.05 were considered significant.
RESULTS
Redox-state-dependency of the pro-apoptotic function of cyt c
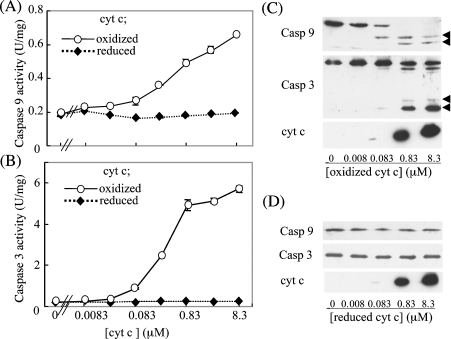
Since the issue of whether the redox state of haem affects the pro-apoptotic function of cyt c is currently under debate, we first examined the relationship between the redox state and the ability of cyt c to activate the caspase cascade in a cell-free system. Cyt c was first fully oxidized by treatment with 10 μM K3[Fe(CN)6], and used with or without reduction by 0.2 mM DTT. The redox state of cyt c was confirmed by scanning the wavelength in a spectrophotometer (results not shown). The ability of cyt c to activate the caspase cascade was examined in a cell-free reconstitution system using the cytosolic fraction prepared from HepG2 cells. As a result, oxidized cyt c dose-dependently activated both caspase-9 and -3 (Figures 1A and 1B). However, reduction of the oxidized cyt c with DTT totally abolished this ability. Conversion into processed, active forms of these enzymes was confirmed by immunoblotting (Figures 1C and 1D). Thus these data are consistent with the view that only oxidized cyt c serves as a pro-apoptotic molecule [31].
Figure 1. Effects of oxidized and reduced cyt c on activation of caspases.
After incubation of cytosolic fractions from HepG2 cells with oxidized or reduced cyt c, the activities of caspase-9 (A) and -3 (B) were measured. An immunoblot analysis was performed for the caspase assay mixture containing either oxidized (C) or reduced (D) cyt c using specific antibodies against caspase-9, -3 or cyt c (1:1000 dilution). The arrowheads in (C) indicate the processed, active form of the caspases. Abbreviation: U, units.
Effects of cyt c reduction by GSH or cysteine on caspase activation
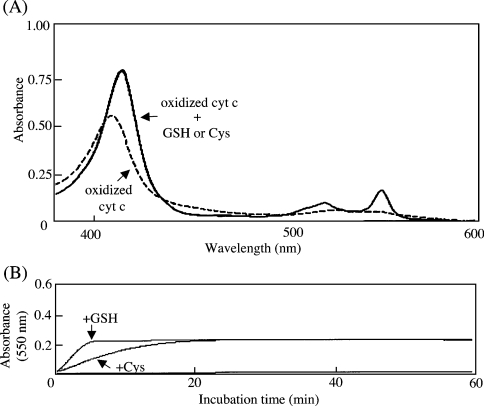
We examined the effects of the physiological reductants, GSH and cysteine, on the spectral change of the oxidized cyt c in the range of 370–600 nm. The absorbance at 550 nm (α-band) characteristic of the reduced form was observed for reduced cyt c, but not for oxidized cyt c (Figure 2A). Both GSH and cysteine reduced the oxidized cyt c under these conditions. The characteristic absorbance at 550 nm was continuously monitored to evaluate the redox potency of GSH and cysteine on cyt c during the incubation (Figure 2B). GSH was found to be more potent than cysteine, as is generally accepted [32].
Figure 2. Effects of GSH and cysteine on redox state of cyt c.
(A) After incubation of oxidized cyt c with 1 mM DTT, GSH or cysteine for 1 h at 37 °C, spectral changes between 370 and 600 nm were measured. The dotted line shows the spectrum of oxidized cyt c in the absence of a reductant; the solid line shows superimposed spectra of oxidized cyt c treated with GSH or cysteine. (B) Absorbance at 550 nm of oxidized cyt c was continuously recorded in the presence of 1 mM GSH or cysteine for 1 h. The spectra corresponded to oxidized cyt c (9 μM) that was incubated with GSH (top trace), cysteine (middle trace) or no reductant (bottom trace).
