Abstract
We prepared CHO (Chinese hamster ovary) cells expressing both IR (insulin receptor) and A1R (A1 adenosine receptor). Treatment of the cells with insulin or PIA [N6-(2-phenylisopropyl)adenosine], a specific A1R agonist increased Akt activity in the cells in a PI3K- (phosphoinositide 3-kinase) dependent manner. Transfection of p110β into the cells augmented the action of PIA with little effect on insulin. Introduction of a pH1 vector producing shRNA (short hairpin RNA) that targets p110β abolished PIA-induced Akt activation. By contrast, an shRNA probe targeting p110α did not impair the effects of PIA. The effect of PIA in p110α-deficient cells was attenuated effectively by both Δp85 and βARK-CT (β-adrenergic receptor kinase-C-terminal peptide). A Δp85-derived protein possessing point mutations in its two SH2 domains did not impair PIA action. These results suggest that tyrosine-phosphorylated proteins and Gβγ (βγ subunits of GTP-binding protein) are necessary for the specific function of p110β in intact cells. The p110β-middle (middle part of p110β) may play an important role in signal reception from GPCRs (GTP-binding-protein-coupled receptor), because transfection of the middle part impaired PIA sensitivity.
Keywords: adenosine, Akt, Gβγ subunit, GTP-binding-protein-coupled receptor (GPCR), p110β, phosphoinositide 3-kinase, short-hairpin RNA (shRNA)
Abbreviations: A1R, A1 adenosine receptor; βARK-CT, β-adrenergic receptor kinase-C-terminal peptide; CHO, Chinese hamster ovary; DTT, dithiothreitol; EGF, epithelial growth factor; FBS, foetal bovine serum; Gβγ, βγ subunits of GTP-binding protein; GPCR, GTP-binding-protein-coupled receptor; GFP, green fluorescent protein; IGF-1, insulin growth factor-1; IR, insulin receptor; LPA, lysophosphatidic acid; p110β-middle, middle part of p110β; PDGF, platelet-derived growth factor; PIA, N6-(2-phenylisopropyl)adenosine; PI3K, phosphoinositide 3-kinase; PtdIns(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate; PP, pyrazolopyrimidine; RTK, receptor tyrosine kinase; RT-PCR, reverse transcription-PCR; shRNA, short hairpin RNA; siRNA, small interfering RNA
INTRODUCTION
PI3K (phosphoinositide 3-kinase) is a lipid kinase that phosphorylates the D-3 position of the inositol ring of phosphoinositides. Class I PI3Ks are under the control of cell-surface receptors, including RTKs (receptor tyrosine kinase) and GPCRs (G protein-coupled receptors) and produce PtdIns(3,4,5)P3 (phosphatidylinositol 3,4,5-trisphosphate) in cells. The catalytic subunits of class IA isoforms p110α, β and δ form a complex with the p85 regulatory subunit, whereas the only member of class IB subclass p110γ, is associated with the p101 subunit (for review see [1]).
Cross-linking of RTKs produces tyrosine-phosphorylated proteins including the receptors themselves and adaptor proteins, such as IR (insulin receptor) substrates. Specific binding of these phosphorylated proteins to the SH2 domains of p85 causes translocation of the class IA PI3Ks to the plasma membrane, thereby facilitating access to their lipid substrate. At the same time, this binding causes an increase in the specific activity of p110 by attenuating the inhibitory effect of p85 on the catalytic subunit [2–4]. The catalytic subunit of class IB PI3K subclass p110γ, does not form a complex with p85 but is instead activated directly by Gβγ (βγ subunits of GTP-binding protein). The tightly associated p101 subunit seems not to be essential for Gβγ-induced activation of p110γ but plays some role in recruitment of p110γ to the plasma membrane [5]. The non-catalytic p101 subunit is also known to determine the substrate specificity of p110γ [6,7]. The molecular mechanism of the Gβγ-induced activation of p110γ has been shown to involve the direct interaction of Gβγ with both the NH2- and COOH-terminals of p110γ [8].
The above features suggest that the major isoform of PI3K transmitting the signal from GPCR is p110γ. This must also be true in haematopoietic cells known to express high levels of p110γ, as shown by the observation that neutrophils from mice lacking p110γ show impaired sensitivity to agonists that activate pertussis toxin-sensitive GTP-binding proteins [9]. On the other hand, there are several lines of evidence suggesting that GPCRs cause activation of class IA PI3K in many cell lines including haematopoietic cells [7,10–15]. As one of the possible mechanisms of this activation, we and another group have previously reported that p110β, one of the catalytic subunits of class IA PI3Ks, are activated by Gβγ in cell-free systems [7,16]. Other members of class IA PI3Ks, p110α and p110δ, showed no sensitivity to Gβγ. However, the role of p110β in mediating the GPCR signal should be addressed further in intact cell systems because we have reported previously that the Gβγ sensitivity to PI3K activity is highly susceptible to assay conditions [10]. Moreover, the structural basis for the Gβγ sensitivity of p110β has not been examined.
In the present study, we prepared CHO (Chinese hamster ovary) cells expressing both IR and A1R (A1 adenosine receptor), as representatives of RTKs and GPCRs respectively, and examined the effects of manipulations that alter the ratio of p110β/p110α in these cells. The results confirmed that the p110β subtype is essential for the GPCR-mediated activation of PI3K in intact cells. Both the tyrosine-phosphorylated proteins and Gβγ are necessary for this activation. The results also suggest that the structure of p110β in its middle part is important for its specific function.
MATERIALS AND METHODS
Reagents and antibodies
The materials were obtained from the following sources: [3H]cyclopentyl-1,3-dipropylxanthine, 32Pi and [γ-32P]ATP were from Perkin Elmer Life Sciences (Norwalk, CT, U.S.A.); PIA [N6-(phenylisopropyl)-adenosine], insulin and anti-Myc antibody (9E10) were from Sigma (St. Louis, MO, U.S.A.); AG1296, AG1478, 1-O-Me-AG538, PP (pyrazolopyrimidine) 2, PP3 and genistein were from Calbiochem (La Jolla, CA, U.S.A.). Crosstide was from Upstate Biotechnology (Charlottesville, MO, U.S.A.); anti-phospho-Akt (anti-pT308 and anti-pS473) antibodies were from Cell Signaling Technology (Beverly, MA, U.S.A.); anti-p110α and anti-p110β antibodies were from B.D. Biosciences Clontech (Franklin Lakes, NJ, U.S.A.) and Santa Cruz Biotechnology (CA, U.S.A.) respectively. Anti-(mouse IgG) agarose beads were from American Qualex Antibodies (San Clemente, CA, U.S.A.); glutathione-Sepharose 4B beads were from Amersham Pharmacia (Uppsala, Sweden); wortmannin was from Kyowa Medex (Tokyo, Japan) and LY294002 was from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). All other reagents from commercial sources were of analytical grade.
