Abstract
Based on BLAST analysis of the human and mouse genome databases using the human CMP sialic acid; α2,8-sialyltransferase cDNA (hST8Sia I; EC 2.4.99.8), a putative sialyltransferase gene, was identified on human chromosome 10. The genomic organization was found to be similar to that of hST8Sia I and hST8Sia V. Transcriptional expression analysis showed that the newly identified gene was constitutively expressed at low levels in various human tissues and cell lines. We have isolated a full-length cDNA clone from the breast cancer cell line MCF-7 that encoded a type II membrane protein of 398 amino acid residues with the conserved motifs of sialyltransferases. We have established a mammary cell line (MDA-MB-231) stably transfected with the full-length hST8Sia VI and the analysis of sialylated carbohydrate structures expressed at the cell surface clearly indicated the disappearance of Neu5Acα2-3-sialylated structures. The transient expression of a truncated soluble form of the enzyme in either COS-7 cells or insect Sf-9 cells led to the production of an active enzyme in which substrate specificity was determined. Detailed substrate specificity analysis of the hST8Sia VI recombinant enzyme in vitro, revealed that this enzyme required the trisaccharide Neu5Acα2-3Galβ1-3GalNAc (where Neu5Ac is N-acetylneuraminic acid and GalNAc is N-acetylgalactosamine) to generate diSia (disialic acid) motifs specifically on O-glycans.
Keywords: α2,8-sialyltransferase (ST8Sia); O-glycosylproteins; disialic acid (diSia); enzymatic characterization; MDA-MB-231 cells; Sf-9
Abbreviations: BSM, bovine submaxillary mucin; diSia, disialic acid; DMB, 1,2-diamino-4,5-methylene dioxybenzene; DMEM, Dulbecco's modified Eagle's medium; DP, degree of polymerization; EGT, ecdysone-S-glycosyltransferase; EST, expressed sequence tag; FCS, foetal calf serum; Gal, β-D-galactopyranose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; HTG, High-Throughput Genomic; MAA, digoxigenin-labelled Maackia amurensis agglutinin; MEM, minimal essential medium; NCAM, neural cell adhesion molecule; NCBI, National Center for Biotechnology Information; NBEC, normal breast epithelial cells; Neu5Ac, N-acetylneuraminic acid; oligoSia, oligosialic acid; OSM, ovine submaxillary mucin; PGNase F, peptide N-glycosidase F; PSA, polysialic acid; RT-PCR, reverse transcription-PCR; SNA, digoxigenin-labelled Sambucus nigra agglutinin; (h)ST8Sia, (human) α2,8-sialyltransferase
INTRODUCTION
Sialic acids are negatively charged monosaccharides found at the non-reductive terminal position of the carbohydrate groups of glycoconjugates or oligosaccharides. In mammals they are α2,3- or α2,6-linked to a β-D-galactopyranose (Gal) residue or α2,6-linked to a β-D-N-acetylglucosamine (GlcNAc) or a β-D-N-acetylgalactosamine (GalNAc) residue. Sialic acid residues can also form linear homopolymers of α2,8-linked sialic acids that are named according to their DP (degree of polymerization) diSia (disialic acid) for DP=2, oligosialic acid (oligosia) for 3≥DP≤7 and PSA [polySia (polysialic acid)] for DP≥8 [2]. Until now, PSA has been described on a small group of N-glycosylproteins including the extensively studied NCAM (neural cell adhesion molecule), the α-subunit of the voltage-sensitive sodium channels from Electrophorus electricus and most recently the O-glycosylprotein CD-36 of human milk [3–7]. PSAs confer anti-adhesive properties to the polysialylated glycoproteins that attenuate cell–cell interactions [8]. Associated with NCAM in the developing nervous system, PSA can be extended to as many as 60 sialic acid residues and is implicated in various biological processes such as neural cell migration, axonal growth or synaptogenesis [6,9,10]. diSia and oligoSia chains are commonly found on gangliosides of the b and c series that are known to play important roles in differentiation, signal transduction and cell adhesion [11–13]. More recently, these shorter sialic acid chains were also described on both N- and O-glycosylproteins. diSia motifs were found on the N-glycans of Band 3 of human erythrocytes, fetuin and α2-macroglobulin of calf serum, and on the O-glycans of human glycophorin, bovine adipo-Q, CD-166 of Neuro2A cells and the bovine chromogranins [14–19]. OligoSia motifs were described on the N-glycans of ceruloplasmin and integrin α5 subunit [20,21] and on the O-glycans of megalin [22,23].
