Abstract
Smooth muscle contraction is activated by phosphorylation at Ser-19 of LC20 (the 20 kDa light chains of myosin II) by Ca2+/calmodulin-dependent MLCK (myosin light-chain kinase). Diphosphorylation of LC20 at Ser-19 and Thr-18 is observed in smooth muscle tissues and cultured cells in response to various contractile stimuli, and in pathological circumstances associated with hypercontractility. MLCP (myosin light-chain phosphatase) inhibition can lead to LC20 diphosphorylation and Ca2+-independent contraction, which is not attributable to MLCK. Two kinases have emerged as candidates for Ca2+-independent LC20 diphosphorylation: ILK (integrin-linked kinase) and ZIPK (zipper-interacting protein kinase). Triton X-100-skinned rat caudal arterial smooth muscle was used to investigate the relative importance of ILK and ZIPK in Ca2+-independent, microcystin (phosphatase inhibitor)-induced LC20 diphosphorylation and contraction. Western blotting and in-gel kinase assays revealed that both kinases were retained in this preparation. Ca2+-independent contraction of calmodulin-depleted tissue in response to microcystin was resistant to MLCK inhibitors [AV25 (a 25-amino-acid peptide derived from the autoinhibitory domain of MLCK), ML-7, ML-9 and wortmannin], protein kinase C inhibitor (GF109203X) and Rho-associated kinase inhibitors (Y-27632 and H-1152), but blocked by the non-selective kinase inhibitor staurosporine. ZIPK was inhibited by AV25 (IC50 0.63±0.05 μM), whereas ILK was insensitive to AV25 (at concentrations as high as 100 μM). AV25 had no effect on Ca2+-independent, microcystin-induced LC20 mono- or di-phosphorylation, with a modest effect on force. We conclude that direct inhibition of MLCP in the absence of Ca2+ unmasks ILK activity, which phosphorylates LC20 at Ser-19 and Thr-18 to induce contraction. ILK is probably the kinase responsible for myosin diphosphorylation in vascular smooth muscle cells and tissues.
Keywords: integrin-linked kinase, microcystin, myosin light-chain phosphatase, myosin phosphorylation, vascular smooth muscle, zipper-interacting protein kinase
Abbreviations: AV25, 25-amino-acid peptide derived from the autoinhibitory domain of MLCK; [Ca2+]i, cytosolic free Ca2+ concentration; CaMKI/II, Ca2+/calmodulin-dependent protein kinases I and II respectively; DTT, dithiothreitol; GST, glutathione S-transferase; ILK, integrin-linked kinase; LC20, 20 kDa light chains of myosin II; MAPKAPK2, mitogen-activated-protein-kinase-activated protein kinase-2; MLCK, myosin light-chain kinase; MLCP, myosin light-chain phosphatase; MYPT1, myosin-targeting subunit of MLCP; PGF2α, prostaglandin F2α; PKC, protein kinase C; CPI-17, PKC-potentiated inhibitory protein for PP1 (protein phosphatase type 1) of 17 kDa; ROK, Rho-associated kinase; ZIPK, zipper-interacting protein kinase
INTRODUCTION
Smooth muscle contraction is activated primarily by phosphorylation at Ser-19 of LC20 (the 20 kDa light chains of myosin II) catalysed by Ca2+/calmodulin-dependent MLCK (myosin light-chain kinase) in response to a variety of stimuli, including membrane depolarization, agonists that act on seven-transmembrane-domain-containing G-protein-coupled receptors such as the α1-adrenoceptor, and shear stress, all of which trigger an increase in [Ca2+]i (cytosolic free Ca2+ concentration) [1,2]. Numerous contractile stimuli also elicit Ca2+ sensitization (increased force at a given [Ca2+]i) [3] through protein-kinase-mediated inhibition of MLCP (myosin light-chain phosphatase), a type 1 protein serine/threonine phosphatase [4], either directly by phosphorylation of MYPT1 (the myosin-targeting subunit of MLCP) [5], or indirectly via phosphorylation of CPI-17 [PKC (protein kinase C)-potentiated inhibitory protein for PP1 (protein phosphatase type 1) of 17 kDa], which becomes a potent MLCP inhibitor when phosphorylated at Thr-38 [6].
The type 1 and 2A phosphatase inhibitor microcystin, which directly inhibits these phosphatases by interaction with the catalytic subunit [7], when added to Triton X-100-skinned rat caudal arterial smooth muscle elicited a Ca2+-independent contraction, which correlated with phosphorylation of LC20 at Ser-19 and Thr-18 [8]. Thiophosphorylated CPI-17, a more specific MLCP inhibitor [9], has a similar effect [6,10]. Since MLCK is absolutely dependent on Ca2+ and calmodulin for activity [8], and only phosphorylates Thr-18 of LC20 when present at very high (non-physiological) concentrations [11], the possibility arose that a distinct kinase, which is Ca2+-independent, is responsible for the observed microcystin-induced phosphorylation of LC20 and contraction. This was confirmed by the demonstration that microcystin-induced LC20 phosphorylation and contraction at pCa 9 were unaffected by a peptide inhibitor of MLCK, AV25 (a 25-amino-acid peptide derived from the autoinhibitory domain of MLCK), and that Ca2+-independent myosin kinase activity could be separated from MLCK by differential extraction from smooth muscle myofilaments and by affinity chromatography on immobilized calmodulin [8]. We concluded that inhibition of MLCP by microcystin unmasks a basal activity of Ca2+-independent myosin kinase, which then phosphorylates LC20 at Ser-19 and Thr-18 to activate cross-bridge cycling and force development. We proceeded to isolate the Ca2+-independent LC20 kinase, and identified it as ILK (integrin-linked kinase) [12]. Independently, Murata-Hori et al. [13] and Niiro and Ikebe [14] demonstrated that ZIPK (zipper-interacting protein kinase) can also phosphorylate LC20 at Ser-19 and Thr-18 in a Ca2+/calmodulin-independent manner. However, it was unclear from the latter study whether the effect of ZIPK was due to direct phosphorylation of LC20 or inhibition of MLCP via phosphorylation of MYPT1 [15].
In the present paper, we describe a series of experiments designed to determine whether ILK, ZIPK or both kinases are responsible for microcystin-induced, Ca2+-independent phosphorylation of LC20 and contraction of vascular smooth muscle (rat caudal artery).