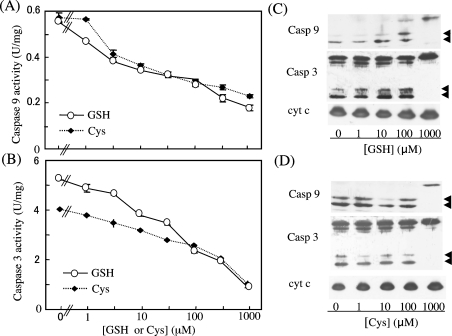
When the effects of treatment with these reductants on the ability of oxidized cyt c to activate the caspase cascade were examined, both GSH and cysteine treatment decreased the pro-apoptotic ability of the oxidized cyt c in a dose-dependent manner (Figures 3A and 3B). Consistency of the activities and processed, active forms of caspase-9 and -3 were confirmed by immunoblotting (Figures 3C and 3D). This suggests that the suppression of caspase activation occurred at the initial step of the caspase cascade, and support the view that cyt c in cells with a high redox potential is unable to activate the caspase cascade [13].
Figure 3. Dose-dependent inhibition by GSH and cysteine of the caspase-activating ability of oxidized cyt c.
After pre-incubation of oxidized cyt c with various concentrations of GSH or cysteine for 1 h at 37 °C, a cell-free caspase (Casp)-activation assay was performed. The activities of caspase-9 (A) and -3 (B) are shown. An immunoblot analysis was performed for the caspase assay mixture containing cyt c treated with either GSH (C) or cysteine (D) using specific antibodies against caspase-9, -3 or cyt c (1:1000 dilution). The arrowheads indicate the processed, active form of the enzymes.
Effects of singlet oxygen on the pro-apoptotic function of cyt c
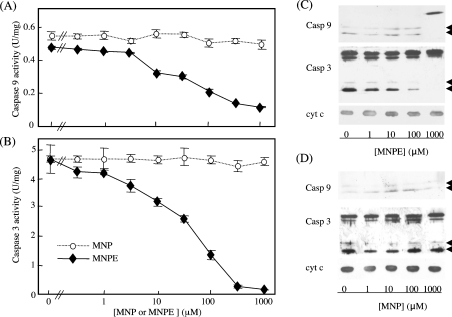
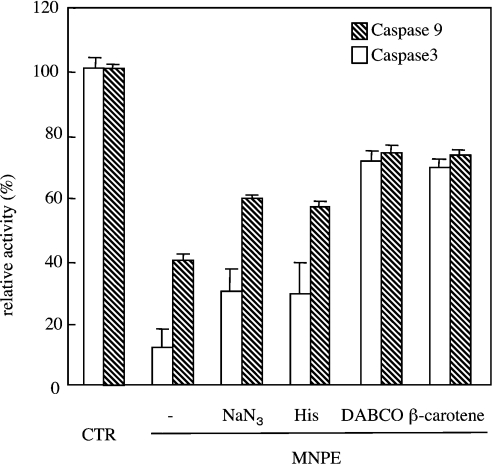
We have recently shown that a singlet-oxygen donor, MNPE, induced atypical cell death in which the caspase cascade was only slightly activated, despite the release of cyt c from mitochondria into the cytosol [27]. To determine whether singlet oxygen affects the pro-apoptotic function of cyt c, the effects of singlet oxygen on the ability of oxidized cyt c to activate the caspase cascade was examined by incubating cyt c with MNPE. Treatment of the oxidized cyt c with MNPE dose-dependently abolished the proapoptotic ability, whereas a precursor, MNP, had virtually no effect (Figures 4A and 4B). The levels of active forms of caspase-9 and -3 as detected by an immunoblot analysis were consistent with the activity (Figures 4C and 4D). To rationalize involvement of singlet oxygen in suppression of pro-apoptotic ability of cyt c by MNPE, we examined protective action of singlet oxygen inhibitors (sodium azide, histidine, DABCO and β-carotene) during treatment with cyt c (Figure 5). The assay of caspase activity in the reconstitution system indicated that all four compounds significantly suppressed the effect of MNPE on the pro-apoptotic ability of oxidized cyt c. This suggests that singlet oxygen released from MNPE converted the oxidized, pro-apoptotic cyt c into the inactive form, in view of the activation of the caspase cascade.
Figure 4. Effects of MNPE treatment on the caspase-activating ability of oxidized cyt c.
After incubation of oxidized cyt c with various concentrations of MNPE or MNP for 1 h at 37 °C, a cell-free caspase (Casp)-activation assay was performed. The activities of caspase-9 (A) and -3 (B) are shown. An immunoblot analysis was performed for the caspase assay mixture containing cyt c treated with MNPE (C) or MNP (D) using specific antibodies against caspase-9, -3 or cyt c (1:1000 dilution). The arrowheads indicate the processed, active form of the enzymes.