Plasmids
Expression vectors for epitope-tagged mouse Akt (pCMV5-Myc–Akt), bovine p110α (pCMV5-p110α), human p110β (pCMV5-p110β), and epitope-tagged bovine p85α (pCMV5-FLAG–p85α) were provided by Dr T. Katada (University of Tokyo, Japan). The expression vector for canine A1R (pCDL-SRα296-A1R) was from Dr F. Okajima (Gunma University, Maebashi City, Japan). A plasmid encoding the C-terminal (CT) region of βARK1 (β-adrenergic receptor kinase1) pcDNA3-βARK1-CT, a plasmid encoding a dominant negative mutant of bovine p85 (pCMV5-FLAG-Δp85) and the pH1 vector for expression of shRNAs (short hairpin RNA) were kindly provided by Dr H. Kurose (Kyushu University, Fukuoka, Japan), Dr M. Kasuga (Kobe University, Kobe, Japan) and Dr N. Inoue (Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan) respectively.
Cell lines
CHO cells expressing human IRs (CHO-IR) were kindly given by Dr Y. Ebina (University of Tokushima, Japan) and were maintained in Ham's F-12 medium (Sigma) supplemented with 10% FBS (foetal bovine serum), 100 μg/ml of streptomycin and 100 U/ml penicillin under an atmosphere of 5% CO2 at a temperature of 37 °C. The cells in 90 mm dishes were transfected with 5.4 μg of the pCDL-SRα296 expression vector containing the A1R cDNA insert together with 0.6 μg of pSV2bsr, a blasticidin-S deaminase expression plasmid, using the LipofectAMINE Plus™ reagent (Invitrogen) in accordance with the manufacturer's instructions. The cells were then cultured in the presence of 5 μg/ml blasticidin-S, and resistant colonies were replated after 14 days of treatment. The cloned cells thus obtained (CHO-IR-A1R cells) were examined for the level of A1R expression by a radioligand-binding assay before use.
Radioligand binding
Crude membranes from CHO-IR-A1R cells were suspended in 50 mM Tris/HCl (pH 7.4) at a protein concentration of 2–4 mg/ml and stored in aliquots at −80 °C. For saturation-binding experiments, 5 μg aliquots of membrane protein were incubated in buffer containing 0.2–10 nM [3H]cyclopentyl-1,3-dipropylxanthine and 1 unit/ml adenosine deaminase. After incubation at 25 °C for 60 min, the reaction was terminated by rapid filtration under suction over glass fibre filters, which had been treated with 0.3% polyethylenimine. The radioactivity that bound to the filter even in the presence of 1 mM theophylline was regarded as non-specific binding. Scatchard analysis of the results indicated that the cells used in the present study possessed the A1R with a Bmax value of 6.6 pmol/mg of membrane protein and Kd value of 8.8 nM.
cAMP accumulation
CHO-IR-A1R cells were grown to confluence in six-well plates and washed twice with an incubation buffer consisting of 130 mM NaCl, 4.7 mM KCl, 1 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM Hepes-NaOH (pH 7.4) and 0.1% (w/v) BSA. The cells were incubated at 37 °C for 10 min in the presence or absence of PIA and for a further 10 min with the addition of 50 μM forskolin. The reaction was stopped by removing the buffer and addition of cold 0.1 M HCl. The cells were transferred to sample tubes, boiled for 3 min and then centrifuged at 7500 g for 5 min. A 50 μl aliquot of the supernatant was used for measurement of cAMP using a radio-immunoassay kit (Yamasa, Tokyo, Japan) according to the manufacturer's instructions.
PtdIns(3,4,5)P3 production
Serum-starved CHO-IR-A1R cells (1.6×106 cells) in six-well plates were washed twice with Pi-free RPMI 1640 medium supplemented with 0.1% (w/v) BSA and incubated for 30 min in the same medium with the addition of 50 μCi/ml carrier-free 32Pi. The cells were washed twice with buffer. After incubation at 37 °C for 5 min with or without insulin or PIA, the reaction was stopped by mixing with 0.6 ml of 1% (w/v) HClO4. To the mixture was added to 2.25 ml of chloroform/methanol (1:2, v/v), stirred vigorously and then mixed successively with 0.75 ml of chloroform and 0.75 ml of 1% (w/v) HClO4. The organic phase was washed once with chloroform-saturated solution containing 0.5 M NaCl and 1% HClO4 before drying. The extract was spotted onto Silica Gel 60 plates (Merck, Darmstadt, Germany), which had been impregnated with 1.2% (w/v) potassium oxalate in methanol/water (2:3, v/v) and heated at 110 °C for 20 min. The plates were developed in chloroform/methanol/acetic acid/acetone/water (70:50:20:20:20, by vol.) and the radioactivity in the PtdIns(3,4,5)P3 spot was located using a Fuji BAS2000 analyser (Fuji, Tokyo, Japan).
Akt activation
CHO-IR-A1R cells grown on six-well plates were transfected with pCMV5-Myc–Akt, together with the indicated plasmids as necessary, using FuGENE 6 reagent (Roche Diagnostics, Tokyo, Japan) or LipofectAMINE Plus™ reagent (Invitrogen). The total amount of DNA was adjusted to 1.0–2.0 μg/well. After stimulation with insulin or PIA for 4 min, the cells were washed twice and lysed on ice using 0.2 ml of cold buffer consisting of 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 0.27 M sucrose, 1% (w/v) Nonidet P 40, 1 mM EGTA, 1 mM EDTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulphonyl fluoride and 1 mM DTT (dithiothreitol). The lysate was centrifuged for 10 min at 6500 g and the supernatant was incubated with 3 μg of anti-Myc antibody at 4 °C for 1.5 h. The immune complex was collected using anti-(mouse IgG)-conjugated agarose beads, which were washed twice with the same buffer and then twice with 50 mM Tris/HCl (pH 7.4) containing 0.1 mM EGTA and 1 mM DTT. The immunoprecipitates were resuspended in 20 μl of buffer consisting of 50 mM Tris/HCl (pH 7.4), 0.25 mCi/ml [γ-32P]ATP, 0.1 mM ATP, 10 mM MgCl2, 30 μM Crosstide, 0.1 mM EGTA and 1 mM DTT. After incubation at 30 °C for 15 min, the reaction mixture was cooled in an ice-bath and centrifuged at 6500 g for 20 s. An aliquot of the sample was spotted onto P81 paper (Whatman, Brentford, Middlesex, U.K.), which was washed 5 times with 1% phosphoric acid and the radioactivity remaining on the paper was determined.