EXPERIMENTAL
Materials
[γ-32P]ATP (>5000 Ci/mmol) and trifluoperazine were purchased from ICN Biomedical Inc. (Aurora, OH, U.S.A.), microcystin-LR was from Alexis Biochemicals (San Diego, CA, U.S.A.) and Y-27632 was from BioMol (Plymouth Meeting, PA, U.S.A.). H-1152, GF109203X, wortmannin and anti-ZIPK were purchased from Calbiochem–Novabiochem Corp. (La Jolla, CA, U.S.A.), ML-7 and ML-9 were from Toronto Research Chemicals Inc. (North York, ON, Canada) and benzamidine was from Eastman Kodak (Rochester, NY, U.S.A.). ILK [12], MLCP [16] and LC20 [8] were purified from chicken gizzard, as described previously. In one specified instance, ILK containing a small amount of ZIPK was used, which was obtained during the purification of ILK [12]. GST (glutathione S-transferase)–ZIPK (or GST–recombinant myosin-phosphatase-associated kinase or GST–rMYPT1K) [15] and GST–ILK [12] were expressed and purified as described previously. Monoclonal antibodies specific for MYPT1 phosphorylated at Thr-697 (anti-[phospho-Thr-697]-MYPT1; rat numbering) were purified as described previously [16]. Polyclonal antibodies against ILK were raised in rabbits by injection of a synthetic peptide (residues 428–447: CMNEDPAKRPKFDMVIPILE) coupled with keyhole-limpet haemocyanin. ILK(428–447) was synthesized by Macromolecular Resources (Fort Collins, CO, U.S.A.) and confirmed by MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry). Antiserum was used at a 1:3000 dilution. Antibody specificity was confirmed by recognition of a 60 kDa protein in HEK-293 cells transfected with His6-tagged ILK, but not in untransfected cell lysates. Secondary antibodies used were anti-rabbit IgG coupled with Alexa 680 (Invitrogen, Carlsbad, CA, U.S.A.) and anti-rabbit IgG coupled with Alexa 780 (Rockland, Gilbertsville, PA, U.S.A.). AV25, a 25-amino-acid synthetic peptide inhibitor of MLCK (sequence: AKKLAKDRMKKYMARRKLQKAGHAV) was produced by the Peptide Synthesis Core Facility at the University of Calgary, confirmed by amino acid analysis and shown to be >95% pure by analytical HPLC. This peptide corresponds to the autoinhibitory domain of MLCK (residues 783–807 of chicken gizzard MLCK) with three amino acid substitutions: Trp-800 was replaced by leucine to eliminate calmodulin binding, and Ser-787 and Thr-803 were replaced by alanine to remove potential phosphorylation sites in the peptide. These substitutions did not affect the potency of inhibition of MLCK [8]. Other chemicals were purchased from VWR (Edmonton, AB, Canada) or Sigma–Aldrich (Oakville, ON, Canada) and were of analytical grade, or better.
Gel electrophoresis and Western blotting
SDS/PAGE using 7.5–20% acrylamide gradient gels [17], urea/glycerol gel electrophoresis to separate unphosphorylated, mono- and di-phosphorylated forms of LC20 [8], and Western blotting [16] were performed as described previously. Secondary antibodies were anti-rabbit IgG coupled with Alexa 680 (with anti-ILK as the primary antibody) or Alexa 780 (with anti-ZIPK as primary antibody), used at a 1:10000 dilution. Fluorochrome signals were detected and analysed using the Odyssey Near Infrared Imaging System (Li-Cor Biosciences, Lincoln, NB, U.S.A.).
In-gel kinase assay
The endothelium was removed from rat caudal artery before skinning for 2 h with 1% (v/v) Triton X-100. Proteins from two strips (1.5 mm×6 mm each) of skinned rat caudal artery were extracted with 0.2 ml of 1% (w/v) SDS in 50 mM Tris/HCl, pH 7.5, containing 100 μM di-isopropylfluorophosphate. The mixture was warmed to 70 °C, followed by mixing for 60 min on a vortex platform. Tissue samples, ILK containing a small amount of ZIPK (30 μl), or recombinant ZIPK (125 ng) were mixed with an equal volume of 2×SDS gel sample buffer [50 mM Tris/HCl (pH 6.8)/1% (w/v) SDS/30% (v/v) glycerol/0.01% (w/v) Bromophenol Blue] and incubated at 70 °C for 5 min, before SDS/PAGE using a 7.5–20% acrylamide gradient with or without LC20 (20 μg/ml gel solution) incorporated throughout the 0.75-mm-thick gels. Following electrophoresis, gels were washed at room temperature (≈20 °C) with 25 mM Tris/HCl, pH 7.5, containing 60 mM KCl, 10 mM MgCl2, 10 mM DTT (dithiothreitol), 10 mM EGTA and 2.5% (v/v) Triton X-100 with five changes of buffer over 2 h to remove the SDS. Gels were washed for a further 2 h (with five changes of buffer) with 20 mM Tris/HCl, pH 7.5, 60 mM KCl, 10 mM MgCl2, 10 mM DTT, 10 mM EGTA and 0.1% (v/v) Tween 80 (kinase assay buffer). The buffer was replaced with 20 ml of fresh kinase assay buffer, and protein phosphorylation was initiated by addition of 20 μM ATP containing 200 μCi of [γ-32P]ATP. After incubation at 20 °C for 3 h, the gel was washed extensively with 5% (w/v) trichloroacetic acid/1% (w/v) sodium pyrophosphate until the radioactivity in the wash solution was negligible. The gel was stained and destained, and radioactively labelled bands were detected using a Storm Phosphorimaging System (Molecular Dynamics).
ILK activity assay
For LC20 phosphorylation, ILK (10%, v/v) was incubated for 60 min at 30 °C in 25 mM Tris/HCl, pH 7.5, containing 10 mM EGTA, 10 mM MgCl2, 50 mM KCl, 10 mM DTT, 0.1% (v/v) Tween 80, 1 μM microcystin-LR, 5 μM LC20 and 0.2 mM [γ-32P]ATP (200–500 c.p.m./pmol) in the absence or presence of AV25 (100 μM) or staurosporine (2 μM) in a reaction volume of 100 μl. Reactions were started by addition of ATP and stopped by spotting 95 μl on to P81 phosphocellulose paper squares (Whatman, Springfield Mill, Maidstone, Kent, U.K.), which were washed and radioactivity quantified by Cerenkov counting, as described previously [8]. Identical reactions were stopped by addition of 4×SDS gel sample buffer (50 μl) and boiling, before SDS/PAGE and autoradiography [8]. For MYPT1 phosphorylation, ILK (10%, v/v) was incubated for 60 min at 30 °C with MLCP (5.3 nM) in 25 mM Tris/HCl, pH 7.5, containing 10 mM EGTA, 50 mM KCl, 100 mM MgCl2, 10 mM DTT, 0.1% (v/v) Tween 80, 20 μM microcystin, 0.2 mM Pefabloc SC and 1 mM benzamidine in the absence or presence of ATP (0.2 mM) and AV25 (50 μM) in a reaction volume of 100 μl. Reactions were stopped by addition of an equal volume of 2×SDS gel sample buffer and boiling, before SDS/PAGE and Western blotting with anti-[phospho-Thr-697]-MYPT1.