Until now, six members of the ST8Sia (α2,8-sialyltransferase) family have been described and cloned from various animal species. However, the role of each enzyme in the biosynthesis of diSia, oligoSia and PSA in vivo remains unclear. ST8Sia I and ST8Sia V are both involved in the α2,8-sialylation of gangliosides. ST8Sia I is responsible for the biosynthesis of GD3 from GM3 whereas ST8Sia V synthesizes mainly GT3 [24,25]. ST8Sia II and ST8Sia IV are polysialyltransferases catalysing the polymerization of α2,8-sialic acid residues onto the Neu5Acα2-3(6)Galβ1-4GlcNAc structures found on the N-glycans of NCAM [26]. A third α2,8-sialyltransferase, ST8Sia III, transfers sialic acid residues to a terminal α2,3-linked sialic acid of glycoproteins and glycolipids [27]. Interestingly, h (human) ST8Sia III as well as ST8Sia II and ST8Sia IV are capable of autopolysialylation [28,29]. This autopolysialylation has been suggested to be implicated in the formation of active polysialyltransferases in vivo and in vitro [29–31]. However, nothing is known about the enzymes driving the synthesis of diSia, oligoSia or PSA chains on mammalian O-glycans.
In the present study, we describe the molecular cloning, genomic organization and the enzymatic characterization of the hST8Sia VI, which is orthologous to the recently cloned mouse enzyme [32]. The expression of an active, soluble recombinant enzyme clearly shows that the hST8Sia VI synthesizes mainly diSia motifs on the α2,3-sialylated core 1 structure (Neu5Acα2-3Galβ1-3GalNAcα1-O-Ser) found in O-glycosylproteins. In contrast with the mouse enzyme, hST8Sia VI exhibits almost no activity towards either gangliosides or N-glycans.
EXPERIMENTAL
Materials
CMP-[14C]Neu5Ac (CMP-[14C]N-acetylneuraminic acid, 10.7 GBq/mmol) and First Strand cDNA Synthesis kit were from Amersham Pharmacia Biotech (Little Chalfont, U.K.). NucleoSpin® RNA II kit was from Macherey-Nagel (Düren, Germany). Oligo nts were synthesized and purified by Eurogentec (Seraing, Belgium) and dNTP were from Promega Biosciences (Son Luis Obispo, CA, U.S.A.). EXTRA-POL I DNA polymerase and DyNazyme Ext DNA polymerase were from Eurobio (Courtaboeuf, France) and Ozyme (Saint-Quentin-en-Yvelines, France). DMEM (Dulbecco's modified Eagle's medium) containing 4.5 g/l glucose without glutamine was from BioWhittaker Europe. TC100 medium, MEM (minimal essential medium), L-glutamine, antibiotics, geneticin G418, FCS (foetal calf serum) used in cell culture, LIPOFECTAMINE™ Plus reagent and TOPO TA-cloning kit were from Invitrogen (Cergy-Pontoise, France). Foetal bovine serum was from D. Deutscher (Issyles-Moulineaux, France). DOTAP transfection reagent, MAA (digoxigenin-labelled Maackia amurensis agglutinin), SNA (digoxigenin-labelled Sambucus nigra agglutinin), PGNase F (peptide N-glycosidase F) and anti-digoxigenin fluorescein Fab fragments were from Roche (Meylan, France). HiSpeed Plasmid Midi kit was from Qiagen (Courtaboeuf, France). CMP-Neu5Ac, α1-acid glycoprotein, fetuin, pFLAG-CMV-1, DMB (1,2-diamino-4,5-methylene dioxybenzene) dihydrochloride, aryl glycosides and Triton CF-54 were from Sigma-Aldrich (St Louis, MO, U.S.A.). Glyco® Sialidase S, Glyco® Sialidase C and Glyco® Sialidase A™ were from Glyko Inc. (Novato, CA, U.S.A.). Colon cancer cell lines HT-29 and Caco-2 were generously given by Dr G. Huet (INSERM U560, Lille, France) and NBEC (normal breast epithelial cells) were kindly provided by Dr Xuefen Le Bourhis (UPRES-EA 1033, Université des Sciences et Technologies de Lille, France) [33]. mRNA of lung cancer cell line NCI and the cDNA of Dami megakaryocyte cell line were given by Dr I. Van Seuningen (INSERM U560) and Dr A. Pierce (CNRS UMR 8576, Université des Sciences et Technologies de Lille, France) respectively. GT1b, GD1b and GM3 were a generous gift from J.-P. Zanetta (CNRS UMR 8576).