Figure 5. Effects of singlet oxygen ‘quenchers’ on pro-apoptotic ability of reduced cyt c.
Cyt c in PBS was first oxidized and treated with 1 mM MNPE or the control condition (CTR) in the presence of 20 mM NaN3, 20 mM histidine, 5 mM DABCO or 10 μM β-carotene at 37 °C for 1 h. After gel filtration of cyt c on the PD10 column, caspase activity was measured in a cell-free reconstitution system. Data represent relative activities of caspase-9 and -3 to control experiments that were performed with fully oxidized cyt c (CTR).
Relationship between the suppressed pro-apoptotic function and the redox state of cyt c by treatment with singlet oxygen
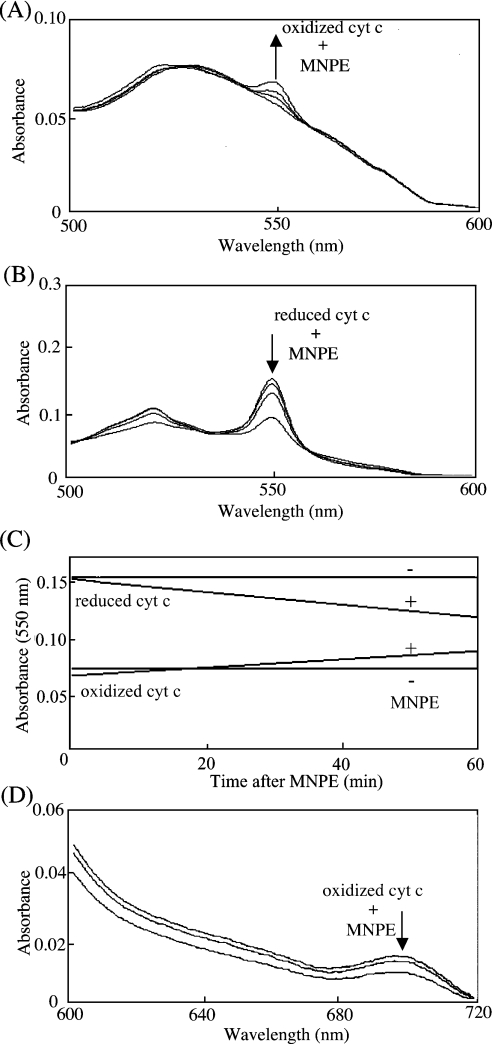
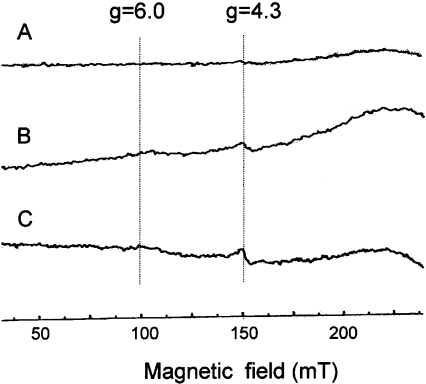
Since the redox state affects the pro-apoptotic function of cyt c, we examined the effects of singlet oxygen on the redox state of cyt c spectrophotometrically. MNPE at less than 0.5 mM, which fully inactivated the pro-apoptotic ability of cyt c, affected less than 3% of the redox state of haem, as judged from the spectra of both oxidized and reduced cyt c (results not shown). Either oxidized (Figure 6A) or reduced (Figure 6B) cyt c was then treated with 1, 3 and 5 mM MNPE for 1 h, and the change in absorbance was monitored. The absorbance at a unique peak maximum of 550 nm was increased in oxidized cyt c, but decreased in reduced cyt c time-dependently (Figure 6C). Thus singlet oxygen affected haem not by shifting the redox balance, but in a more complex manner. To understand better the role of singlet oxygen, we measured spectral changes from 370–720 nm and presented data in the range of 600–720 nm (Figure 6D), because only spectral changes of this range were informative. Treatment of oxidized cyt c with MNPE caused the disappearance of the characteristic 695 nm absorbance maximum. The conversion of the low-spin state into the high-spin state occurred at concentrations higher than 1 mM, as judged by EPR spectral results (Figure 7). A similar profile was observed in cyt c by nitration [33], and was attributed to the loss of Met-80–haem-iron interactions [6].