In some experiments, the immune complexes were subjected to electrophoresis on 10% SDS/PAGE. The fractionated proteins were transferred onto PVDF membranes (Millipore, Bedford, MA, U.S.A.) and analysed by Western blotting using active Akt-directed or anti-Myc antibodies.
RNA interference
The partial nt sequences of CHO versions of p110α and p110β were determined (GenBank® accession number DQ073082 and DQ073081) and the target sequence suitable for the RNA interference technique was selected according to the literature [17,18]. The sequence 5′-GCACATCTACAACAAGTTA-3′ was selected to interfere with p110α expression, whereas the sequence 5′-GCTTAACACAGAAGAAACT-3′ was selected for p110β. For each of these sequences a pair of oligonucleotides was synthesized with sequences 5′-CCC(X)19TTCAAGAGA(Y)19TTTTTGGAAA-3′ and 5′-CTAGTTTCCAAAAA(Y)19TCTCTTGAA-(X)19GGGTGCA, where (X)19 is the coding sequence and (Y)19 is the complementary sequence. The oligonucleotide pair was annealed and ligated downstream of the H1-RNA promoter at the PstI and XbaI sites of the pH1 vector. The vector was transfected into cells with LipofectAMINE Plus™ reagent. The cells were maintained in the presence of 8 μg/ml puromycin for 7 days and resistant colonies were replated.
RT-PCR (reverse transcription-PCR)
Total RNA of cells was isolated and purified using an RNeasy Protect kit (Qiagen, Hilden, Germany). Samples of 2 μg were constructed in nuclease-free water, heated for 5 min at 70 °C, and mixed with First-Strand Buffer (Invitrogen) containing 10 units/μl Moloney-murine leukaemia virus reverse transcriptase (Invitrogen), 10 mM DTT, 1 mM dNTP mixture, 1 unit/μl RNasin (Promega, Madison, WI, U.S.A.), and 25 μg/ml random primers (Promega). The mixture was incubated at 37 °C for 90 min and cooled to 4 °C before analysis using a PCR system (Applied Biosystems, Foster City, CA, U.S.A.). For species-specific determination of p110α and p110β the primers 5′-AAAGAAGCTGTGGATCTGC-3′ forward and 5′-CAGCATGCTCCGAGTC-3′ reverse; 5′-TTGTAAAGAAGCTGTGGATCTTA-3′ forward and 5′-TTGTTCAGATGATAGCAACATACTT-3′ reverse; 5′-CACCGGAGCATGAGG-3′ forward and 5′-GGATGTTCTCCAAATACATACTCC-3′ reverse; 5′-CATATCCACCAGAGCATGAAC-3′ forward and 5′-GGATGATCACCAAAAACATATTCT-3′ reverse were used for CHO-p110α, bovine p110α, CHO-p110β and human p110β respectively. PCR was performed for 13 to 28 cycles, during which the exponential phase of the reaction was included. The size of the PCR products was checked by electrophoresis on 8.0% polyacrylamide gels.
RESULTS
Preparation of CHO-IR cells expressing A1R
CHO-IR cells were transfected with the pCDL-SRα296 expression vector containing a canine A1R cDNA insert together with a blasticidin-S resistance pSV2-bsr plasmid using LipofectAMINE Plus™ reagent. The cells showing resistance to blasticidin-S were cloned and examined for specific binding of [3H]cyclopentyl-1,3-dipropylxanthine, a specific radioligand for A1Rs. The clone expressing the highest number of receptors (6.6 pmol/mg of membrane protein with a Kd value of 8.8 nM) was used in the present study.
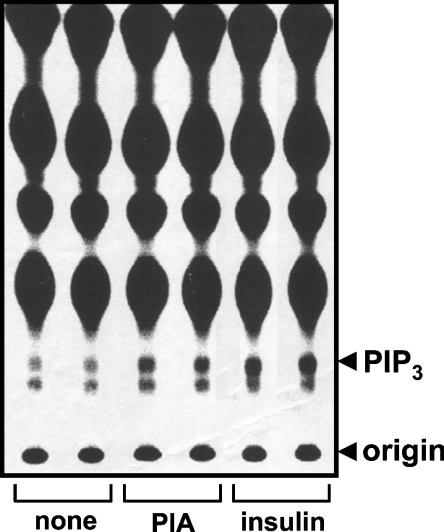
The functional integrity of the receptors was first checked by monitoring their ability to control the adenylate cyclase activity of the cells. Treatment of the cells with 50 μM forskolin increased the cAMP level from 1 to 40 pmol/105 cells. The specific A1R agonist, N6-(2-phenylisopropyl)adenosine, inhibited this increase in a dose–dependent manner with an IC50 value of approx, 4 nM (results not shown). The cells were then examined for their ability to produce PtdIns(3,4,5)P3, a product of PI3Ks. Stimulation of 32Pi-labelled cells with PIA resulted in a marked increase in the incorporation of radioactivity into the PtdIns(3,4,5)P3 fraction, indicating that the A1Rs were functional in stimulating PI3K activity in these cells (Figure 1). The PtdIns(3,4,5)P3 spot disappeared in cells treated with the PI3K inhibitors, wortmannin or LY294002 (results not shown).
Figure 1. Effects of PIA and insulin on PtdIns(3,4,5)P3 production in CHO-IR-A1R cells.
CHO-IR-A1R cells, labelled with 32Pi, were stimulated with 1 μM PIA or 100 nM insulin for 5 min. Phospholipids were extracted and separated on oxalate-impregnated TLC plates. An autoradiogram of a TLC plate is shown. PIP3, PtdIns(3,4,5)P3.
Reciprocal effects of p110α and p110β on the PIA-induced activation of Akt
cAkt, also known as protein kinase B, is a downstream target of PI3K that plays pivotal roles in the regulation of cell survival, proliferation, angiogenesis and glucose metabolism (for review, see [19]). We reported previously that activation of A1Rs in rat adipocytes increases Akt activity under certain conditions [20]. Therefore we examined whether PIA activates Akt activity in the newly established CHO-IR-A1R cells. Firstly, we measured the protein kinase activity of Myc–Akt transiently transfected into the cells (Figure 2, open bars). Stimulation of the cells with PIA increased the protein kinase activity in a dose–dependent manner. However, the effect of 1 μM PIA was still much weaker than that of 0.1 μM insulin. The effects of both PIA and insulin were completely inhibited by wortmannin and LY294002 (results not shown), indicating that the Myc–Akt activity reflected that of PI3K in the cells.
