Abstract
Sal1p is a mitochondrial protein that belongs to the SCaMC (short calcium-binding mitochondrial carrier) subfamily of mitochondrial carriers. The presence of calcium-binding motifs facing the extramitochondrial space allows the regulation of the transport activity of these carriers by cytosolic calcium and provides a new mechanism to transduce calcium signals in mitochondria without the requirement of calcium entry in the organelle. We have studied its transport activity, finding that it is a carboxyatractyloside-resistant ATP-Mg carrier. Mitochondria from a disruption mutant of SAL1 have a 50% reduction in the uptake of ATP. We have also found a clear stimulation of ATP-transport activity by calcium, with an S0.5 of approx. 30 μM. Our results also suggest that Sal1p is a target of the glucose-induced calcium signal which is non-essential in wild-type cells, but becomes essential for transport of ATP into mitochondria in yeast lacking ADP/ATP translocases.
Keywords: ATP transport, calcium, calcium-dependent mitochondrial carrier (CaMC), glucose-sensing, short calcium-binding mitochondrial carrier (SCaMC), Saccharomyces cerevisiae
Abbreviations: AAC, ADP/ATP carrier; CaMC, calcium-binding mitochondrial carrier; CAT, carboxyatractyloside; CPY, carboxypeptidase Y; GFP, green fluorescent protein; RGS, regulator of heterotrimeric G-protein signalling; SCaMC, short CaMC; SM, synthetic minimal medium; yEGFP, yeast enhanced GFP
INTRODUCTION
Calcium is a second messenger with important functions, of which one is calcium signalling in mitochondria. This function is performed thanks to the transport of calcium from the cytosol to mitochondria across the calcium uniporter, followed by activation of calcium-sensitive dehydrogenases [1,2]. A second mechanism to transduce calcium signals to mitochondria is through CaMCs (calcium-binding mitochondrial carriers), the AGCs (aspartate/glutamate carriers) aralar and citrin [3,4], and the SCaMC (short CaMC) [5] that correspond to ATP-Mg/Pi carriers [6]. CaMCs possess N-terminal extensions with EF-hand motifs (EF does not stand for elongation factor, but corresponds to α-helices E and F of parvalbumin, the archetypal calcium-binding protein). These extensions face the intermembrane space, and CaMCs have been shown to transduce calcium signals to mitochondria without the need of calcium uptake into the organelle [7,8].
Saccharomyces cerevisiae is an exception in terms of calcium signalling in mitochondria. These yeasts do not have a mitochondrial calcium uniporter equivalent to the mammalian one [9] and, accordingly, also lack calcium-sensitive dehydrogenases [10]. Moreover, yeast Agc1p does not have calcium-binding motifs in its long N-terminal extension, indicating that this additional mechanism of calcium signalling is absent from S. cerevisiae [11].
However, S. cerevisiae possesses a gene of the SCaMC family, YNL083w. It was identified during the sequencing of the genome of S. cerevisiae, and it was found to be non-essential [12,13]. Since the sequences of human SCaMCs and Sal1p (the protein encoded by YNL083w, renamed SAL1 by Chen [14]) are very similar, with 34–37% identity at the amino acid level along the 300-amino-acid long C-terminus [5], we have attempted to study the function and calcium regulation of Sal1p as a first step to study that of the various human SCaMC isoforms.
Glucose sensing in yeast is required to tune growth and metabolic activity to the amount and type of sugars available. Even brief exposure to glucose leads to cell-cycle-related changes [15]. Glucose sensing involves a signal transduction pathway similar to that employed by hormones in higher eukaryotes, in which both cAMP and calcium are early second messengers (see [16] for review), and glucose uptake and phosphorylation are required to obtain a full response [17]. At present, it is unknown which are the relevant targets, if any, of the glucose-induced calcium transient.
We have verified that the Sal1p is localized to mitochondria, and we have studied the transport activity of the protein, finding that it is a CAT (carboxyatractyloside)-resistant ATP-Mg carrier of mitochondria different from the ADP/ATP translocase. It drives net ATP uptake, as found for SCaMCs reconstituted in proteoliposomes [6], a transport activity probably corresponding to that of the ATP-Mg/Pi carrier of mitochondria described by Aprille and Austin [18] and Aprille [19]. Furthermore, transport activity is calcium-sensitive, with an S0.5 of approx. 30 μM.
Our results also indicate that calcium binding to Sal1p is a component of the glucose-sensing system that becomes essential in yeast that lack adenine nucleotide translocases when no carrier other than Sal1p catalyses adenine nucleotide transport in mitochondria.
MATERIALS AND METHODS
Yeast strains
The agc1Δ cells were prepared as described in Cavero et al. [11]. The sal1Δ haploid cells (W303; MATa, ura3-1, trp1-Δ2, leu2-3,112, his3-11, ade2-1, can1-100; sal1::kanMX4) were obtained by sporulation from a W303 diploid strain in which one copy of SAL1 was disrupted by replacing its complete coding sequence with the kanMX4 module [13]. The triple aac mutant in which the three isoforms of the ADP/ATP translocator are disrupted was obtained from Dr Jordan Kolarov (Comenius University, Bratislava, Slovakia) [20]. A diploid strain which contains disruptions in SAL1 and all three AAC (ADP/ATP carrier) genes (W303; a/α, leu2-3/aac1::LEU2-3, his3-11/aac2::HIS3-11, ura3-1/aac3::URA3-1, trp1-Δ2/trp1-Δ2, ade2-1/ade2-1, can1-100/can1-100; SAL1/sal1::kanMX4) was generated by crossing the triple aac mutant and the sal1Δ strain.