ZIPK activity assay
LC20 (50 μM) was incubated at 25 °C with ZIPK (62.5 nM) in 25 mM Hepes/NaOH, pH 7.4, containing 2.5 mM MgCl2, 0.1 mM [γ-32P]ATP (5 c.p.m./pmol) and various concentrations of AV25. Reactions (50 μl) were started by addition of ATP, quenched after 10 min by addition of 20 mM H3PO4, and spotted on to P81 phosphocellulose paper for quantification as described above. Reactions were linear with respect to time and ZIPK concentration under these conditions, as determined in preliminary experiments. IC50 for AV25 was determined using a non-linear least-squares regression method [18].
Force measurements
De-endothelialized rat caudal arterial smooth muscle helical strips (1.5 mm×6 mm) were demembranated (skinned) with 1% (v/v) Triton X-100 for 2 h. In some experiments, calmodulin was then removed by treatment with 0.4 mM trifluoperazine in the presence of Ca2+. Tissues were prepared for force measurements as described previously [19]. pCa 9 solution is composed of 20 mM Tes, 4 mM K2EGTA, 5.83 mM MgCl2, 7.56 mM potassium propionate, 3.9 mM Na2ATP, 0.5 mM dithioerythritol, 16.2 mM phosphocreatine and 15 units/ml creatine kinase, pH 6.9. The free Ca2+ concentration in pCa 9 solution was determined using fura-2, and was found to be less than 6 nM [19].
Statistical analysis
Values are presented as the means±S.E.M., with n indicating the number of independent experiments (tissue isolated from different rats) for a given treatment. Statistical differences were determined using Student's t test, with P<0.05 considered to be statistically significant.
RESULTS
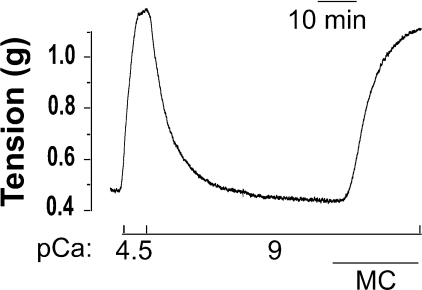
Figure 1 depicts a typical microcystin-induced contraction of a Triton X-100-skinned rat caudal arterial smooth muscle helical strip in the absence of Ca2+. Triton-skinned tissue contracted in response to an increase in [Ca2+] (pCa 4.5), and relaxed upon return to Ca2+-free solution (pCa 9). The Ca2+-induced contraction could be repeated with similar maximal force generation, which was again reversed upon removal of Ca2+. Only the second Ca2+-induced contraction–relaxation cycle is shown in Figure 1. Addition of microcystin (1 μM) then elicited a contractile response in the absence of Ca2+ (pCa 9).
Figure 1. Ca2+-independent, microcystin-induced contraction of Triton-skinned rat caudal arterial smooth muscle.
Triton-skinned smooth muscle strips contracted in pCa 4.5 and relaxed in pCa 9 solution. Ca2+-induced contraction was repeated and relaxation effected again by a return to pCa 9 solution. Only the second Ca2+-induced contraction is shown here. Subsequent addition of microcystin (MC; 1 μM) at pCa 9 elicited a contractile response (n=9).
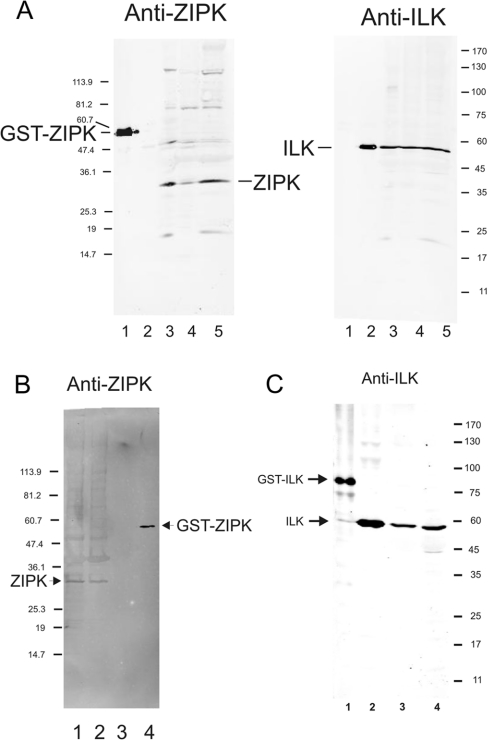
Antibodies specific for ZIPK and ILK were used to investigate the presence of these kinases in rat smooth muscle tissues and their association with the cytoskeleton/contractile machinery. Figure 2(A) demonstrates the presence of both kinases in rat bladder, aorta and caudal artery (lanes 3–5), and confirms that the antibodies do not cross-react (lanes 1 and 2). Both ZIPK and ILK are retained in Triton-skinned rat caudal artery preparations (Figures 2B and 2C respectively), suggesting that, like MLCK, which is anchored to actin filaments [20], they are tightly bound to a component of the cytoskeleton/contractile machinery. Association of these Ca2+-independent kinases with the contractile machinery would be consistent with microcystin-induced contraction and LC20 phosphorylation catalysed by ZIPK, ILK or both.
Figure 2. ZIPK and ILK are retained in Triton-skinned rat caudal artery.
(A) Western blots probed with anti-ZIPK (left panel) or anti-ILK (right panel): GST–ZIPK (lane 1), chicken gizzard ILK (lane 2), rat bladder (lane 3), rat aorta (lane 4) and rat caudal artery (lane 5). (B) Western blot probed with anti-ZIPK: intact rat caudal artery (one strip; lane 1), Triton-skinned rat caudal artery (one strip; lane 2), chicken gizzard ILK (lane 3) and GST–ZIPK (lane 4). (C) Western blot probed with anti-ILK: GST–ILK (lane 1), chicken gizzard ILK (lane 2), Triton-skinned rat caudal artery (one strip; lane 3) and intact rat caudal artery (one strip; lane 4). Numbers at the right or left of each panel indicate the sizes of molecular-mass markers (in kDa).