Figure 6. Effects of singlet oxygen on redox state of cyt c.
After incubation of either oxidized (A) or reduced (B) cyt c (8 μM) with 0, 1, 3 or 5 mM MNPE for 1 h at 37 °C, spectral changes between 370 and 800 nm were measured. Informative spectral ranges are shown. (A) Spectral traces shown from bottom to top (↑) correspond to oxidized cyt c that was treated with 0, 1, 3 or 5 mM MNPE. (B) Spectral traces shown from top to bottom (↓) correspond to reduced cyt c that was treated with 0, 1, 3 or 5 mM MNPE. (C) Absorbance changes at 550 nm of oxidized or reduced cyt c (8 μM) in the presence of 1 mM MNPE were continuously recorded for 60 min. (D) Spectral changes between 600 and 720 nm are shown. Spectral traces shown from top to bottom (↓) correspond to oxidized cyt c (50 μM) that was incubated with 0 (top), 1 (middle) or 5 (bottom) mM MNPE for 1 h at 37 °C.
Figure 7. EPR spectra of oxidized cyt c treated with or without MNPE at 77 K.
(A) The spectrum of oxidized cyt c (4 mM) without MNPE, (B) the spectrum of oxidized cyt c with 1 mM MNPE, and (C) the spectrum of oxidized cyt c with 5 mM MNPE are shown. Cyt c (4 mM) was incubated in PBS. Instrumental conditions were: sweep time, 8 min; time constant, 0.3 s; modulation amplitude, 0.5 mT; modulation frequency, 100 KHz; and microwave frequency, ≈9.2 GHz.
Characterization of the modification of cyt c by singlet oxygen
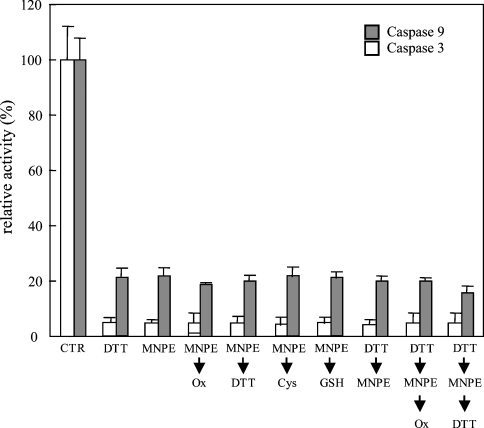
To characterize this process further, we examined whether the effects of singlet oxygen on the pro-apoptotic function of cyt c were reversible or not (Figure 8). Oxidized cyt c was first treated with MNPE, and then with the oxidant K3[Fe(CN)6], or the reductants DTT, GSH and cysteine. Neither treatment led to the recovery of the pro-apoptotic activity of cyt c. This implies that an irreversible modification was induced by singlet oxygen, and cyt c was converted into the inactive form.
Figure 8. Irreversible dysfunction of cyt c by treatment with singlet oxygen.
Cyt c was first fully oxidized, and then treated as indicated. After the treatments, a cell-free caspase-activation assay was performed. Data represent relative activities of caspase-9 and -3 to control experiments that were performed with fully oxidized (Ox) cyt c. CTR, control.
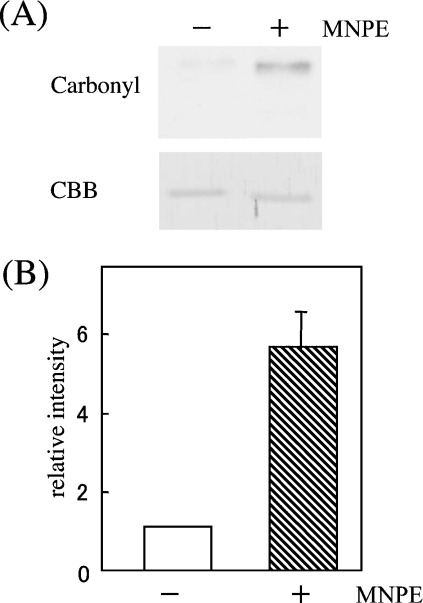
Since the oxidative modification of proteins by ROS generally causes an increase in the levels of carbonyl compounds, we compared the carbonyl contents after treatment of cyt c with 1 mM MNPE for 1 h. Analysis of carbonyl contents in cyt c by an Oxyblot kit indicated that treatment with singlet oxygen increased the carbonyl contents approx. 6 times over a control sample (Figure 9). This suggests that singlet oxygen oxidatively modified cyt c (see Supplementary Table 1; http://www.BiochemJ.org/bj/392/bj3920399add.htm), resulting in the elimination of pro-apoptotic ability.