Media and growth conditions
For the selection of geneticin (G418; Life Technologies) resistance, cells were spread on YPD (1% yeast extract, 2% Bacto peptone and 2% glucose) plates containing 200 μg·ml−1 G418 [13]. For isolation of mitochondria, yeast cells were pre-cultured on minimal medium [11], diluted in YPD and grown until late exponential phase for the ATP transport experiments and for the Sal1p expression studies. Mating and selection of diploid cells heterozygous for disruptions in SAL1 and all three AAC genes were carried out on SM (synthetic minimal medium) with 100 mM sodium acetate as a carbon source, supplemented with 20 μg·ml−1 L-adenine and 20 μg·ml−1 L-tryptophan. Sporulation and tetrad analysis of these diploids was performed using standard methods. Asci were dissected on to YPD medium and incubated at 30 °C for 2–3 days, and the resulting meiotic progenies were grown on YPD with G418 and SM with glucose as a carbon source and lacking uracil, histidine and leucine, to select for the SAL1 and AAC disruptions respectively.
Construction of expression plasmids
The coding sequence of SAL1 (corresponding to open reading frame YNL083w) was amplified from S. cerevisiae genomic DNA by PCR using primers corresponding to the extremities of the coding sequence without the termination codon and with additional BamHI and EcoRI sites. For the amplification assay, the oligonucleotides 5′-aggatccATGCTGATGAAAAATTGCG-3′ and 5′-agaattcGCTGATGGAAACTGCTGGAC-3′ (sites for restriction endonucleases are underlined, and the start codon is in bold) were used as forward and reverse primers respectively. The amplified product was cloned into pSTBlue-1 Blunt vector (Novagen) and verified by sequencing. The BamHI–EcoRI fragment encoding amino acids 1–540 was then cloned into the pUG35 vector, kindly provided by Dr Johannes H. Hegemann (Heinrich-Heine-Universität, Düsseldorf, Germany), in frame with the yEGFP [yeast enhanced GFP (green fluorescent protein)] coding sequence. Finally, the plasmids pYeDP-yEGFP and pYeDP-SAL1-yEGFP were obtained by subcloning the DNA fragments including, respectively, the entire yEGFP open reading frame and coding sequence for Sal1p (amino acids 1–531) C-terminally fused in-frame with yEGFP, Sal1p-yEGFP, into the pYeDP vector [21] downstream of the inducible Gal-10 promoter. The sequences of the inserts were verified by sequencing.
Generation of specific antibodies against Sal1p
Antibodies against the N-terminal extension of Sal1p were generated as follows. An expression construct encoding a fusion protein with a His6 tag at the N-terminus was made by subcloning the 5′-end of SAL1 DNA into pQE30 vector (Qiagen). The overexpressed sequence corresponded to amino acids 1–188. Procedures for overexpression and purification of the recombinant protein, and generation of rabbit antibodies were as described previously [3].
Intracellular localization
The yeast strain W303 was transformed with each of the pYeDP-yEGFP fusion constructs. Cells were grown in YPD to mid-exponential phase and transferred to YPG (2% galactose) for the expression of yEGFP and Sal1p-yEGFP. Then, the cells were incubated for 30 min at 30 °C in the presence of 50 nM MitoTracker Red CMXRos (Molecular Probes), washed twice with PBS and viewed under a microscope. We employed a Leica DM IRB inverted epifluorescence microscope equipped with a Plan-Neofluar 100× oil objective and with a JVC CCD (charge-coupled device) camera using the Leica IM 1000 software.
Western blot analysis
Mitochondria were isolated as described by Arechaga et al. [21]. Briefly, protoplasts were prepared by enzymatic digestion with zymolyase (Seikagaku), and mitochondria were isolated by differential centrifugation after homogenization of protoplasts. The resuspension of final protoplast pellet and all subsequent centrifugations were performed in mitochondrial buffer containing 0.6 M mannitol, 10 mM Tris/maleate, 0.5 mM Na2HPO4, 0.2% BSA, pH 6.8, and protease inhibitors (1 mM PMSF and 1 μg/ml pepstatin A) [11]. The mitochondrial pellet was resuspended in the same buffer, and protein was determined by standard procedures. Proteins were separated by SDS/PAGE and analysed by Western blotting with anti-Sal1p, anti-GFP (green fluorescent protein) (Chemicon) and anti-porin (Molecular Probes) antibodies (diluted 1:5000). We also used monoclonal antibodies against: CPY (carboxypeptidase Y), a vacuolar protein (Molecular Probes); Vps10p, a protein from the late Golgi compartment (Molecular Probes) at 1:10000 and 1:500 dilutions respectively; and supernatant of 3BH5 hybridoma against P0 (provided by Dr J. P. G. Ballesta, Centro de Biología Molecular Severo Ochoa), a ribosomal protein, at 1:10 dilution. Horseradish-peroxidase-conjugated goat anti-rabbit was used at 1:10000 dilution as a secondary antibody for Sal1p and horseradish-peroxidase-conjugated horse anti-mouse antibody was used at 1:1000 dilution as a secondary antibody for porin, GFP, CPY, Vps10p and P0.
ATP transport assays
Mitochondria were obtained as described above. Mitochondria (2 mg of protein/ml) were incubated in the mitochondrial buffer, but containing 2 mM Na2HPO4, and supplemented with 5 mM succinate, 5 mM MgCl2 (unless indicated otherwise) and 4 mM ATP for 0, 5 or 10 min at 30 °C, with mild orbital shaking. CAT (10 μM) was added to inhibit adenine nucleotide transport via the ADP/ATP translocases. The reaction was stopped by adding 1.5 ml of ice-cold buffer (same as incubation buffer but without ATP, succinate and CAT). Mitochondria were collected by centrifugation at 14000 g for 5 min in a benchtop centrifuge, washed with 1.5 ml of the same buffer and stored at −70 °C. Perchloric acid extracts were prepared as described in [11]. ATP and ADP levels were determined enzymatically [22] with the CLSII ATP bioluminescence assay kit (Roche Applied Science). ATP uptake was also determined in similar experiments in the presence of [2,5′,8-3H]ATP (3–6 μCi/ml). The medium also contained [14C]sucrose (1–2 μCi/ml) to correct for external ATP in mitochondrial pellets. The amount of radioactivity was determined by liquid-scintillation counting, using an Optiphase Hisafe 2 (Wallac).