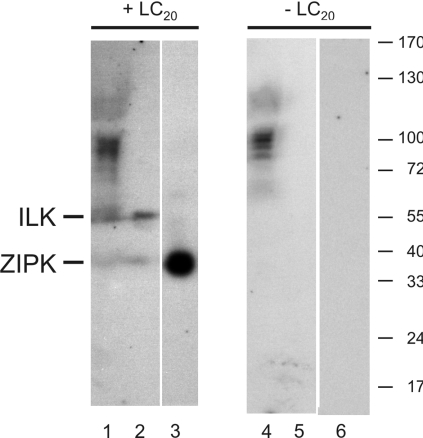
In-gel kinase assays were used to confirm the retention of ILK and ZIPK activities in Triton-skinned rat caudal arterial smooth muscle, and to determine if any additional LC20 kinase activity could be detected in this preparation. Proteins from Triton-skinned tissue, ILK and ZIPK were subjected to SDS/PAGE in a 7.5–20% acrylamide gradient gel (control) or in an identical gel containing purified LC20 throughout, which is achieved by polymerization of the separating gel in the presence of LC20. Proteins were renatured following electrophoretic separation, and Ca2+-independent kinase activity was detected by incubation of the gels in the presence of buffer containing [γ-32P]ATP and EGTA, followed by autoradiography (Figure 3). Only two unique radiolabelled bands were detected in the Triton-skinned tissue in the presence (Figure 3, lane 1) but not the absence (lane 4) of LC20, which correspond to ILK (lane 2) and ZIPK (lane 3). Additional radiolabelled bands were detected in both the absence and presence of LC20 (Figure 3, lanes 1 and 4), indicating autophosphorylation of kinases or phosphorylation of endogenous substrate proteins by kinases of identical molecular mass. Autophosphorylation of ILK and ZIPK was not detectable at the loading levels utilized (Figure 3, lanes 5 and 6). The only Ca2+-independent LC20 kinases detected in Triton-skinned rat caudal arterial smooth muscle, therefore, corresponded to ILK and ZIPK. It is possible, however, that other Ca2+-independent LC20 kinases were retained in the Triton-skinned preparation, but were undetected, due to either an inability to refold properly following SDS/PAGE or a requirement for additional subunits or cofactors for activity.
Figure 3. In-gel kinase assays.
Triton-skinned rat caudal arterial smooth muscle (lanes 1 and 4; two muscle strips), tissue-purified ILK containing a small amount of ZIPK (lanes 2 and 5; 30 μl) and recombinant ZIPK (lanes 3 and 6; 0.125 μg) were subjected to SDS/PAGE in a gel containing purified LC20 (lanes 1–3) or an identical gel without LC20 (lanes 4–6). Following electrophoresis, proteins were renatured in the gels, which were then incubated in the presence of [γ-32P]ATP and absence of Ca2+. Phosphorylated proteins were detected by autoradiography (n=3). Numbers at the right indicate the sizes of molecular-mass markers (in kDa).
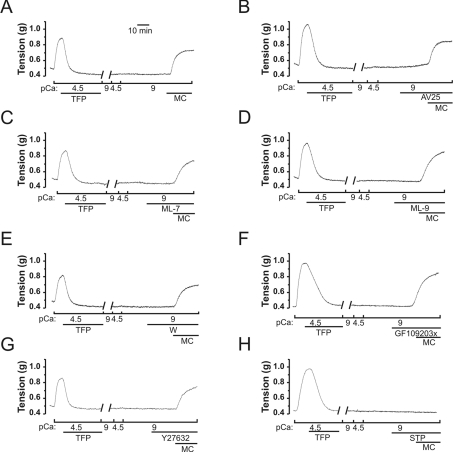
The conclusion that MLCK is not responsible for the Ca2+-independent, microcystin-induced contraction [8] was confirmed by the demonstration that prior removal of endogenous calmodulin by treatment of the tissue with the calmodulin antagonist trifluoperazine at the peak of a Ca2+-induced contraction [19] did not affect the Ca2+-independent, microcystin-induced contraction, although it abolished Ca2+-induced contraction (Figure 4A). The MLCK inhibitor peptide AV25 (Figure 4B) and the small molecule MLCK inhibitors ML-7 (Figure 4C), ML-9 (Figure 4D) and wortmannin (Figure 4E) did not block the Ca2+-independent, microcystin-induced contraction of calmodulin-depleted tissue, confirming that MLCK is not responsible for the microcystin-induced contraction. In control experiments, the MLCK inhibitors were shown to inhibit Ca2+-induced contractions effectively at the concentrations used in Figure 4 (results not shown). The PKC inhibitor GF109203X (Figure 4F) and the ROK (Rho-associated kinase) inhibitor Y-27632 (Figure 4G) also failed to block Ca2+-independent, microcystin-induced contraction. The efficacy of these inhibitors was confirmed by demonstration of their ability to inhibit phorbol-ester-induced contraction and U46619-induced contraction respectively of intact rat caudal arterial smooth muscle [16]. Likewise, H-1152 (100 nM and 1 μM), another ROK-selective inhibitor, had no effect on Ca2+-independent, microcystin-induced contraction (results not shown). On the other hand, the non-selective kinase inhibitor staurosporine effectively inhibited Ca2+-independent, microcystin-induced contraction following removal of endogenous calmodulin (Figure 4H).
Figure 4. Effects of kinase inhibitors on Ca2+-independent, microcystin-induced contraction of Triton-skinned, calmodulin-depleted rat caudal artery.
(A) Addition of trifluoperazine (TFP; 0.4 mM) at the peak of a Ca2+-induced contraction of Triton-skinned strips relaxed the tissue as calmodulin was dissociated [19]. TFP and dissociated calmodulin were removed by several washes in pCa 9 solution. Addition of Ca2+ then failed to elicit contraction, confirming the removal of calmodulin, but subsequent addition of microcystin (MC; 1 μM) at pCa 9 activated contraction (n=8). (B–H) Microcystin-induced contraction at pCa 9 was unaffected by the MLCK inhibitors AV25 (100 μM; B) (n=5), ML-7 (100 μM; C) (n=3), ML-9 (300 μM; D) (n=4) or wortmannin (W; 10 μM; E) (n=4), the PKC inhibitor GF109203X (5 μM; F) (n=4) and the ROK inhibitor Y-27632 (10 μM; G) (n=3), but was blocked by the broad spectrum kinase inhibitor staurosporine (STP; 5 μM; H) (n=4).