Figure 9. Elevated carbonyl contents in singlet oxygen-treated cyt c.
(A) Carbonyl contents of oxidized cyt c (100 ng) that were treated with or without 1 mM MNPE for 1 h at 37 °C were assessed by an Oxyblot kit. Protein staining by Coomassie Brilliant Blue (CBB) was performed to show equal protein loading. (B) Quantification of the band intensity was performed for five assays by densitometric scanning of X-ray films. Data represent the mean values±S.D. relative to the control sample (without MNPE treatment).
DISCUSSION
We have recently shown that singlet oxygen triggered the release of cyt c from mitochondria, and consequently led to cell death, which was, however, different from typical apoptosis [27]. This was caused by impairment in the caspase cascade by singlet oxygen. One mechanism involves the direct action of singlet oxygen on caspases, probably via the oxidative modification of the enzyme. There also appears to be an action of singlet oxygen on the process of activation of the caspase cascade in which an assembly of apoptosomes is required. Cyt c plays a pivotal role in the initiation of this process by interacting with Apaf-1 [4,5]. Since cyt c is a haem-containing electron-transferring protein, a causal connection between the redox state of haem and apoptosome-forming ability is an interesting issue.
Cells are resistant to apoptotic stimuli when they are in a high redox potential with abundant thiol compounds, such as GSH, present [8–11]. The rapid efflux of GSH and its resultant depletion occur prior to apoptosis triggered by Fas ligand [34]. It has been reported that reduced cyt c functions to activate the caspase cascade in the cytoplasm, although this process does not rely on electron transfer from cyt c [12]. On the contrary, Pan et al. [31] showed the activation of apoptosis machinery by oxidized cyt c. Here, we have clearly shown that cyt c with oxidized haem is capable of activating the caspase cascade, but cyt c with reduced haem is not (Figure 1). We also found that the physiological reductants cysteine and GSH were able to reduce the oxidized cyt c, and abolished the ability of cyt c to induce caspase activation (Figure 2). GSH, abundantly present in cells, appears to be involved in the Bcl-2-induced suppression of apoptosis that is caused by granzyme B [10] and radiation [11]. It has been hypothesized that the redox control of cyt c acts as a failsafe mechanism in the regulation of programmed cell death [13]. This suggests that a functional interaction is induced among apoptosomal proteins by oxidized cyt c, and the apoptotic signal is transduced to downstream caspases without the transfer of electrons.
When oxidized cyt c was treated with MNPE, a singlet-oxygen donor, even cyt c with oxidized haem was unable to activate the caspase cascade. Regarding the redox state of haem, singlet oxygen that is generated during photosensitization using Methylene Blue changes the haem electronic state of cyt c from low spin to high spin [35]. In the present study, however, inability of oxidized cyt c to activate the caspase cascade was caused by submillimolar concentrations of endoperoxide (Figure 6), which had a slight effect (less than 3%) on the haem redox state, whereas the conversion of the low-spin state into the high-spin state occurred at concentrations higher than 1 mM, as judged from the EPR spectral results (Figure 7). This change in spin state could be related to a loss of Met-80–haem-iron interactions [6]. This suggests that the lack of activation of the caspase cascade by singlet oxygen was a separate function from the redox state of haem.
Activation of the caspase cascade requires the interaction of cyt c with Apaf-1. Since ionic strength is critical, an electrostatic interaction has been postulated [36]. Nϵ-Trimethylation of a specific lysine residue has been reported to abrogate the pro-apoptotic ability of cyt c [37]. A mutational analysis of the epitope for cyt c binding to Apaf-1 suggests the importance of some lysine residues in cyt c [38]. In addition, cardiolipin, an acidic phospholipid, appears to play a pivotal role in the retention of cyt c by the inner mitochondrial membrane [39]. It is possible that singlet oxygen converts the cyt c protein conformation into one similar to the reduced form, because redox-dependent conformational changes commonly occur in cyt c from various species [40,41]. However, the disappearance of the pro-apoptotic function of cyt c by singlet oxygen did not always accompany the reduction of haem (cf. Figures 4 and 6). Similarly, the haem-independent prevention of apoptosome formation has been observed for apo-cyt c that lacks haem [42,43]. This suggests that even apo-cyt c is incorporated into apoptosomes, and interferes with the formation of active machinery by preventing holo-cyt c from functioning.