To study calcium dependence of ATP transport, calcium or EGTA was added so as to obtain the free calcium concentrations indicated in the Figures. The concentrations of free calcium were determined fluorimetrically in the presence of mitochondria, all reactants and 0.1 μM Calcium Green 5N (Molecular Probes) and calculated by established procedures for non-ratiometric probes as [Ca2+]free=Kd (F−Fmin)/(Fmax−F) [23]. A Kd of 14 μM for Calcium Green was used. The basal calcium concentration in the transport assays (when no extra calcium is added to the medium) is 6.42 μM. All the calcium points below 6.42 μM required the presence of EGTA (up to 5 mM).
To study the efflux of adenine nucleotides from mitochondria, it was necessary to increase the mitochondrial nucleotide pool size. To this end, mitochondria (10 mg of protein/ml) were pre-incubated in 0.6 M mannitol, 10 mM Tris/maleate, 2 mM Na2HPO4, 5 mM MgCl2, 5 mM succinate and 0.2% BSA, pH 6.8, with 30 μM free calcium and 4 mM [2,5′,8-3H]ATP (3–6 μCi/ml) for 15 min at 30 °C, with mild orbital shaking. The reaction was stopped by adding 1 ml of ice-cold buffer (same as incubation buffer but without ATP, succinate and CAT), and the mitochondria were collected by centrifugation at 14000 g for 5 min in a benchtop centrifuge and transferred (0.5 mg of protein/ml) to incubation medium (0.6 M mannitol, 10 mM Tris/maleate, 5 mM MgCl2, 5 mM succinate and 0.2% BSA, pH 6.8) with or without 10 mM Pi. The efflux reaction was stopped at zero time, and 5 and 10 min later, as described for adenine nucleotide influx studies.
Kinetics of bud emergence
Yeasts were grown on YPD and maintained in stationary phase for 2 days. Then, cells were washed three times and resuspended in a small volume of water, water plus 2% glucose or water plus 2% glucose and 10 mM EGTA for 2 h at 30 °C. The final density of cells was approx. 2×107 cells/ml. Then the cells were pelleted at 4000 g for 5 min and transferred to YPD medium (at the same density) and incubated at 30 °C. The percentage of cells with buds was determined at various times after transfer to rich medium [15,24].
RESULTS
Sal1p is localized to mitochondria
To determinate the cellular localization of Sal1p in yeast cells, we fused yEGFP to its C-terminus to express a C-terminal end yEGFP-tagged Sal1p. In contrast with a cytosolic distribution showed by yEGFP, Sal1p–yEGFP displayed a more restricted distribution and was found exclusively in mitochondria. Fluorescence microscopy revealed the co-localization of the Sal1p–yEGFP signal with that of the mitochondrial marker MitoTracker (Figure 1A).
Figure 1. Sal1p–yEGFP and endogenous Sal1p localize to mitochondria.
(A) Fluorescence microscopy of wild-type W303 cells expressing Sal1p–yEGFP (yeast Sal1p carrier fused to yEGFP from the pYeDP-SAL1-yEGFP vector) or yEGFP (from pYeDP-yEGFP vector) during exponential growth in 2% galactose. MitoTracker Red was used to locate mitochondria in the cells expressing Sal1p–yEGFP (MitoTracker), and phase-contrast microscopy was used to monitor the integrity of the cells. The same cells were visualized first with the MitoTracker filter set and then with a GFP filter set. Identical fields are presented. Scale bar, 5 μm. BF, bright field. (B) Immunoblot of subcellular fractions of wild-type W303 cells expressing Sal1p–yEGFP (left-hand panel) or yEGFP (right-hand panel) during exponential growth on 2% galactose. Blots were probed with antibodies against GFP and porin. M, mitochondria; PM, post-mitochondrial supernatant. Molecular-mass sizes are given in kDa. (C) Endogenous Sal1p was detected in the mitochondrial fraction. Subcellular fractions of wild-type W303 cells during exponential growth on 2% galactose were analysed by Western blotting with antibodies against Sal1p, Porin, P0, CPY and Vps10p. H, total homogenate; M, mitochondria; PM, post-mitochondrial supernatant.
Subcellular fractionation studies also confirmed that Sal1p is a mitochondrial protein (Figure 1B). When whole-cell extracts from wild-type cells expressing Sal1p–yEGFP and yEGFP were separated into post-mitochondrial supernatant and mitochondrial pellet fractions, Sal1p–yEGFP was found in the mitochondrial pellet, along with the integral outer mitochondrial membrane protein porin (Figure 1B). In contrast, Sal1p–yEGFP was not detected at all in the post-mitochondrial supernatant, which contained mainly the expressed yEGFP. The size of the fusion protein detected with anti-GFP antibodies, approx. 85–90 kDa, matched well with the molecular mass for yEGFP, 27 kDa, plus the additional 61 kDa predicted for Sal1p.
To verify that endogenous Sal1p also localizes to mitochondria, mitochondrial and post-mitochondrial fractions from W303 yeast cells were tested for the presence of Sal1p, porin and marker proteins from yeast vacuole, CPY [25], late Golgi compartment, Vps10p [25] and rough endoplasmic reticulum, P0 [26] (Figure 1C). Sal1p and Porin are localized in the mitochondrial fraction, which is free from markers of Golgi, vacuoles and rough endoplasmic reticulum (Figure 1C).