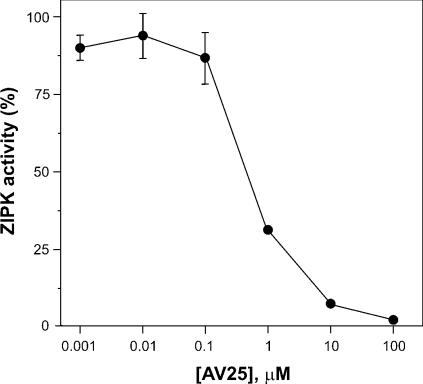
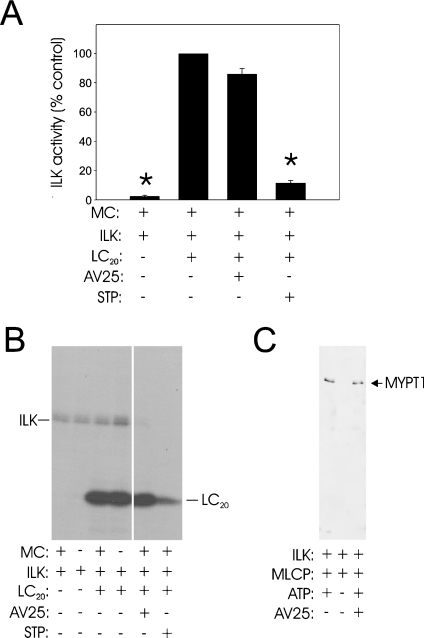
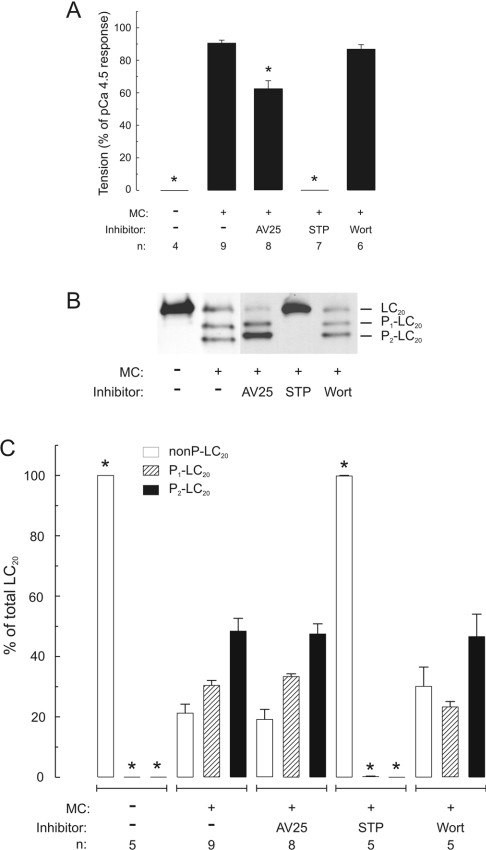
To distinguish between the effects of ZIPK and ILK, we sought a kinase inhibitor that would inhibit one kinase but not the other. Since MLCK and ZIPK have closely related catalytic domain sequences [13], which are quite distinct from ILK [12], we considered that the MLCK inhibitory peptide AV25, based on the autoinhibitory domain of MLCK, would be likely to inhibit ZIPK but not ILK. Purified ZIPK was inhibited by AV25 with an IC50 of 0.63±0.05 μM (Figure 5), compared with 0.2 μM for MLCK [8]. On the other hand, AV25, even at very high concentrations (50–100 μM), had no effect on ILK activity using either LC20 (Figures 6A and 6B) or MYPT1 (MLCP; Figure 6C) as substrate. The observation that AV25 (100 μM) did not block Ca2+-independent, microcystin-induced contraction of calmodulin-depleted tissue (Figure 4B) suggests, therefore, that ILK and not ZIPK is the kinase responsible for Ca2+-independent phosphorylation of myosin LC20 and contraction in response to microcystin-mediated phosphatase inhibition. This was confirmed in Triton-skinned rat caudal arterial smooth muscle that had not been treated with trifluoperazine and, therefore, retained the pool of calmodulin that remains associated with the contractile machinery [19]. As shown in Figure 7(A), microcystin-induced steady-state tension at pCa 9 was partially inhibited by AV25 (100 μM). However, AV25 had no inhibitory effect on LC20 mono- or di-phosphorylation in the same tissue samples (Figures 7B and 7C). The small decrease in force attributable to AV25 is therefore not due to inhibition of LC20 phosphorylation, but rather the peptide acts downstream of LC20 phosphorylation. The non-selective kinase inhibitor staurosporine, which inhibits both ILK (Figure 6A) and ZIPK [14], inhibited Ca2+-independent, microcystin-induced contraction in the absence (Figure 4H) or presence (Figure 7A) of endogenous contractile calmodulin. This can be explained by complete inhibition of LC20 phosphorylation (Figures 7B and 7C). Neither Ca2+-independent, microcystin-induced force (Figure 7A) nor LC20 mono- and di-phosphorylation (Figures 7B and 7C) were affected by the MLCK inhibitor wortmannin (10 μM). Even at 150 μM, wortmannin had no effect on either parameter in the presence of microcystin (results not shown). Likewise, Ca2+-independent, microcystin-induced contraction and LC20 mono- and di-phosphorylation were unaffected by the ROK inhibitors Y-27632 (10 μM; Figure 4G and results not shown) and H-1152 (100 nM and 1 μM; results not shown), or the PKC inhibitor GF109203X (100 nM and 5 μM; Figure 4F and results not shown).
Figure 5. Inhibition of ZIPK activity by AV25.
The activity of purified recombinant ZIPK was assayed with LC20 as substrate in the presence of the indicated concentrations of AV25 as described in the Experimental section. Values are expressed relative to the control (100%=5.4 nmol of Pi/min per mg) in the absence of AV25. Error bars indicate S.E.M. (n=3). The absence of error bars indicates they are smaller than the symbols.
Figure 6. AV25 does not inhibit ILK activity.
(A) ILK activity was measured with isolated LC20 as substrate under the indicated conditions, as described in the Experimental section (n=3). Where present, AV25 was at a concentration of 100 μM and staurosporine (STP) at 2 μM; microcystin (MC) was at 1 μM. Values are expressed relative to the control (100%=0.1 nmol of Pi/min per ml) in the absence of kinase inhibitors. Asterisks indicate significant difference from control (P<0.05). (B) Reaction mixtures with LC20 as substrate were analysed by SDS/PAGE and autoradiography. A representative autoradiogram is shown. (C) ILK activity with MLCP (MYPT1) as substrate was assayed by Western blotting with anti-[phospho-Thr-697]-MYPT1 under the indicated conditions. Where present, AV25 was at a concentration of 50 μM.
Figure 7. Effect of AV25 on contraction and LC20 mono- and di-phosphorylation in Triton-skinned rat caudal arterial smooth muscle containing contractile calmodulin.