The effects of singlet oxygen on the pro-apoptotic function of cyt c can be reasonably explained by the oxidative modification of some amino acid residues of cyt c by singlet oxygen. Based on calculations, approx. 70% of the singlet oxygen is consumed by proteins due to their abundance and high reactivity [15]. ROS and reactive nitrogen oxide species are known to inhibit cysteine proteases [44]. Cathepsin K, a cysteine protease in lysosomes, is inhibited by nitric oxide donors [45]. The oxidative modification of cyt c by singlet oxygen, however, appears to be neither simple disulphide formation nor oxidation to sulphenic acid, because two cysteine residues in cyt c are covalently bound to haem, and DTT treatment had no effect on the recovery of the pro-apoptotic function of cyt c (Figure 8). A similar irreversible inactivation was observed in the singlet-oxygen-treated cysteine proteases cathepsins B and L/S [46]. Whereas caspase and cathepsins are cysteine proteases, and have reactive cysteines at their catalytic centre, cyt c does not contain such a reactive cysteine. A spectrophotometric analysis suggested that Met-80 is dissociated from haem iron by treatment with singlet oxygen (Figure 6D). Since methionine is one of the most reactive amino acids with respect to singlet oxygen, the oxidative modification of Met-80 may occur, thus eliminating the pro-apoptotic ability of cyt c. An elevation in the carbonyl content in MNPE-treated cyt c (Figure 9) supports oxidative modification of amino acids by singlet oxygen.
The findings herein show that singlet oxygen modifies cyt c, converting it into a form that is unable to activate the subsequent caspase cascade. Dysfunction of common apoptotic pathways, including caspases [27] and cyt c (the present study), leads a cell to more necrotic cell-like death. Intracellular components are released during necrotic cell death, and may enhance inflammatory responses [47,48]. An impaired removal of dead cells by apoptosis and following scavenging cause autoimmune diseases, as reported in mice lacking a gene responsible for phagocytosis by macrophages [49]. The development of a marker specific for modification by singlet oxygen is awaited to evaluate the participation of singlet oxygen in pathogenesis.
Online data
Acknowledgments
This work was supported, in parts, by Grant-in-Aid for Scientific Research (C) and the 21st Century COE Program from the JSPS (Japan Society for the Promotion of Science) and by the Cosmetology Research Foundation. We thank Professor Kiharu Igarashi, Department of Bioresource Engineering, Faculty of Agriculture, Yamagata University, Yamagata, Japan, for amino acid analysis.
References
- 1.Leist M., Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 2.Green D., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner M. O. The biochemistry of apoptosis. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 5.Kluck R. M., Martin S. J., Hoffman B. M., Zhou J. S., Green D. R., Newmeyer D. D. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 1997;16:4639–4649. doi: 10.1093/emboj/16.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson R. E., Timkovich R. Cytochromes c. In: Boyer P. D., editor. The Enzymes. XI: Oxidation–Reduction. New York, NY: Academic Press; 1975. pp. 397–547. [Google Scholar]
- 7.Jo S. H., Son M. K., Koh H. J., Lee S. M., Song I. H., Kim Y. O., Lee Y. S., Jeong K. S., Kim W. B., Park J. W., et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 8.Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 9.Backway K. L., McCulloch E. A., Chow S., Hedley D. W. Relationships between the mitochondrial permeability transition and oxidative stress during ara-C toxicity. Cancer Res. 1997;57:2446–2451. [PubMed] [Google Scholar]
- 10.Voehringer D. W., McConkey D. J., McDonnell T. J., Brisbay S., Meyn R. E. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirkovic N., Voehringer D. W., Story M. D., McConkey D. J., McDonnell T. J., Meyn R. E. Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene. 1997;15:1461–1470. doi: 10.1038/sj.onc.1201310. [DOI] [PubMed] [Google Scholar]