Sal1p is a mitochondrial ATP-Mg carrier
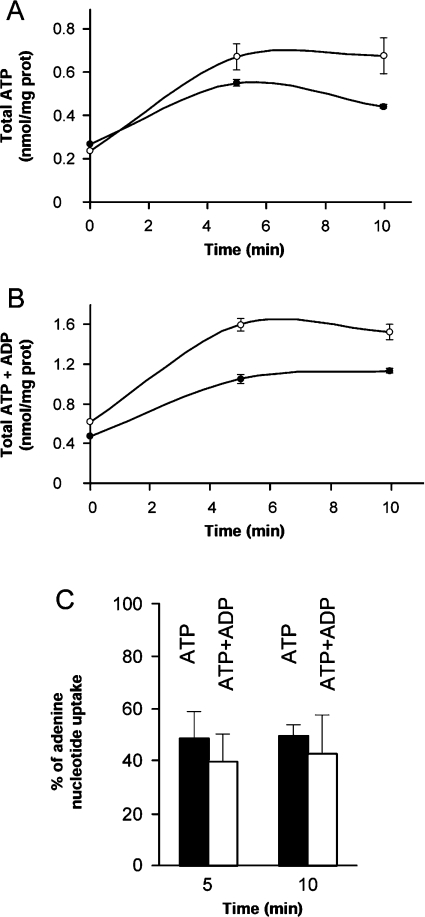
We have tested whether Sal1p catalyses ATP-Mg transport in mitochondria, as shown for the ATP-Mg/Pi carrier identified by Aprille and colleagues [18,19,27] more than 20 years ago. To this end, mitochondria were isolated from sal1Δ and wild-type (W303) yeasts, and were assayed for the transport of ATP-Mg in the presence of CAT, to block adenine nucleotide translocases. Freshly isolated wild-type yeast mitochondria contained approx. 0.23 nmol of ATP per mg of protein, and this increased to 0.7 nmol/mg of protein after 5–10 min of incubation with 4 mM ATP (Figure 2A). The content of ATP+ADP increased in parallel. sal1Δ yeast mitochondria had similar levels of endogenous ATP or ATP+ADP to that from the wild-type strain, but net uptake of adenine nucleotides was substantially diminished, with net uptake of ATP+ADP of 0.98 and 0.58 nmol/mg of protein, for wild-type or sal1Δ yeast mitochondria respectively, after 5 min of incubation (Figure 2B). The mean reduction in adenine nucleotide uptake in sal1Δ mitochondria was highly significant, between 40 and 50% reduction in ATP or ATP+ADP uptake after 5 or 10 min of incubation (Figure 2C).
Figure 2. ATP transport in mitochondria.
Mitochondria (2 mg of protein/ml) from wild-type (○) or sal1Δ (●) yeast were incubated with 4 mM ATP for 0, 5 or 10 min at 30 °C, and the levels of ATP (A) and ATP+ADP (B) were determined enzymatically. The free calcium concentration of the assay was 6.42 μM. Results are the means±S.E.M. for a representative experiment performed in triplicate. (C) Percentage of nucleotide uptake by the sal1Δ strain after incubation with 4 mM ATP for 5 or 10 min at 30 °C. The uptake of the wild-type strain was considered to be 100%. Results are means±S.E.M. for three independent experiments. The difference between wild-type and sal1Δ strains was significant (P<0.05; Student's t test).
The effects of Mg2+ on ATP transport were studied with the aac1,2,3Δ strain, which lacks all three yeast adenine nucleotide translocases [20]. Table 1 shows that when MgCl2 was omitted from the incubation medium, the uptake of [3H]ATP was significantly reduced (40%). However, the absence of MgCl2 did not change the uptake in mitochondria from sal1Δ yeast (1.07±0.05 and 0.97±0.18 nmol of ATP/mg of protein taken up after 5 min with or without Mg2+ respectively), indicating that the effect of Mg2+ is only observed in the presence of Sal1p.
Table 1. Effects of Mg2+ and Pi on ATP transport.
Influx (a) and efflux (b) of 3H-labelled ATP were assayed for 5 and 10 min in the presence or absence of 5 mM MgCl2 for influx studies, and in the presence or absence of 10 mM Pi, for efflux studies. Results (uptake or loss) are means±S.E.M. for one or two experiments performed in triplicate.
| (a) | |||
|---|---|---|---|
| Mitochondrial ATP (nmol/mg of protein) | |||
| MgCl2 concentration (mM) | Incubation time (min)… | 5 | 10 |
| 0 | 0.73±0.11 | 1.22±0.09 | |
| 5 | 1.26±0.06 | 1.96±0.09 | |
| (b) | |||
| Mitochondrial ATP (nmol/mg of protein) | |||
| Pi concentration (mM) | Incubation time (min)… | 5 | 10 |
| 0 | −0.26±0.04 | −0.41±0.08 | |
| 10 | −0.91±0.09 | −1.04±0.04 | |
To verify the requirement for Pi in ATP transport through Sal1p, we have tested its effect on [3H]ATP efflux in mitochondria from aac1,2,3Δ yeast. Table 1 shows that ATP efflux is greatly enhanced when 10 mM Pi is present in the incubation medium, suggesting that Pi is exchanged for ATP-Mg through the carrier. Taken together, these results show that Sal1p is an ATP-Mg/Pi carrier in mitochondria.