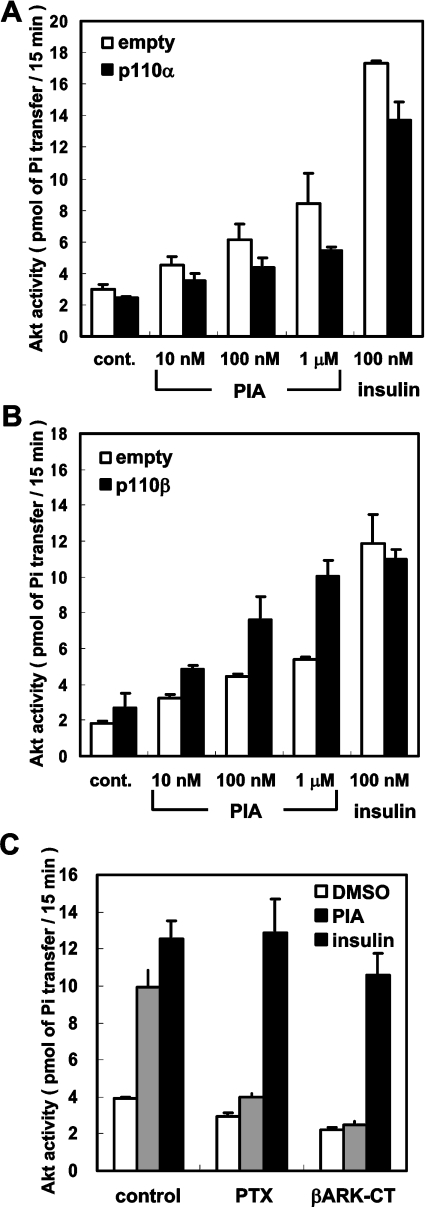
Figure 2. Effects of p110β expression on PIA-induced Akt activation.
CHO-IR-A1R cells transfected with Myc–Akt were stimulated with PIA or insulin for 4 min. The cell lysate was mixed with anti-Myc antibody and the immune complex was assayed for protein kinase activity with Crosstide as the substrate. Each bar represents the mean±S.D. of triplicate determinations. In (A) p110α or control empty vector was co-transfected with Myc–Akt. In (B) p110β or control empty vector was co-transfected with Myc–Akt. In (C) p110β together with βARK-CT or control vector was co-transfected with Myc–Akt. Treatment with pertussis toxin (PTX) was performed by incubating the cells with 10 ng/ml of the toxin for 16 h before stimulation.
We and another group have previously reported that p110β is activated by the Gβγ in cell-free systems, whereas p110α is not [7,17]. In a cell-line derived from NIH3T3 cells apparently lacking p110β, GPCR agonists such as carbachol and LPA (lysophosphatidic acid) fail to activate Akt [14]. Therefore we examined whether overexpression of each p110 subtype had any effect on PIA action. Transfection of p110α or p110β did not change the action of insulin (Figure 2). The effect of PIA was augmented by transfection with p110β and the effect of 1 μM PIA was as prominent as that for insulin after the transfection (Figure 2B, solid bars). By contrast, transfection with p110α decreased the PIA-induced Akt activation (Figure 2A, solid bars), suggesting that a decrease in the p110β/p110α ratio is sufficient to impair the action of PIA. The effect of PIA in the p110β-transfected cells was abolished by treatment with pertussis toxin or by transfection with βARK1-CT, a scavenger of Gβγ (Figure 2C). By contrast, the effect of insulin was not affected by these treatments. These results indicate that the mechanism by which PIA activates Akt includes liberation of Gβγ from pertussis toxin-sensitive GTP-binding proteins and is different from that utilized by insulin.
Inhibition of PIA-induced Akt activation by p110β-specific shRNA
To further examine the mechanism of PIA-induced Akt activation, we prepared cells lacking either the p110α or p110β subtype of PI3K using a vector-based expression system that produces shRNAs. For this purpose, we first determined the nt sequence of p110α and p110β in our CHO cells, and selected suitable sites for the knockout technique (Figure 3A) based on the information available in the literature [17,18].
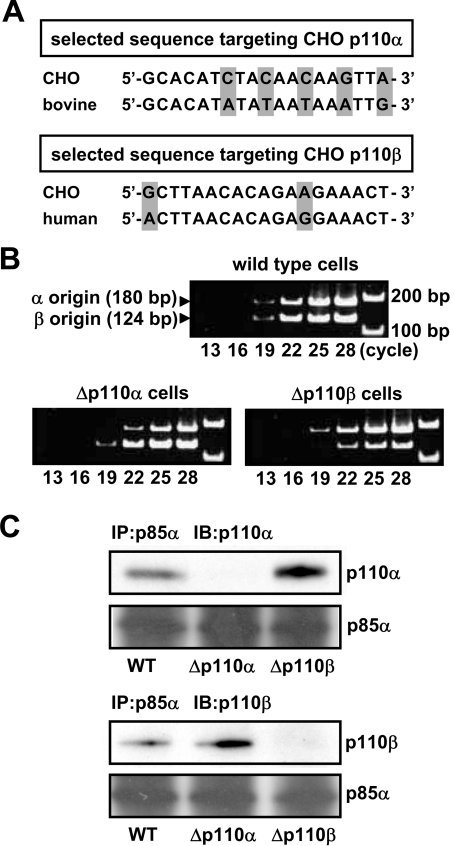
Figure 3. Preparation of Δp110α and Δp110β cells.
(A) The panel shows the sense sequences of the CHO versions of p110α and p110β genes selected as targets of the siRNA gene-silencing technique. The corresponding sequences of bovine p110α and human p110β are also shown. (B) CHO-IR-A1R cells were transfected with the pH1 expression vector and producing shRNA targeting the sequences of CHO-p110α and CHO-p110β using LipofectAMINE Plus™, cells resistant to 8 μg/ml puromycin were selected. Total RNA from the cells was analysed for the mRNAs of p110α and p110β by RT-PCR using gene-specific primers. (C) The lysate from Δp110α or Δp110β cells was mixed with the anti-serum against p85 and the immune complex was subjected to Western blotting using the specific antibody against p110α or p110β. IB, immunoblot; IP, immunoprecipitation.