(A) Steady-state force developed in Triton-skinned strips at pCa 9 in the absence or presence of microcystin (MC; 1 μM). Where indicated, tissues were incubated with AV25 (100 μM), staurosporine (STP; 5 μM) or wortmannin (Wort; 10 μM) for 10 min prior to addition of microcystin. (B) Once steady-state force was attained, tissues were quick-frozen for analysis of LC20 phosphorylation by urea/glycerol-PAGE, which separates unphosphorylated, mono- and di-phosphorylated forms of LC20. (C) LC20 bands were quantified by scanning densitometry of gels such as are shown in (B). Different exposure times were used for quantification of these data to ensure that signals lay within the linear range of the relationship between protein amount and signal intensity in each case. Results are expressed as percentages of total LC20 for unphosphorylated (nonP-LC20; open bars), monophosphorylated (P1-LC20; hatched bars) and diphosphorylated (P2-LC20; black bars) bands. Phosphorylation stoichiometry was calculated from the following equation: mol of Pi/mol of LC20=(y+2z)/(x+y+z), where x, y and z are the signal intensities of unphosphorylated, mono- and di-phosphorylated LC20 bands respectively. The following phosphorylation stoichiometries were determined: 1.26±0.07 mol of Pi/mol of LC20 (MC alone), 1.28±0.07 mol of Pi/mol of LC20 (MC+AV25) and 1.16±0.14 mol of Pi/mol of LC20 (MC+Wort). n values are indicated below each histogram. Asterisks indicate P<0.001 compared with values at pCa 9 in the presence of microcystin; in all other cases, P>0.3.
DISCUSSION
Several protein kinases have been shown to phosphorylate smooth muscle LC20 in vitro. Of these, only a subset phosphorylate the light chain in intact myosin: MLCK [2], CaMKI and CaMKII (Ca2+/calmodulin-dependent protein kinases I and II respectively) [21,22], ROK [23], PKC [24], MAPKAPK2 (mitogen-activated-protein-kinase-activated protein kinase-2) [25], MAPK-activated protein kinase-1b (or RSK-2) [26], citron kinase [27], ILK [12] and ZIPK [13,14]. In most cases (MLCK, CaMKI, CaMKII, ROK, MAPKAPK2 and RSK-2 [2,21–23,25,26]), phosphorylation is restricted to Ser-19, and PKC does not phosphorylate either Ser-19 or Thr-18 [24]. Similarly, γ-PAK (p21-activated kinase) has been shown to phosphorylate LC20 of non-muscle myosin II exclusively at Ser-19 [28]. Citron kinase, a target of activated RhoA that has been implicated in cytokinesis and is localized to the cleavage furrow, was shown to phosphorylate non-muscle myosin II at Ser-19 and Thr-18 [27]; there is no evidence, however, for a role of citron kinase in regulation of smooth muscle contraction. For these and other reasons, we can rule out these kinases as playing a role in Ca2+-independent, microcystin-induced contraction. ILK and ZIPK, therefore, have emerged as candidate kinases for the diphosphorylation of LC20 at Ser-19 and Thr-18 and Ca2+-independent contraction. Phosphorylation at Ser-19 markedly increases the actin-activated MgATPase activity of smooth muscle myosin [29], and this is enhanced further (approx. 3-fold) by additional phosphorylation at Thr-18 [30]. Replacement of LC20 with an S19A mutant to restrict phosphorylation to Thr-18 resulted in approx. 15-fold less activation of the actomyosin MgATPase activity than is achieved with phosphorylation at Ser-19 [31]. In apparent contrast with these effects on the actomyosin MgATPase activity, the rate of movement of actin filaments over immobilized myosin in the in vitro motility assay was the same for mono- and di-phosphorylated LC20 [32], and this was comparable for diphosphorylated myosin and S19A-LC20-containing myosin phosphorylated exclusively at Thr-18 [31]. On the other hand, the rate of contraction of rabbit aortic smooth muscle was enhanced in response to PGF2α (prostaglandin F2α), which induced significant diphosphorylation compared with other stimuli [33], supporting the notion that phosphorylation of LC20 at Thr-18 and Ser-19 has additive effects.
Our results demonstrate that inhibition of phosphatase activity in Triton-skinned vascular smooth muscle (rat caudal artery) by microcystin-LR (a type 1 and 2A phosphatase inhibitor) unmasks endogenous Ca2+-independent LC20 kinase activity that is associated with the contractile machinery, since it is not extracted by non-ionic detergent treatment. Similar results have been obtained with other smooth muscle tissues and other phosphatase inhibitors, suggesting a common mechanism (e.g. see [14,34,35]). The Ca2+-independent LC20 kinase is not MLCK for the following reasons: (i) its activity is Ca2+- and calmodulin-independent [8]; (ii) it phosphorylates LC20 at both Ser-19 and Thr-18, whereas phosphorylation by endogenous levels of MLCK occurs exclusively at Ser-19 [8]; (iii) its activity is resistant to a variety of MLCK inhibitors, including AV25 [8], ML-7, ML-9 and wortmannin (the present study); (iv) it could be separated from MLCK by differential extraction from myofilaments and by affinity chromatography on calmodulin–Sepharose [8]; and (v) Ca2+-independent, microcystin-induced contraction of Triton-skinned rat caudal arterial smooth muscle was retained following removal of the endogenous contractile pool of calmodulin by treatment with trifluoperazine in the presence of Ca2+ (the present study). The principal objective of our study, however, was to determine whether ILK, ZIPK or both kinases are responsible for Ca2+-independent, microcystin-induced contraction (Figure 1). Western blot analysis (Figure 2) and in-gel kinase assays (Figure 3) showed that both kinases are retained after non-ionic detergent treatment. The Ca2+-independent, microcystin-induced contraction was blocked by the broad spectrum kinase inhibitor staurosporine, but not by several other kinase inhibitors, i.e. AV25, ML-7, ML-9, wortmannin, GF109203X and Y-27632 (Figure 4), or H-1152 (results not shown). AV25, a synthetic peptide inhibitor of MLCK based on the autoinhibitory domain of the kinase, was found to inhibit ZIPK (Figure 5) with an IC50 of 0.6 μM, but had no effect on ILK activity at concentrations as high as 100 μM (Figure 6). AV25 therefore provided a very useful tool to determine the relative importance of ILK and ZIPK in Ca2+-independent LC20 phosphorylation and microcystin-induced contraction of Triton-skinned rat caudal arterial smooth muscle. As shown in Figure 7(A), AV25 had a small but significant inhibitory effect on this contraction. However, since no inhibition of LC20 phosphorylation was observed in the presence of AV25 (Figures 7B and 7C), we conclude that the Ca2+-independent diphosphorylation of LC20 can be attributed exclusively to ILK. Furthermore, the fact that ML-7 (100 μM) did not inhibit microcystin-induced contraction at pCa 9 (Figure 4C) supports the conclusion that ZIPK is not responsible for LC20 phosphorylation, since ML-7 has recently been shown to inhibit ZIPK in vitro over the concentration range of 10–100 μM [14].