- 12.Hampton M. B., Zhivotovsky B., Slater A. F., Burgess D. H., Orrenius S. Importance of the redox state of cytochrome c during caspase activation in cytosolic extracts. Biochem. J. 1998;329:95–99. doi: 10.1042/bj3290095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock J. T., Desikan R., Neill S. J. Does the redox status of cytochrome c act as a fail-safe mechanism in the regulation of programmed cell death? Free Radical Biol. Med. 2001;31:697–703. doi: 10.1016/s0891-5849(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B., Gutteridge J. M. C. 3rd edn. Oxford: Clarendon Press; 1999. Free Radical Biology and Medicine. [Google Scholar]
- 15.Davies M. J. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Godar D. E. Singlet oxygen-triggered immediate preprogrammed apoptosis. Methods Enzymol. 2000;319:309–330. doi: 10.1016/s0076-6879(00)19032-9. [DOI] [PubMed] [Google Scholar]
- 17.Berneburg M., Grether-Beck S., Kurten V., Ruzicka T., Briviba K., Sies H., Krutmann J. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J. Biol. Chem. 1999;274:15345–15349. doi: 10.1074/jbc.274.22.15345. [DOI] [PubMed] [Google Scholar]
- 18.Tatsuzawa H., Maruyama T., Hori K., Sano Y., Nakano M. Singlet oxygen 1ΔgO2 as the principal oxidant in myeloperoxidase-mediated bacterial killing in neutrophil phagosome. Biochem. Biophys. Res. Commun. 1999;262:647–650. doi: 10.1006/bbrc.1999.1265. [DOI] [PubMed] [Google Scholar]
- 19.Pellieux C., Dewilde A., Pierlot C., Aubry J. M. Bactericidal and virucidal activities of singlet oxygen generated by thermolysis of naphthalene endoperoxides. Methods Enzymol. 2000;319:197–207. doi: 10.1016/s0076-6879(00)19020-2. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad N., Mukhtar H. Mechanism of photodynamic therapy-induced cell death. Methods Enzymol. 2000;319:342–358. doi: 10.1016/s0076-6879(00)19034-2. [DOI] [PubMed] [Google Scholar]
- 21.Morita A., Werfel T., Stege H., Ahrens C., Karmann K., Grewe M., Grether-Beck S., Ruzicka T., Kapp A., Klotz L. O., et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J. Exp. Med. 1997;186:1763–176. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochevar I. E., Lynch M. C., Zhuang S., Lambert C. R. Singlet oxygen, but not oxidizing radicals, induces apoptosis in HL-60 cells. Photochem. Photobiol. 2000;72:548–553. doi: 10.1562/0031-8655(2000)072<0548:sobnor>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Klotz L. O., Briviba K., Sies H. Singlet oxygen mediates the activation of JNK by UVA radiation in human skin fibroblasts. FEBS Lett. 1997;408:289–291. doi: 10.1016/s0014-5793(97)00440-7. [DOI] [PubMed] [Google Scholar]
- 24.Klotz L. O., Pellieux C., Briviba K., Pierlot C., Aubry J. M., Sies H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem. 1999;260:917–922. doi: 10.1046/j.1432-1327.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang S., Demirs J. T., Kochevar I. E. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 26.Chan W. H., Yu J. S., Yang S. D. Apoptotic signalling cascade in photosensitized human epidermal carcinoma A431 cells: involvement of singlet oxygen, c-Jun N-terminal kinase, caspase-3 and p21-activated kinase 2. Biochem. J. 2000;351:221–232. doi: 10.1042/0264-6021:3510221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsu K., Sato K., Ikeda Y., Imai H., Nakagawa Y., Ohba Y., Fujii J. Abortive apoptotic pathway by singlet oxygen due to the suppression of caspase activation. Biochem. J. 2005;389:197–206. doi: 10.1042/BJ20042067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubry J. M., Cazin B., Duprat F. Chemical sources of singlet oxygen. 3. Peroxidation of water-soluble singlet oxygen carriers with the hydrogen peroxide-molybdate system. J. Org. Chem. 1989;54:726–728. [Google Scholar]
- 29.Liu W., Ogata T., Sato K., Ohba Y., Sakurai K., Igarashi T. Syntheses of water-soluble endoperoxides as a singlet oxygen source. ITE Letters on Batteries, New Technol. Med. 2001;2:98–101. [Google Scholar]
- 30.Stennicke H. R., Salvesen G. S. Biochemical characteristics of caspases-3, -6, -7 and -8. J. Biol. Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z., Voehringer D. W., Meyn R. E. Analysis of redox regulation of cytochrome c-induced apoptosis in a cell-free system. Cell Death Differ. 1999;6:683–688. doi: 10.1038/sj.cdd.4400544. [DOI] [PubMed] [Google Scholar]