Oligonucleotides containing the selected sequences were inserted downstream of the H1-RNA promoter of the pH1 expression vector to produce shRNAs. The vectors were transfected into CHO-IR-A1R cells, and the puromycin-resistant cells were used for subsequent analyses. Figure 3(B) shows the results of semi-quantitative RT-PCR for determination of the mRNA levels of p110α and p110β. The results indicated that each shRNA caused decreases in the expression of either subtype of more than 70%. In Figure 3(C), cell lysates were treated with the anti-serum against p85 and the immune complex was examined by specific antibodies against p110α and p110β (Figure 3C). The results confirmed the specific knockdown of either subtype at the protein level. Figure 4(A) shows the results when these cells were stimulated with PIA or insulin and examined for the level of active Akt using an antibody against the phosphorylated Thr-308 of Akt. Deficiency of p110β (Δp110β cells) markedly impaired the effects of PIA without altering the action of insulin indicating that the PIA-induced effect was highly dependent on p110β activity in the cells. By contrast, PIA caused marked activation of Akt in p110α-deficient (i.e. p110β-dominant) Δp110α cells. The use of another active Akt-directed antibody directed against phosphorylated Ser-473 showed similar results (Figure 4B).
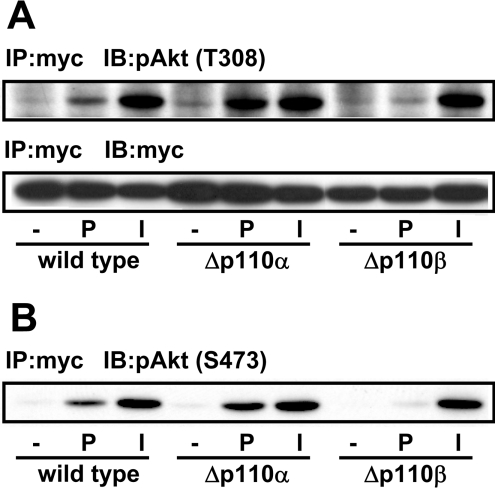
Figure 4. Effect of p110β knockdown on PIA-induced Akt activation.
(A) The Δp110α or Δp110β cells transfected with Myc–Akt were stimulated with 1 μM PIA (P) or 0.1 μM insulin (I). The cell lysate was subjected to immunoprecipitation (IP) with anti-Myc antibody followed by Western blotting with anti-Myc and anti-pAkt (pT308) antibodies (IB). (B) The blot in (A) was reprobed with anti-pAkt (pS473) antibody.
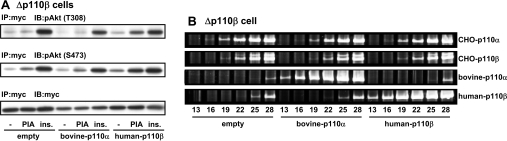
In order to check the specificity of the action of the anti-p110β probe, we examined whether the PIA sensitivity of Δp110β cells was recovered by expression of the shRNA-resistant p110β. For this purpose, we attempted to use a human version of p110β because its nt sequence is different from that of the CHO version (see Figure 3A). Expression of human p110β effectively rescued the PIA-induced activation of Akt, whereas expression of p110α (the bovine version was used here as the human version was not available) did not increase the action of PIA (Figure 5A). One possible explanation for the results is that expression of human p110β prevented the function of the anti-CHO-p110β probe. However, semi-quantitative RT-PCR analysis indicated that expression of human p110β did not increase mRNA levels of the CHO version (Figure 5B). Thus the catalytic activity of human p110β was considered to be important for the recovery of PIA action. These results indicate the crucial role of p110β in transmitting the GPCR signal to Akt in intact cells.
Figure 5. Recovery of PIA action by transfection of shRNA-resistant p110β.
(A) The Δp110β cells were transfected with Myc–Akt, together with bovine version of p110α or human version of p110β. The cells were then stimulated with 1 μM PIA or 0.1 μM insulin (ins.). The cell lysate was subjected to immunoprecipitation with anti-Myc antibody (IP) followed by Western blotting with anti-pAkt (pT308), anti-pAkt (pS473) and anti-Myc antibodies (IB). (B) Total RNA from the cells was analysed for the mRNAs of CHO-p110α, CHO-p110β, bovine p110α and human p110β by RT-PCR using gene-specific primers.
The mechanism of PIA-induced p110β activation
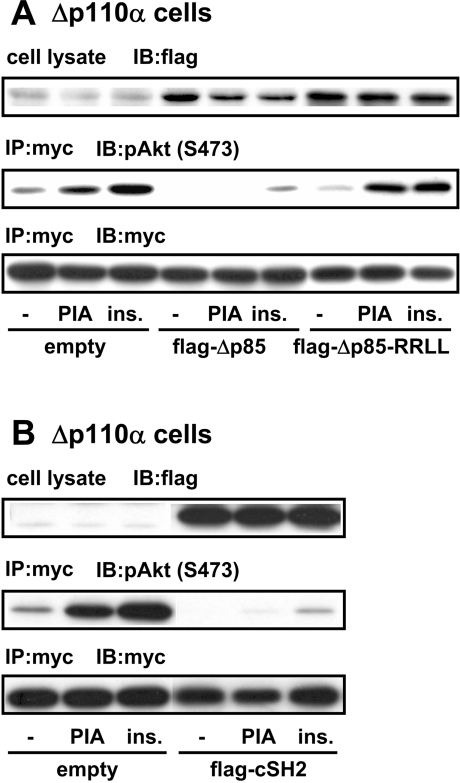
We next tried to gain further insight into a mechanism of GPCR-induced activation of p110β. Both p110α and p110β are known to be complexed with a common regulatory subunit p85, the absence of which greatly impaired the stabiltity of the catalytic subunits in cells. The binding of tyrosine-phosphorylated proteins to SH2 domains in p85 is one of the mechanisms leading to p110 activation. It has been demonstrated in several cell lines that Δp85, a mutant p85 regulatory subunit lacking the binding site to p110, inhibits GPCR-induced cellular events [21–24]. This observation suggests that the GPCR-derived signal is transmitted through p85 to p110β. Thus we examined the effect of Δp85 on p110β-dominant (Δp110α) cells. Transfection of Δp85 effectively impaired the Akt activation induced by both PIA and insulin (Figure 6A). Transfection of the SH2 domain of p85 showed a similar inhibitory effect on the action of PIA (Figure 6B). By contrast, Δp85-R358L/R649L, a Δp85-derived protein lacking the ability to bind tyrosine-phosphorylated proteins due to mutations in two SH2 domains, did not attenuate the Akt activation (Figure 6A).