In addition to their ability to phosphorylate LC20 in vitro, ILK and ZIPK have been implicated in the regulation of MLCP. Thus both kinases have been shown to phosphorylate MYPT1 [36,37] and CPI-17 [10,38] in vitro, resulting in inhibition of MLCP activity. Ongoing studies are focused on the relative importance of ILK- and ZIPK-catalysed phosphorylation of MYPT1 and CPI-17.
While LC20 monophosphorylation is most commonly observed in smooth muscle tissues in response to physiological contractile stimuli, low levels of LC20 diphosphorylation have been reported in a variety of vascular and other smooth muscles. For example, neural or carbachol stimulation of bovine tracheal smooth muscle resulted in peaks of 5% and 11% diphosphorylated LC20 respectively [39,40]. Likewise, LC20 diphosphorylation peaked at 7.4% in rabbit thoracic aorta in response to PGF2α treatment [33,41], and was almost completely inhibited by fasudil (HA-1077) or hydroxyfasudil, selective inhibitors of ROK [42]. LC20 diphosphorylation is more commonly observed in pathological situations, however. For example, maximal PGF2α-induced force in hyperplastic rabbit carotid artery was found to be significantly higher than control, and correlated with higher levels of both mono- and di-phosphorylation of LC20 [43]. During the early stages of vasospasm in a rat femoral artery model, LC20 mono- and di-phosphorylation were significantly greater in the vasospastic artery that had been exposed to whole blood than in the control contralateral artery [44]. Similarly, increased LC20 phosphorylation was observed in a dog model of cerebral vasospasm [45], although no distinction was made between mono- and diphosphorylation in that study. In a porcine model of coronary artery spasm, LC20 diphosphorylation was observed in response to serotonin in the spastic segment, but not in the control segment, of the artery [46], and was inhibited by hydroxyfasudil [47]. These observations have led to the suggestion that diphosphorylation of LC20 is associated with hypercontractility. Since ROK, PKC, MYPT1 and CPI-17 have been implicated, through inhibitor studies, in Ca2+-sensitization, a potential mechanism to explain the observed increase in LC20 diphosphorylation in vasospastic arteries involves increased activity of the Ca2+-sensitizing kinases, ROK and PKC. Phosphorylation of CPI-17 and MYPT1 is known to result in inhibition of MLCP, and is predicted to cause not only Ca2+-sensitization, but also an increase in the rate of contraction and diphosphorylation of LC20 at Ser-19 and Thr-18. Furthermore, since ILK has demonstrated unusual resistance to numerous kinase inhibitors and may be involved in disease states involving vascular hypercontractility, this kinase emerges as a potentially useful therapeutic target.
Acknowledgments
This work was supported by grants from the CIHR (Canadian Institutes of Health Research) to J.A.M. (MOP-62720) and M.P.W. (MOP-13101). D.P.W. was recipient of Fellowships from the HSFC (Heart and Stroke Foundation of Canada) and the AHFMR (Alberta Heritage Foundation for Medical Research). M.A.B. is recipient of a Fellowship from HSFC. J.A.M. is recipient of a Canada Research Chair (Tier II) in Smooth Muscle Pathophysiology, the Protein Engineering Network of Centres of Excellence Chair in Protein Sciences and a Scholarship from HSFC. M.P.W. is an AHFMR Medical Scientist, Director of the CIHR Group in Regulation of Vascular Contractility and recipient of a Canada Research Chair (Tier I) in Biochemistry. The authors are grateful to Janina Ostrander for expert technical assistance.
References
- 1.Somlyo A. P., Somlyo A. V. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. (Cambridge, U.K.) 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamm K. E., Stull J. T. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 3.Somlyo A. P., Somlyo A. V. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 4.Hartshorne D. J., Ito M., Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J. Biol. Chem. 2004;279:37211–37214. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- 5.Ito M., Nakano T., Erdödi F., Hartshorne D. J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 6.Li L., Eto M., Lee M. R., Morita F., Yazawa M., Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J. Physiol. (Cambridge, U.K.) 1998;508:871–881. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honkanen R. E., Zwiller J., Moore R. E., Daily S. L., Khatra B. S., Dukelow M., Boynton A. L. Characterization of microcystin-LR, a potent inhibitor of type 1 and 2A protein phosphatases. J. Biol. Chem. 1990;265:19401–19404. [PubMed] [Google Scholar]
- 8.Weber L. P., Van Lierop J. E., Walsh M. P. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J. Physiol. (Cambridge, U.K.) 1999;516:805–824. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eto M., Kitazawa T., Brautigan D. L. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J. T., Sutherland C., Brautigan D. L., Eto M., Walsh M. P. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem. J. 2002;367:517–524. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikebe M., Hartshorne D. J., Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-Dalton light chain of smooth muscle myosin. J. Biol. Chem. 1986;261:36–39. [PubMed] [Google Scholar]
- 12.Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P. Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J. Biol. Chem. 2001;276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 13.Murata-Hori M., Suizu F., Iwasaki T., Kikuchi A., Hosoya H. ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells. FEBS Lett. 1999;451:81–84. doi: 10.1016/s0014-5793(99)00550-5. [DOI] [PubMed] [Google Scholar]
- 14.Niiro N., Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 2001;276:29567–29574. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- 15.Borman M. A., MacDonald J. A., Murányi A., Hartshorne D. J., Haystead T. A. J. Smooth muscle myosin phosphatase-associated kinase induces Ca2+ sensitization via myosin phosphatase inhibition. J. Biol. Chem. 2002;277:23441–23446. doi: 10.1074/jbc.M201597200. [DOI] [PubMed] [Google Scholar]
- 16.Wilson D. P., Susnjar M., Kiss E., Sutherland C., Walsh M. P. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855 but not Thr-697. Biochem. J. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J. Biol. Chem. 1990;265:10148–10155. [PubMed] [Google Scholar]