- 32.Jocelyn P. C. The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur. J. Biochem. 1967;2:327–331. doi: 10.1111/j.1432-1033.1967.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 33.Cassina A. M., Hodara R., Souza J. M., Thomson L., Castro L., Ischiropoulos H., Freeman B. A., Radi R. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 34.van den Dobbelsteen D. J., Nobel C. S., Schlegel J., Cotgreave I. A., Orrenius S., Slater A. F. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J. Biol. Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- 35.Estevam M. L., Nascimento O. R., Baptista M. S., Di Mascio P., Prado F. M., Faljoni-Alario A., Zucchi Mdo R., Nantes I. L. Changes in the spin state and reactivity of cytochrome C induced by photochemically generated singlet oxygen and free radicals. J. Biol. Chem. 2004;279:39214–39222. doi: 10.1074/jbc.M402093200. [DOI] [PubMed] [Google Scholar]
- 36.Purring-Koch C., McLendon G. Cytochrome c binding to Apaf-1: the effects of dATP and ionic strength. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11928–11931. doi: 10.1073/pnas.220416197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluck R. M., Ellerby L. M., Ellerby H. M., Naiem S., Yaffe M. P., Margoliash E., Bredesen D., Mauk A. G., Sherman F., Newmeyer D. D. Determinants of cytochrome c pro-apoptotic activity. The role of lysine 72 trimethylation. J. Biol. Chem. 2000;275:16127–16133. doi: 10.1074/jbc.275.21.16127. [DOI] [PubMed] [Google Scholar]
- 38.Yu T., Wang X., Purring-Koch C., Wei Y., McLendon G. L. A mutational epitope for cytochrome c binding to the apoptosis protease activation factor-1. J. Biol. Chem. 2001;276:13034–13038. doi: 10.1074/jbc.M009773200. [DOI] [PubMed] [Google Scholar]
- 39.Nomura K., Imai H., Koumura T., Kobayashi T., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kar L., Sherman S., Johnson M. E. Comparison of protein structures in solution using local conformations derived from NMR data: application to cytochrome c. J. Biomol. Struct. Dyn. 1994;12:527–558. doi: 10.1080/07391102.1994.10508758. [DOI] [PubMed] [Google Scholar]
- 41.Calvert J. F., Hill J. L., Dong A. Redox-dependent conformational changes are common structural features of cytochrome c from various species. Arch. Biochem. Biophys. 1997;346:287–293. doi: 10.1006/abbi.1997.0324. [DOI] [PubMed] [Google Scholar]
- 42.Martin A. G., Fearnhead H. O. Apocytochrome c blocks caspase-9 activation and Bax-induced apoptosis. J. Biol. Chem. 2002;277:50834–50841. doi: 10.1074/jbc.M209369200. [DOI] [PubMed] [Google Scholar]
- 43.Martin A. G., Nguyen J., Wells J. A., Fearnhead H. O. Apocytochrome c inhibits caspases by preventing apoptosome formation. Biochem. Biophys. Res. Commun. 2004;319:944–950. doi: 10.1016/j.bbrc.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 44.Ascenzi P., Salvati L., Bolognesi M., Colasanti M., Polticelli F., Venturini G. Inhibition of cysteine protease activity by NO-donors. Curr. Protein Pept. Sci. 2001;2:137–153. doi: 10.2174/1389203013381170. [DOI] [PubMed] [Google Scholar]
- 45.Percival M. D., Ouellet M., Campagnolo C., Claveau D., Li C. Inhibition of cathepsin K by nitric oxide donors: evidence for the formation of mixed disulfides and a sulfenic acid. Biochemistry. 1999;38:13574–13583. doi: 10.1021/bi991028u. [DOI] [PubMed] [Google Scholar]
- 46.Nagaoka Y., Otsu K., Okada F., Sato K., Ohba Y., Kotani N., Fujii J. Specific inactivation of cysteine protease-type cathepsin by singlet oxygen generated from naphthalene endoperoxides. Biochem. Biophys. Res. Commun. 2005;331:215–223. doi: 10.1016/j.bbrc.2005.03.146. [DOI] [PubMed] [Google Scholar]
- 47.Savill J., Fadok V. Corpse clearance defines the meaning of cell death. Nature (London) 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 48.Cline A. M., Radic M. Z. Apoptosis, subcellular particles and autoimmunity. Clin. Immunol. 2004;112:175–182. doi: 10.1016/j.clim.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.