Figure 6. Effects of Δp85 on the action of PIA in p110α-deficient cells.
Δp110α cells were transfected with Myc–Akt, together with FLAG-Δp85 (in A), FLAG-Δp85-R358L/R649L (FLAG-Δp85-RRLL in A), FLAG-tagged C-SH2 domain of p85 (FLAG-cSH2 in B) or empty vector. After stimulation of the cells with 1 μM PIA or 0.1 μM insulin, the cell lysate was prepared and subjected to immunoprecipitation with anti-Myc antibody (IP). The immune complex was analysed by Western blotting with anti-FLAG, anti-pAkt (pS473) and anti-Myc antibodies (IB).
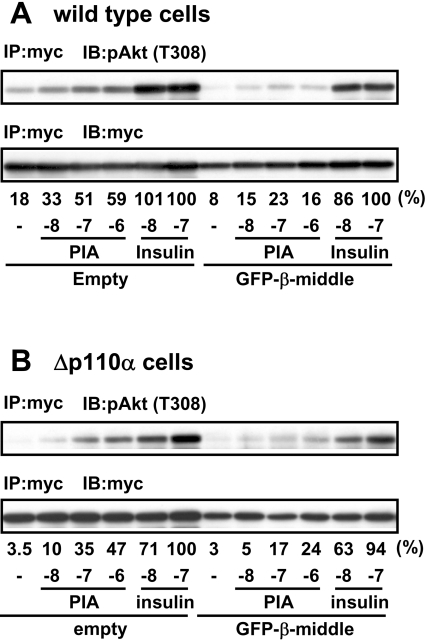
The above result suggested that binding of some tyrosine-phosphorylated protein to the SH2 domain of p85 is necessary for the PIA-induced activation of p110β. One critical question regarding this p85-mediated activation of p110β is why PIA does not activate p110α, which is also regulated by this common regulatory subunit. As a possible basis for the specific activation, we and another group have previously reported that p110β, but not p110α, can be activated by Gβγ in cell-free systems [7,17]. Thus it is speculated that direct interaction of p110β with Gβγ is responsible for its specific function. If so, it can also be considered that there exists a specific binding site for Gβγ in the structure of p110β. Although the overall structures of p110α and p110β are similar, alignment of their amino acid sequences indicated a lower level of sequence identity in their middle parts (24–352 and 34–349 of p110α and p110β respectively). The result of structural analysis suggested that this region appears on the surface of the p110 proteins [25]. Thus we examined whether the middle part of p110β impaired the PIA signal by acting as a dominant negative protein. Because the small protein itself was not produced in CHO cells (results not shown), a fusion protein with GFP (green fluorescent protein) was used in the experiment. As shown, expression of the GFP–p110β-middle (middle part of p110β) into wild-type (Figure 7A) and Δp110α (Figure 7B) cells impaired PIA-induced Akt activation, suggesting that the middle region is important in receiving a signal from the GPCR.
Figure 7. Effect of p110β-middle protein on the action of PIA.
Wild-type CHO-IR-A1R cells (A) or Δp110α cells (B) were transfected with Myc–Akt, together with GFP-p110β-middle or empty vector and then stimulated with PIA or insulin. The cell lysate was subjected to immunoprecipitation with anti-Myc antibody (IP) followed by Western blotting with anti-pAkt (pT308) and anti-Myc antibodies (IB). The blots were analysed by densitometer and the density of pAkt was normalised to that of Myc. The relative values are presented just below the autoradiogram, as percentage of 0.1 μM insulin in vector-control cells; the values below this are the concentrations of PIA or insulin (mole/l), represented by logarithm unit {log[M]}.
DISCUSSION
The members of the class I PI3K family play pivotal roles in the transmission of signals from membrane receptors (for review, see [26]). Three members of this family possess the binding site to an adaptor protein, p85, and are grouped into the class IA subfamily. The only member of the class IB subfamily (p110γ) does not bind p85 but associates with p101 protein.
Originally, these two subclasses were considered to correspond to the receptor families from which they receive signals [27]. Class IA PI3K is activated by RTKs through interaction between p85 and tyrosine-phosphorylated motifs. Class IB PI3K is activated by GPCRs through direct interaction with Gβγ. However, there is accumulating evidence that class IA PI3K is able to transmit the signals derived from GPCRs.
In the present study, we showed the specific role of p110β in transmitting a GPCR signal to Akt by use of a siRNA (small interfering RNA) gene-silencing technique. Specific knockdown of p110β abolished PIA-induced Akt activation (Figure 4). In agreement with this result, a previous report showed that GPCR agonists fail to activate Akt in a unique cell line derived from NIH3T3 cells that apparently lack p110β [14]. In contrast with GPCR, the IR-derived signal is transmitted by both p110α and p110β, because specific knockdown of either subtype did not abolish the insulin-induced Akt activation (Figure 4). This insulin action is considered to function through p85, a common regulatory subunit of class IA PI3Ks, as proved by a well-known fact that a mutant p85 lacking the binding site to p110s (Δp85) behaves as a dominant negative protein [21]. We observed in the present study that Δp85 attenuated the action of insulin in both Δp110α and Δp110β cells (Figure 6).
In several cell lines, the βARK-CT that traps Gβγ has been shown to inhibit GPCR-induced Akt activation [14,28–31]. The PIA-induced activation of Akt in CHO-IR-A1R cells was again inhibited by the Gβγ scavenger (Figure 2C). The PIA action is abolished by cell treatment with pertussis toxin (Figure 2C). Thus it should be considered that PIA activates pertussis toxin-sensitive GTP-binding protein and produces Gβγ, which in turn activates p110β. In this scenario, an intriguing question is whether or not Gβγ transmits its signal through p85.
It has been reported that expression of Δp85 effectively attenuates the action of GPCR agonists [13,15]. We observed in the present study that the effect of PIA is attenuated by Δp85 in p110β-dominant Δp110α cells (Figure 6A). A Δp85-derived protein lacking the ability to bind tyrosine-phosphorylated proteins did not abolish the PIA-induced Akt activation (Figure 6A). Thus one possible explanation is that Gβγ produces tyrosine-phosphorylated proteins, which in turn activate p110β by binding to the SH2 domains of p85. The inhibition of the PIA action by expression of the C-SH2 domain of p85 (Figure 6B) supports this possibility.