Activation by calcium of ATP uptake
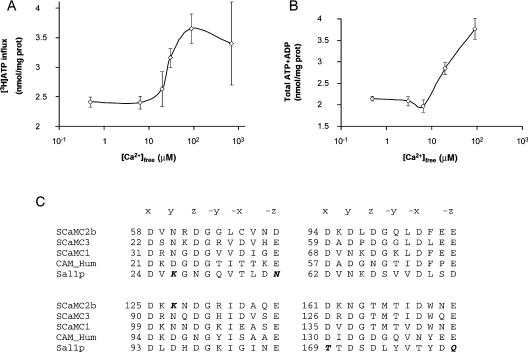
The effects of external calcium on ATP transport were studied with the [3H]ATP method (Figure 3A) and also after analysis of the ATP and ADP content in mitochondria (Figure 3B). ATP entering the mitochondria can be hydrolysed to ADP by the ATPase or via other reactions. Most of the ATP turns to ADP (compare the increase in internal ATP with that of internal ATP+ADP in Figure 2). When unlabelled ATP is used in the assay, ATP uptake is measured as the increase in ATP+ADP, which detects the ATP that has turned into ADP. When labelled ATP is used, the influx of [3H]ATP reports all forms of ATP in mitochondria, including that turned to ADP. Figures 3(A) and 3(B) show that the results are very similar for each method.
Figure 3. Activation by calcium of ATP uptake.
Mitochondria (2 mg of protein/ml) from wild-type cells were incubated with [3H]ATP (A) or unlabelled ATP (B) for 5 min at 30 °C and the indicated calcium concentrations. ATP influx in (A) was corrected for external ATP with [14C]sucrose. The content of ATP+ADP in (B) was determined enzymatically. Results are means±S.E.M. for three independent experiments (A) and for a representative experiment performed in triplicate (B). (C) Alignment of the four putative EF-hand domains in Sal1p, SCaMC1, SCaMC2b, SCaMC3 and human calmodulin (GenBank® accession numbers P48233, AJ619987, AJ619990, AJ619988 and P02593 respectively). The alignment was carried out with the ClustalW program. The amino acids that contribute to the octahedral calcium co-ordination cage are labelled x, y, z, −x, −y and −z. The amino acids that do not satisfy the co-ordination bond of canonical EF-hands are in italic bold type.
A clear stimulation of ATP transport activity at free calcium concentrations above 10 μM was observed for both methods, with an S0.5 of approx. 30 μM. The S0.5 for calcium-activation of yeast Sal1p is somewhat higher than that reported for the rat liver carrier [28,29]. This is perhaps not so surprising as Sal1p lacks two (out of four) canonical EF-hand motifs that are present in human SCaMCs (Figure 3C) [5]. EF-hands 1 and 4 each have two amino acids that do not satisfy the co-ordination bond of canonical EF-hands. EF-hands 2 and 3 are canonical, and Chen [14] showed that they are required for Sal1p function. Thus the lower affinity for calcium of Sal1p with respect to the liver ATP-Mg/Pi carrier may be explained on this basis. On the other hand, mammalian ATP-Mg/Pi carriers probably also include forms with different calcium sensitivities, as SCaMC-2 variants, arising from alternative splicing, lack some of the canonical EF-hand motifs [5].
Sal1p is expressed during growth in glucose and is required for growth in the absence of adenine nucleotide translocases
There is a large number of mitochondrial functions that require ATP in the mitochondrial matrix. Drgon et al. [20] found that a triple deletant of all three adenine nucleotide translocases (aac1,2,3Δ) is still able to grow on glucose, and proposed the existence of another transporter which could supply the mitochondria with adenine nucleotides in these conditions. This transporter would be responsible for the non-lethality of the triple aac mutant and it would be expected that, in its absence, these mutations would be lethal. Since the closest homologues of Sal1p are the adenine nucleotide translocases (42%, 43% and 41% similarity to AAC2, AAC3 and AAC1 respectively), we have analysed whether this protein is responsible for adenine nucleotide transport in aac1,2,3Δ yeast.
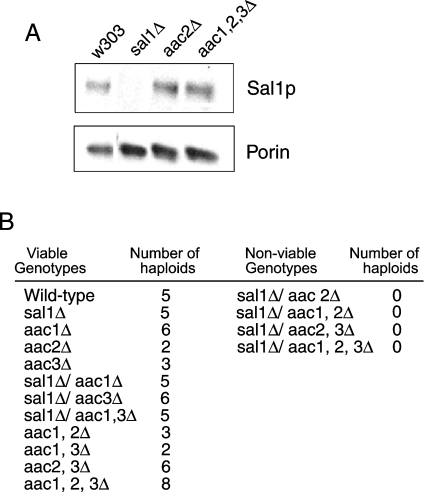
Wild-type and aac1,2,3Δ yeast express Sal1p during growth on glucose (Figure 4A). Thus Sal1p could be responsible for adenine nucleotide transport in the absence of all three translocases during growth on glucose. On the other hand, when diploid yeast containing one single copy of the translocases and SAL1 were induced to sporulate, it was found that spores lacking the three translocases and SAL1 were non-viable (Figure 4B). Moreover, haploid yeast with combinations of aac2Δ and sal1Δ were not obtained. This was not due to a sporulation problem, since each sporulation event produced four spores. A similar finding was reported by Chen [14], who also found that expression of SAL1, AAC2 or AAC3 on a monocopy plasmid was able to rescue the lethality of the aac2Δ sal1Δ mutant. These results support the idea that Sal1p is the ATP carrier that compensates for the lack of translocases during growth on glucose.
Figure 4. Sal1p levels in glucose-grown yeast and effects of SAL1 and AAC deletions on viability.
(A) Western-blot analysis of mitochondria (10 μg) extracted from W303, sal1Δ, aac2Δ and aac1,2,3Δ strains grown in YPD medium. (B) Genotypes of the meiotic progeny of diploid yeast heterozygous for SAL1 and AAC1, AAC2 and AAC3 gene disruptions. Three independent sporulation experiments were performed, with a total of 95 tetrads dissected. The results of one representative experiment are shown. Sporulation of heterozygous strains for AGC1 (the aspartate–glutamate carrier) and AAC1,2,3 gene disruptions gave rise to all possible combinations (results not shown), showing that the lethality of the simultaneous disruption in SAL1 and AAC2 is specific.