- 18.Brooks S. P. J. A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques. 1992;13:906–911. [PubMed] [Google Scholar]
- 19.Wilson D. P., Sutherland C., Walsh M. P. Ca2+ activation of smooth muscle contraction: evidence for the involvement of calmodulin that is bound to the Triton-insoluble fraction even in the absence of Ca2+ J. Biol. Chem. 2002;277:2186–2192. doi: 10.1074/jbc.M110056200. [DOI] [PubMed] [Google Scholar]
- 20.Smith L., Su X., Lin P., Zhi G., Stull J. T. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J. Biol. Chem. 1999;274:29433–29438. doi: 10.1074/jbc.274.41.29433. [DOI] [PubMed] [Google Scholar]
- 21.Suizu F., Fukuta Y., Ueda K., Iwasaki T., Tokumitsu H., Hosoya H. Characterization of Ca2+/calmodulin-dependent protein kinase I as a myosin II regulatory light chain kinase in vitro and in vivo. Biochem. J. 2002;367:335–345. doi: 10.1042/BJ20020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelman A. M., Lin W.-H., Osterhout D. J., Bennett M. K., Kennedy M. B., Krebs E. G. Phosphorylation of smooth muscle myosin by type II Ca2+/calmodulin-dependent protein kinase. Mol. Cell. Biochem. 1990;97:87–98. doi: 10.1007/BF00231704. [DOI] [PubMed] [Google Scholar]
- 23.Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 24.Ikebe M., Hartshorne D. J., Elzinga M. Phosphorylation of the 20,000-Dalton light chain of smooth muscle myosin by the calcium-activated, phospholipids-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J. Biol. Chem. 1987;262:9569–9573. [PubMed] [Google Scholar]
- 25.Komatsu S., Hosoya H. Phosphorylation by MAPKAP kinase 2 activates Mg2+-ATPase activity of myosin II. Biochem. Biophys. Res. Commun. 1996;223:741–745. doi: 10.1006/bbrc.1996.0966. [DOI] [PubMed] [Google Scholar]
- 26.Suizu F., Ueda K., Iwasaki T., Murata-Hori M., Hosoya H. Activation of actin-activated MgATPase activity of myosin II by phosphorylation with MAPK-activated protein kinase-1b (RSK-2) J. Biochem. (Tokyo) 2000;128:435–440. doi: 10.1093/oxfordjournals.jbchem.a022771. [DOI] [PubMed] [Google Scholar]
- 27.Yamashiro S., Totsukawa G., Yamakita Y., Sasaki Y., Madaule P., Ishizaki T., Narumiya S., Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew T.-L., Masaracchia R. A., Goeckeler Z. M., Wysolmerski R. B. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (γ-PAK) J. Muscle Res. Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 29.Walsh M. P. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem. Cell Biol. 1991;69:771–800. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]
- 30.Ikebe M., Hartshorne D. J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985;260:10027–10031. [PubMed] [Google Scholar]
- 31.Bresnick A. R., Wolff-Long V. L., Baumann O., Pollard T. D. Phosphorylation on threonine-18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry. 1995;34:12576–12583. doi: 10.1021/bi00039a012. [DOI] [PubMed] [Google Scholar]
- 32.Umemoto S., Bengur A. R., Sellers J. R. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J. Biol. Chem. 1989;264:1431–1436. [PubMed] [Google Scholar]
- 33.Seto M., Sasaki Y., Sasaki Y. Stimulus-specific patterns of myosin light chain phosphorylation in smooth muscle of rabbit thoracic artery. Pflügers Arch. 1990;415:484–489. doi: 10.1007/BF00373627. [DOI] [PubMed] [Google Scholar]
- 34.Obara K., Takai A., Rüegg J. C., de Lanerolle P. Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflügers Arch. 1989;414:134–138. doi: 10.1007/BF00580954. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D., Hartshorne D. J. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J. Pharmacol. Exp. Therap. 1989;250:388–396. [PubMed] [Google Scholar]
- 36.Murányi A., MacDonald J. A., Deng J. T., Wilson D. P., Haystead T. A. J., Walsh M. P., Erdödi F., Kiss E., Wu Y., Hartshorne D. J. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem. J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald J. A., Borman M. A., Murányi A., Somlyo A. V., Hartshorne D. J., Haystead T. A. J. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald J. A., Eto M., Borman M. A., Brautigan D. L., Haystead T. A. J. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 2001;493:91–94. doi: 10.1016/s0014-5793(01)02277-3. [DOI] [PubMed] [Google Scholar]
- 39.Miller-Hance W. C., Miller J. R., Wells J. N., Stull J. T., Kamm K. E. Biochemical events associated with activation of smooth muscle contraction. J. Biol. Chem. 1988;263:13979–13982. [PubMed] [Google Scholar]
- 40.Colburn J. C., Michnoff C. H., Hsu L.-C., Slaughter C. A., Kamm K. E., Stull J. T. Sites phosphorylated in myosin light chain in contracting smooth muscle. J. Biol. Chem. 1988;263:19166–19173. [PubMed] [Google Scholar]
- 41.Seto M., Sasaki Y., Sasaki Y., Hidaka H. Effects of HA-1077, a protein kinase inhibitor, on myosin phosphorylation and tension in smooth muscle. Eur. J. Pharmacol. 1991;195:267–272. doi: 10.1016/0014-2999(91)90545-2. [DOI] [PubMed] [Google Scholar]
- 42.Ito K., Shimomura E., Iwanaga T., Shiraishi M., Shindo K., Nakamura J., Nagumo H., Seto M., Sasaki Y., Takuwa Y. Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F2α-induced contraction of rabbit aortae. J. Physiol. (Cambridge, U.K.) 2003;546:823–836. doi: 10.1113/jphysiol.2002.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seto M., Yano K., Sasaki Y., Azuma H. Intimal hyperplasia enhances myosin phosphorylation in rabbit carotid artery. Exp. Mol. Pathol. 1993;58:1–13. doi: 10.1006/exmp.1993.1001. [DOI] [PubMed] [Google Scholar]
- 44.Harada T., Seto M., Sasaki Y., London S., Luo Z., Mayberg M. The time course of myosin light-chain phosphorylation in blood-induced vasospasm. Neurosurgery. 1995;36:1178–1183. doi: 10.1227/00006123-199506000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Butler W. E., Peterson J. W., Zervas N. T., Morgan K. G. Intracellular calcium, myosin light chain phosphorylation, and contractile force in experimental cerebral vasospasm. Neurosurgery. 1996;38:781–788. doi: 10.1227/00006123-199604000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Katsumata N., Shimokawa H., Seto M., Kozai T., Yamawaki T., Kuwata K., Egashira K., Ikegaki I., Asano T., Sasaki Y., Takeshita A. Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1β. Circulation. 1997;96:4357–4363. doi: 10.1161/01.cir.96.12.4357. [DOI] [PubMed] [Google Scholar]
- 47.Shimokawa H., Seto M., Katsumata N., Amano M., Kozai T., Yamawaki T., Kuwata K., Kandabashi T., Egashira K., Ikegaki I., et al. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]