There is ample evidence indicating that GPCRs activate tyrosine kinases including RTKs and members of the Src family [13,15,32–37]. In the case of GPCR-induced Erk activation the effects of GPCR agonists have been shown to be mediated by the RTKs including EGF (epithelial growth factor), PDGF (platelet-derived growth factor) and IGF-1 (insulin growth factor-1) receptors [13,15,32,33,36,37].
Furthermore, LPA-induced activation of Akt in Vero cells has been reported to accompany the tyrosine-phosphorylation of Gab1 [13,15]. This transactivation mechanism may play a role in the PIA-induced activation of p110β. However, the identity of the tyrosine kinase operating in CHO cells has yet to be clarified because AG1478, AG1296, 1-O-Me-AG538 and PP2 (inhibitors of EGF receptors, PDGF receptors, IGF-1 receptors and Src family tyrosine kinases respectively), which have been shown to inhibit the GPCR-induced activation of Erk in several cell lines, had no effect on Akt activation (results not shown).
A critical question regarding the above scenario is why PIA does not activate p110α despite the observation that p85 is the common regulatory subunit of both p110α and p110β. Thus it is speculated that some difference in the properties of p110α and p110β may be involved in the selective activation by PIA. It is reported that p110α possesses a protein kinase activity phosphorylating a serine residue in p85, whereas p110β is less effective in this phosphorylation [38]. Because the phosphorylation of p85 decreased the lipid kinase activity of the p85–p110 complex, it is intriguing to consider that the p110α-induced phosphorylation of p85 masked a potential action of PIA on p85–p110α. By this mechanism, however, it is difficult to explain why the insulin-induced (p85-mediated) activation of Akt was not impaired in both Δp110α and Δp110β cells (Figure 4).
In cell-free systems, we and another group have previously reported that the lipid kinase activity of p110β is activated by Gβγ whereas that of p110α is not [7,17]. A further in vitro experiment showed that both a monomeric p110β and a p85–p110β complex are activated by Gβγ in the same concentration range [39]. On the other hand, we have reported that the Gβγ-induced activation of class IA PI3K is effectively augmented in the presence of a synthetic tyrosine-phosphorylated peptide, especially when lower concentrations of Gβγ were used for the assay [10]. Another research group has confirmed the augmentation of the Gβγ-induced p85–p110β activation by a tyrosine-phosphorylated peptide [7]. Thus it is intriguing to consider that a direct interaction of Gβγ with p110β is necessary when a signal from GPCR to p85 produces the effective activation of p85–p110β complex in intact cell systems.
A recent study confirmed the direct interaction of Gβγ with both NH2- and COOH-terminal regions of p110γ, a Gβγ-sensitive class IB PI3K [8]. By contrast, the structural background of p110β involved in its Gβγ sensitivity has not been clarified. As a first step to address this problem, in the present study we examined whether a protein derived from p110β (p110β-middle) behaves as a dominant negative protein to the PIA-induced cellular events. The result indicated that expression of p110β-middle inhibited the PIA-induced activation of Akt probably by acting like the βARK-CT (Figure 6). We also attempted to examine whether a chimaera p110β possessing the structure of p110α in its middle part (p110βαβ) loses its sensitivity to Gβγ. This attempt was, however, unsuccessful because the chimaera protein did not possess a kinase activity in vitro (results not shown). A further experiment is necessary to determine whether p110β-middle really has the ability to interact with Gβγ.
In summary, we confirmed the specific role of p110β in mediating a GPCR signal to Akt. It has been reported that an siRNA probe, specific for p110β, impairs the growth of HeLa cells in Matrigel [40]. A study using a microinjection technique with selective antibodies indicated a specific role for p110β in RBL-2H3 cells [41]. Thus further studies are required to determine the role of p110 subtypes in cell functions, that have been examined using non-selective probes such as Δp85 and chemical inhibitors.
Acknowledgments
We thank Dr N. Inoue (Osaka Medical Center for Cancer and Cardiovascular Diseases, Japan) for providing the pH1 vector for the expression of shRNAs. We also thank Dr F. Okajima (Gunma University, Maebashi City, Japan) for the A1R plasmid.
References
- 1.Shepherd P. R., Withers D. J., Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J., White M. F. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter C. L., Auger K. R., Chanudhuri M., Yoakim M., Schaffhausen B., Shoelson S., Cantley L. C. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J. Biol. Chem. 1993;268:9478–9483. [PubMed] [Google Scholar]
- 4.Yu J., Zhang Y., McIlroy J., Rordorf Nikolic T., Orr G. A., Backer J. M. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock C., Schaefer M., Reusch H. P., Czupalla C., Michalke M., Spicher K., Schultz G., Nurnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J. Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens L. R., Eguinoa A., Erdjument Bromage H., Lui M., Cooke F., Coadwell J., Smrcka A. S., Thelen M., Cadwallader K., Tempst P., Hawkins P. T. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 7.Maier U., Babich A., Nurnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J. Biol. Chem. 1999;274:29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- 8.Leopoldt D., Hanck T., Exner T., Maier U., Wetzker R., Nurnberg B. Gβγ stimulates phosphoinositide 3-kinase-γ by direct interaction with two domains of the catalytic p110 subunit. J. Biol. Chem. 1998;273:7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T., Irie-Sasaki J., Jones R. G., Oliveira-dos-Santos A. J., Stanford W. L., Bolon B., Wakeham A., Itie A., Bouchard D., Kozieradzki I., et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science (Washington, DC) 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 10.Okada T., Hazeki O., Ui M., Katada T. Synergistic activation of PtdIns 3-kinase by tyrosine-phosphorylated peptide and βγ-subunits of GTP-binding proteins. Biochem. J. 1996;317:475–480. doi: 10.1042/bj3170475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche S., Downward J., Raynal P., Courtneidge S. A. A function for phosphatidylinositol 3-kinase β (p85α-p110β) in fibroblasts during mitogenesis: requirement for insulin- and lysophosphatidic acid-mediated signal transduction. Mol. Cell. Biol. 1998;18:7119–7129. doi: 10.1128/mcb.18.12.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graness A., Adomeit A., Heinze R., Wetzker R., Liebmann C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase β, and protein kinase Cϵ. J. Biol. Chem. 1998;273:32016–32022. doi: 10.1074/jbc.273.48.32016. [DOI] [PubMed] [Google Scholar]