Calcium binding to Sal1p is a step in the glucose-sensing pathway
Glucose sensing in yeast involves a signal transduction pathway similar to that employed by hormones in higher eukaryotes, in which both cAMP and calcium are early second messengers (see [16] for review). Glucose binds to the G-protein-coupled receptor Gpr1p [30] implicated in glucose-induced transient elevation of cytosolic calcium through a pathway involving phospholipase C stimulation [31,32] and Ins(1,4,5)P3 production [33]. However, the role of calcium signalling in glucose sensing is presently unknown.
In contrast with mammalian cells, in yeast, glucose-induced calcium transient involves mainly calcium influx from the external medium through the Cch1p/Mid1p plasma membrane calcium channel [17]. By using EGTA during exposure to glucose, Granot and Snyder [24] concluded that calcium entry was not required to generate glucose-induced physiological changes as none of them were blocked in the presence of EGTA. We reasoned that Sal1p may be a target of glucose-induced calcium transient revealed only in an aac2Δ background. Thus we have studied the effects of external calcium on a glucose-induced growth-related event, bud formation, in wild-type, and sal1Δ and triple aac mutants. Figure 5 shows the kinetics of bud formation in stationary-phase wild-type yeast and aac1,2,3Δ mutants exposed to water, glucose or glucose and EGTA before transfer to rich medium. Pre-exposure to glucose resulted in a faster rate of bud formation in wild-type yeast, and this was not changed when EGTA was present during pre-exposure to glucose (Figure 5A), as reported by Granot and Snyder [15,24]. Similar results were obtained with sal1Δ yeast (results not shown). However, in cells that lacked all three translocases, the presence of EGTA during pre-exposure to glucose resulted in a total block of the effect of glucose (Figure 5B). In fact, the kinetics of bud emergence were the same as those obtained after pre-exposure to water. Statistical analysis (ANOVA multifactorial test) of the results in Figure 5(A) indicates that there are no significant differences between pre-exposure to EGTA plus glucose and pre-exposure to glucose alone, but the differences between both of these and pre-exposure to water are highly significant (P<0.0001). In Figure 5(B), there are no significant differences between pre-exposure to EGTA plus glucose and pre-exposure to water, but the differences between both and pre-exposure to glucose are highly significant (P<0.0001). These results show that the lack of all three translocases unmasks a role of calcium signalling in glucose sensing in yeast.
Figure 5. Kinetics of bud emergence for W303 (A) and aac1,2,3Δ (B) strains.
Yeast in stationary phase for 2 days were exposed to 2% glucose (□), water (◇) or 2% glucose plus 10 mM EGTA (△) for 2 h and were then transferred to YPD medium. The percentage of cells with buds was determined at various times after transfer to rich medium. Results are means±S.E.M. for three fields, with more than 100 cells/field. The experiment was repeated three times with similar results.
DISCUSSION
The ATP-Mg/Pi carrier was first identified in the early 1980s as a transport system responsible for the uptake and accumulation of adenine nucleotides by rat liver mitochondria within the first 2 h after birth [34]. The rapid increase in glycolysis immediately after birth results in a large rise in ATP that would trigger adenine nucleotide accumulation in mitochondria in response to adrenaline and glucagon [35], a process required for post-natal mitochondrial maturation [18]. The ATP-Mg/Pi carrier is thought to be important in other conditions: for example, in the recovery of the mitochondrial adenine nucleotide content after hypoxia or ischaemia [19]. The carrier catalyses an electroneutral exchange of ATP-Mg for Pi [27] which is activated by submicromolar calcium concentrations from the external side of the mitochondria [28,29]. In humans, the three SCaMCs appear to be isoforms of the ATP-Mg/Pi carrier and make up a complex family of mitochondrial carriers that includes various splicing variants [5,6,36]. We now show that Sal1p is the yeast orthologue of human SCaMCs, and it drives net ATP-Mg uptake in mitochondria. Our results also show that Sal1p is calcium-sensitive, a property not observed previously in assays with human SCaMC isoforms reconstituted in proteoliposomes [6]. The S0.5 for activation of yeast Sal1p is approx. 30 μM, and, at maximal calcium concentrations, ATP transport is activated approx. 2-fold.
Our results show that Sal1p is non-essential in the presence of Aac2p, but it becomes essential in its absence, unmasking a role of calcium in glucose sensing in yeast. Glucose sensing in yeast is required to tune growth and metabolic activity to the amount and type of sugars that are available. Glucose sensing involves a signal transduction pathway similar to that employed by hormones in higher eukaryotes, in which both cAMP and calcium are early second messengers (see [16] for review). Glucose binds to the G-protein-coupled receptor Gpr1p [30], and a Gα protein Gpa2p is thought to act as a stimulatory G-protein on adenylate cyclase. Gpa2p is negatively regulated by a RGS (regulator of heterotrimeric G-protein signalling) protein Rgs2p [16]. Hence, the Gpr1p, Gpa2p and Rgs2p system is proposed to act as a glucose-sensing system to control the cAMP pathway by glucose availability. The same G-protein-coupled receptor system has also been implicated in glucose-induced transient elevation of cytosolic calcium through a pathway involving phospholipase C stimulation [32] and Ins(1,4,5)P3 production [33]. However, differing from mammalian cells, yeast glucose-induced calcium transient involves mainly calcium influx from the external medium through the Cch1p/Mid1p plasma membrane calcium channel, and, as for the cAMP pathway, glucose uptake and phosphorylation are required to obtain a full response [17].
Granot and Snyder [24] concluded that calcium entry was not required to generate glucose-induced physiological changes, as none of them was blocked in the presence of EGTA in the external medium. Thus the relevant targets, if any, of the glucose-induced calcium transient have remained unknown. Our results indicate that calcium binding to Sal1p is a component of the glucose-sensing system that becomes essential in yeast that lack adenine nucleotide translocases. Chen [14] showed that mutations in the EF-hand motifs of Sal1p that eliminated calcium binding also abrogated the ability of the protein to suppress the lethality of aac2Δ sal1Δ double mutants, suggesting that calcium binding by the protein is critical for its function during growth on glucose.
The inducing signals for the glucose-induced early growth events have not yet been identified. One possible inducing signal could be ATP, because it is a product of glycolysis. However, glucose-starved cells contain high levels of ATP [37,38], but do not divide, making ATP an unlikely inducing signal. Our hypothesis is that one of the possible signals is not ATP itself, but the entry of ATP into mitochondria. There are two ways to transport the ATP into the mitochondria: the ADP/ATP translocases (Aac1p, Aac2p and Aac3p) or Sal1p. All three translocases have an R-function (ADP/ATP exchange, required for respiratory growth). In addition, Aac2p and Aac3p (Aac3p only when AAC3 is overexpressed from the AAC2 promoter), but not Aac1p, have a V-function [14] which, as suggested by Chen, could be net transport of adenine nucleotides [14]. Sal1p shares this V-function (but does not have an R-function, because it cannot rescue, when overexpressed, growth in glycerol [14]), and we have demonstrated that it catalyses a net uptake of ATP. We believe this influx of nucleotides (V-function) is responsible for the glucose-induced growth. When Aac2p is functional, the calcium signal generated by glucose is not needed, because the translocases are not calcium-dependent. This could explain why the sal1Δ strain is viable and has no apparent phenotype under all the situations tested. But, in cells lacking Aac2p, the only way to perform net uptake of ATP is through Sal1p, which is calcium-dependent. In this situation, the calcium signal generated by glucose is needed. This is in agreement with the observation that the EF-hand motifs of Sal1p are needed for viability in the absence of Aac2p [14]. Further work is needed to test whether this model is correct.
Acknowledgments
This work was supported by grants from the Spanish Ministerio de Ciencia y Tecnología (PM1998-021 and BMC2002-02072), Ministerio de Sanidad (FIS 01/0395), and Química Farmaceútica Bayer, S.A. The Centro de Biología Molecular Severo Ochoa is the recipient of institutional aid from the Ramón Areces Foundation. The expert technical assistance of Barbara Sesé and Inmaculada Ocaña is gratefully acknowledged. J. T. is a recipient of a I3P CSIC fellowship. We thank Dr Jordan Kolarov for the supply of the aac1,2,3Δ mutant, Dr Johannes H. Hegemann for the supply of pUG35 plasmid, and Dr J. P. G. Ballesta and Dr M. Remacha (Centro de Biología Molecular Severo Ochoa) for the supply of the sal1Δ mutant and monoclonal antibodies against P0, CPY and Vps10p.
References
- 1.Carafoli E. The calcium-signalling saga: tap water and protein crystals. Nat. Rev. Mol. Cell Biol. 2003;4:326–332. doi: 10.1038/nrm1073. [DOI] [PubMed] [Google Scholar]
- 2.Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem. Sci. 2003;28:175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 3.del Arco A., Satrústegui J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J. Biol. Chem. 1998;273:23327–23334. doi: 10.1074/jbc.273.36.23327. [DOI] [PubMed] [Google Scholar]
- 4.del Arco A., Agudo M., Satrústegui J. Characterization of a second member of the subfamily of calcium-binding mitochondrial carriers expressed in human non-excitable tissues. Biochem. J. 2000;345:725–732. doi: 10.1042/0264-6021:3450725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Arco A., Satrústegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 6.Fiermonte G., De Leonardis F., Todisco S., Palmieri L., Lasorsa F. M., Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. J. Biol. Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri L., Pardo B., Lasorsa F. M., del Arco A., Kobayashi K., Iijima M., Runswick M. J., Walker J. E., Saheki T., Satrústegui J., Palmieri F. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasorsa F. M., Pinton P., Palmieri L., Fiermonte G., Rizzuto R., Palmieri F. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J. Biol. Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 9.Carafoli E., Lehninger A. L. A survey of the interaction of calcium ions with mitochondria from different species. Biochem. J. 1971;122:681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols B. J., Rigoulet M., Denton R. M. Comparison of the effects of Ca2+, adenine nucleotides and pH on the kinetic properties of mitochondrial NAD+-isocitrate dehydrogenase and oxoglutarate dehydrogenase from the yeast Saccharomyces cerevisiae and rat heart. Biochem. J. 1994;303:461–465. doi: 10.1042/bj3030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavero S., Vozza A., del Arco A., Palmieri L., Villa A., Blanco E., Runswick M. J., Walker J. E., Cerdán S., Palmieri F., Satrústegui J. Identification and metabolic role of the mitochondrial aspartate–glutamate transporter in Saccharomyces cerevisiae. Mol. Microbiol. 2003;50:1257–1269. doi: 10.1046/j.1365-2958.2003.03742.x. [DOI] [PubMed] [Google Scholar]