- 13.Laffargue M., Raynal P., Yart A., Peres C., Wetzker R., Roche S., Payrastre B., Chap H. An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phosphoinositide 3-kinase by lysophosphatidic acid. J. Biol. Chem. 1999;274:32835–32841. doi: 10.1074/jbc.274.46.32835. [DOI] [PubMed] [Google Scholar]
- 14.Murga C., Fukuhara S., Gutkind J. S. A novel role for phosphatidylinositol 3-kinase β in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- 15.Yart A., Roche S., Wetzker R., Laffargue M., Tonks N., Mayeux P., Chap H., Raynal P. A function for phosphoinositide 3-kinase β lipid products in coupling βγ to Ras activation in response to lysophosphatidic acid. J. Biol. Chem. 2002;277:21167–21178. doi: 10.1074/jbc.M110411200. [DOI] [PubMed] [Google Scholar]
- 16.Kurosu H., Maehama T., Okada T., Yamamoto T., Hoshino S., Fukui Y., Ui M., Hazeki O., Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 17.Ui-Tei K., Naito Y., Takahashi F., Haraguchi T., Ohki-Hamazaki H., Juni A., Ueda R., Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32:936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 19.Vanhaesebroeck B., Alessi D. R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 20.Takasuga S., Katada T., Ui M., Hazeki O. Enhancement by adenosine of insulin-induced activation of phosphoinositide 3-kinase and protein kinase B in rat adipocytes. J. Biol. Chem. 1999;274:19545–19550. doi: 10.1074/jbc.274.28.19545. [DOI] [PubMed] [Google Scholar]
- 21.Hara K., Yonezawa K., Sakaue H., Ando A., Kotani K., Kitamura T., Kitamura Y., Ueda H., Stephens L., Jackson T. R., et al. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimi N., Tremblay E., Elliott B. Phosphatidylinositol 3-kinase activity is required for hepatocyte growth factor-induced mitogenic signals in epithelial cells. J. Biol. Chem. 1996;271:24850–24855. doi: 10.1074/jbc.271.40.24850. [DOI] [PubMed] [Google Scholar]
- 23.Sajan M. P., Standaert M. L., Bandyopadhyay G., Quon M. J., Burke T. R., Jr., Farese R. V. Protein kinase C-ζ and phosphoinositide-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J. Biol. Chem. 1999;274:30495–30500. doi: 10.1074/jbc.274.43.30495. [DOI] [PubMed] [Google Scholar]
- 24.Hsu A. L., Ching T. T., Sen G., Wang D. S., Bondada S., Authi K. S., Chen C. S. Novel function of phosphoinositide 3-kinase in T cell Ca2+ signaling. A phosphatidylinositol 3,4,5-trisphosphate-mediated Ca2+ entry mechanism. J. Biol. Chem. 2000;275:16242–16250. doi: 10.1074/jbc.M002077200. [DOI] [PubMed] [Google Scholar]
- 25.Djordjevic S., Driscoll P. C. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem. Sci. 2002;27:426–432. doi: 10.1016/s0968-0004(02)02136-9. [DOI] [PubMed] [Google Scholar]
- 26.Wymann M. P., Zvelebil M., Laffargue M. Phosphoinositide 3-kinase signaling–which way to target? Trends Pharmacol. Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 27.Stoyanov B., Volinia S., Hanck T., Rubio I., Loubtchenkov M., Malek D., Stoyanova S., Vanhaesebroeck B., Dhand R., Nurnberg B., Gierschik P., Seedorf K., Hsuan J. J., Waterfield M. D., Wetzker R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science (Washington, DC) 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 28.Koch W. J., Hawes B. E., Inglese J., Luttrell L. M., Lefkowitz R. J. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J. Biol. Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 29.Koch W. J., Hawes B. E., Allen L. F., Lefkowitz R. J. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by Gβγ activation of p21ras. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neptune E. R., Bourne H. R. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo H., Sipos K., Go Y. M., Law R., Rong J., McDonald J. M. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gβγ-dependent signaling pathways. J. Biol. Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 32.Herrlich A., Daub H., Knebel A., Herrlich P., Ullrich A., Schultz G., Gudermann T. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeneman S., Haendeler J., Saito Y., Ishida M., Berk B. C. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor β receptor. Key role for the p66 adaptor protein Shc. J. Biol. Chem. 2000;275:15926–15932. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- 34.Luttrell L. M., Della Rocca G. J., van Biesen T., Luttrell D. K., Lefkowitz R. J. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J. Biol. Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 35.Bisotto S., Fixman E. D. Src-family tyrosine kinases, phosphoinositide 3-kinase and Gab1 regulate extracellular signal-regulated kinase 1 activation induced by the type A endothelin-1 G-protein-coupled receptor. Biochem. J. 2001;360:77–85. doi: 10.1042/0264-6021:3600077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., Pyne N. J. Tethering of the platelet-derived growth factor β receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- 37.Zahradka P., Litchie B., Storie B., Helwer G. Transactivation of the IGF-1 receptor by angiotensin II mediates downstream signaling from the AT1 receptor to phosphatidylinositol 3-kinase. Endocrinology. 2004;19:2978–2987. doi: 10.1210/en.2004-0029. [DOI] [PubMed] [Google Scholar]
- 38.Foukas L. C., Beeton C. A., Jensen J., Phillips W. A., Shepherd P. R. Regulation of phosphoinositide 3-kinase by its intrinsic serine kinase activity in vivo. Mol. Cell. Biol. 2004;24:966–975. doi: 10.1128/MCB.24.3.966-975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maier U., Babich A., Macrez N., Leopoldt D., Gierschik P., Illenberger D., Nürnberg B. Gβ5γ2 is a highly selective activator of phospholipid-dependent enzymes. J. Biol. Chem. 2000;275:13746–13754. doi: 10.1074/jbc.275.18.13746. [DOI] [PubMed] [Google Scholar]
- 40.Czauderna F., Fechtner M., Aygun H., Arnold W., Klippel A., Giese K., Kaufmann J. Functional studies of the PI(3)-kinase signaling pathway employing synthetic and expressed siRNA. Nucleic Acids Res. 2003;31:670–682. doi: 10.1093/nar/gkg141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith A. J., Surviladze Z., Gaudet E. A., Backer J. M., Mitchell C. A., Wilson B. S. p110β and p110δ phosphatidylinositol 3-kinases up-regulate Fc(ϵ)RI-activated Ca2+ influx by enhancing inositol 1,4,5-trisphosphate production. J. Biol. Chem. 2001;276:17213–17220. doi: 10.1074/jbc.M100417200. [DOI] [PubMed] [Google Scholar]