- 12.Soler-Mira A., Saiz J. E., Ballesta J. P. G., Remacha M. The sequence of a 17,933 bp segment of Saccharomyces cerevisiae chromosome XIV contains the RHO2, TOP2, MKT1 and END3 genes and five new open reading frames. Yeast. 1996;12:485–491. doi: 10.1002/(sici)1097-0061(199604)12:5<485::aid-yea928>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Zúniga S., Boskovic J., Jiménez A., Ballesta J. P., Remacha M. Disruption of six Saccharomyces cerevisiae novel genes and phenotypic analysis of the deletants. Yeast. 1999;15:945–953. doi: 10.1002/(SICI)1097-0061(199907)15:10B<945::AID-YEA394>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen X. J. Sal1p, a calcium-dependent carrier protein that suppresses an essential cellular function associated with the Aac2 isoform of ADP/ATP translocase in Saccharomyces cerevisiae. Genetics. 2004;167:607–617. doi: 10.1534/genetics.103.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granot D., Snyder M. Glucose induces cAMP-independent growth-related changes in stationary-phase cells of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5724–5728. doi: 10.1073/pnas.88.13.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolland F., Winderickx J., Thevelein J. M. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002;2:183–201. doi: 10.1111/j.1567-1364.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 17.Tökés-Füzesi M., Bedwell D. M., Repa I., Sipos K., Sümegi B., Rab A., Miseta A. Hexose phosphorylation and the putative calcium channel component Mid1p are required for the hexose-induced transient elevation of cytosolic calcium response in Saccharomyces cerevisae. Mol. Microbiol. 2002;44:1299–1308. doi: 10.1046/j.1365-2958.2002.02956.x. [DOI] [PubMed] [Google Scholar]
- 18.Aprille J. R., Austin J. A. Regulation of the mitochondrial adenine nucleotide pool size. Arch. Biochem. Biophys. 1981;212:689–699. doi: 10.1016/0003-9861(81)90413-6. [DOI] [PubMed] [Google Scholar]
- 19.Aprille J. R. Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role. FASEB J. 1988;2:2547–2556. doi: 10.1096/fasebj.2.10.3290024. [DOI] [PubMed] [Google Scholar]
- 20.Drgon T., Sabova L., Nelson N., Kolarov J. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett. 1991;289:159–162. doi: 10.1016/0014-5793(91)81059-h. [DOI] [PubMed] [Google Scholar]
- 21.Arechaga I., Raimbault S., Prieto S., Levi-Meyrueis C., Zaragoza P., Miroux B., Ricquier D., Bouillaud F., Rial E. Cysteine residues are not essential for uncoupling protein function. Biochem. J. 1993;296:693–700. doi: 10.1042/bj2960693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz F., Álvarez G., Ramos M., Hernández M., Bogónez E., Satrústegui J. Cyclosporin A targets involved in protection against glutamate excitotoxicity. Eur. J. Pharmacol. 2000;404:29–39. doi: 10.1016/s0014-2999(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Granot D., Snyder M. Carbon source induces growth of stationary phase yeast cells, independent of carbon source metabolism. Yeast. 1993;9:465–479. doi: 10.1002/yea.320090503. [DOI] [PubMed] [Google Scholar]
- 25.Cooper A. A., Stevens T. H. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos C., Ballesta J. P. Ribosomal protein P0, contrary to phosphoproteins P1 and P2, is required for ribosome activity and Saccharomyces cerevisiae viability. J. Biol. Chem. 1995;269:15689–15696. [PubMed] [Google Scholar]
- 27.Joyal J. L., Aprille J. R. The ATP-Mg/Pi carrier of rat liver mitochondria catalyzes a divalent electroneutral exchange. J. Biol. Chem. 1992;267:19198–19203. [PubMed] [Google Scholar]
- 28.Haynes R. C., Picking R. A., Zaks W. J. Control of mitochondrial content of adenine nucleotides by submicromolar calcium concentrations and its relationship to hormonal effects. J. Biol. Chem. 1986;261:16121–16125. [PubMed] [Google Scholar]
- 29.Nosek M. T., Dransfield D. T., Aprille J. R. Calcium stimulates ATP-Mg/Pi carrier activity in rat liver mitochondria. J. Biol. Chem. 1990;265:8444–8450. [PubMed] [Google Scholar]
- 30.Lemaire K., Van de Velde S., Van Dijck P., Thevelein J. M. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell. 2004;16:293–299. doi: 10.1016/j.molcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Ansari K., Martin S., Farkasovsky M., Ehbrecht I. M., Kuntzel H. Phospholipase C binds to the receptor-like GPR1 protein and controls pseudohyphal differentiation in Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:30052–30058. doi: 10.1074/jbc.274.42.30052. [DOI] [PubMed] [Google Scholar]
- 32.Tisi R., Baldassa S., Belotti F., Martegani E. Phospholipase C is required for glucose-induced calcium influx in budding yeast. FEBS Lett. 2002;520:133–138. doi: 10.1016/s0014-5793(02)02806-5. [DOI] [PubMed] [Google Scholar]
- 33.Tisi R., Belotti F., Wera S., Winderickx J., Thevelein J. M., Martegani E. Evidence for inositol trisphosphate as a second messenger for glucose-induced calcium signalling in budding yeast. Curr. Genet. 2004;45:83–89. doi: 10.1007/s00294-003-0465-5. [DOI] [PubMed] [Google Scholar]
- 34.Pollak J. K., Sutton R. The transport and accumulation of adenine nucleotides during mitochondrial biogenesis. Biochem. J. 1980;192:75–83. doi: 10.1042/bj1920075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton R., Pollak J. K. Hormone-initiated maturation of rat liver mitochondria after birth. Biochem. J. 1980;186:361–367. doi: 10.1042/bj1860361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Arco A. Novel variants of human SCaMC-3, an isoform of the ATP-Mg/Pi mitochondrial carrier, generated by alternative splicing from 3′-flanking transposable elements. Biochem. J. 2005;389:647–655. doi: 10.1042/BJ20050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillies R. J., Ugurbil K., den Hollander J. A., Shulman R. G. 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1981;78:2125–2129. doi: 10.1073/pnas.78.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K.-B., Boronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979;18:4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]