Cell culture
MCF-7 and MDA-MB-231 cells were cultured in MEM medium supplemented with 5% or 10% (v/v) FCS, 100 units/ml penicillin, 100 μg/ml streptomycin and 45 μg/ml gentamycin. COS-7 cells were grown in DMEM supplemented with 10% (v/v) FCS, L-glutamine 20 mM, penicillin and streptomycin at 37 °C under 5% CO2. Sf-9 cells (ATCC, CRL-1711) were cultured in TC100 medium supplemented with 5% (v/v) FCS.
Bio-informatic analysis
BLAST algorithm (http://www.ncbi.nlm.nih.gov/genome/seq/HsBlast.html) [34] was used to retrieve nt sequences highly similar to hST8Sia I (GenBank® accession number D26360), from the human HTG (High-Throughput Genomic) sequences division or the human and mouse EST (expressed sequence tag) divisions of the GenBank®/EBI databases at the NCBI (National Center for Biotechnology Information). The exon-intron junctions were analysed with the internet splice program (http://www.fruitfly.org/cgi-bin/seq_tools/splice.pl). The amino acid sequence analysis was performed using the program of translation for publication (http://www.infobiogen.fr/services/analyseq/cgi-bin/forpub; Infobiogen, France). Hydropathy analyses and determination of potential N-glycosylation sites were performed using the servers TM-Pred Prediction of Transmembrane Regions and orientation (http://www.ch.embnet.org/software/TMPRED_form) and the NetNGlyc 1.0 program (http://www.cbs.dtu.dk/services/NetNGlyc/) from Expert Protein Analysis System (Swiss Institute of Bioinformatics, Switzerland). Sequence alignments were performed using the LFASTAp program (http://www.infobiogen.fr/services/analyseq/cgi-bin/lfastap) and clustalW algorithms (http://www.infobiogen.fr/services/analyseq/cgi-bin/clustalw).
To examine hST8Sia VI gene expression in various human cell lines total RNA from various cell lines (MCF-7, MDA-MB-231, T47-D and NBEC) was extracted using the NucleoSpin® RNA II kit and cellular RNA was quantified by spectrophotometry at 260 nm. For subsequent PCR amplifications, first-strand cDNA was synthesized from total RNA using the First Strand cDNA Synthesis kit according to the manufacturer's instructions.
A specific hST8Sia VI fragment of 834 bp was obtained after RT-PCR (reverse transcription-PCR) of RNAs isolated from various cultured cells, using 200 nM of sense (5′376 AGAGCCAAACTTGCTTCCTGC 3′) and antisense (5′1194 TTAGGCGACTTCACATTTGCT 3′) primers, 200 μM dNTP and 1 unit of EXTRA-POL I DNA polymerase using the following conditions: 95 °C for 1 min, 38 cycles of 1 min at 95 °C, 1 min at 58 °C and 2 min at 72 °C, and 10 min at 72 °C.
Isolation of ST8Sia VI cDNA and construction of expression vectors
For subsequent plasmid constructions, restriction digestion and DNA sequencing (GenoScreen, France) confirmed the insert junctions and the total conservation between the inserted sequence and hST8Sia VI sequence (EMBL accession number: AJ621583). To isolate cDNA clones, RT-PCR was performed using 150 ng of first-strand cDNA synthesized from MCF-7 total RNA. A first cDNA amplification was performed by PCR using a sense (5′−45 GCTGTGCTTCGCCCCGGCAGCAGC 3′) and an antisense (5′1388 TATGAGTCAGATATGGTGTCCATG 3′) primer at 94 °C for 2 min, followed by 40 cycles (94 °C for 1 min, 62 °C for 1 min and 72 °C for 2 min) and an extension step of 10 min at 72 °C. To obtain a cDNA encoding the full-length protein, the amplified products were used as template for a nested PCR using a sense primer containing the restriction site NotI (5′−14 GCCTGGCGGCCGCGATGCGGCCGGGGGGCGCAC 3′) and an antisense primer containing the restriction site XbaI (5′1233 CCTTTGGTGTTTGGAGACATCTAGAATCACCTACTGC 3′). The RT-PCR amplified fragments (1285 bp) were subcloned into PCR2.1 TOPO TA cloning vector giving the TOPO-L-ST8Sia VI plasmid. The inserted fragment was cut out by digestion with NotI and XbaI and inserted into the NotI and XbaI sites of pRc-CMV expression vector. The resulting plasmid (pRc-CMV-L-ST8Sia VI) was further purified and used for stable transfection of MDA-MB-231 cells, as described below.
An expression vector was also prepared for subsequent transient transfection in animal cells. A cDNA encoding a truncated form of hST8Sia VI lacking the first 27 amino acids of the open reading frame was obtained by PCR amplification using the TOPO-L-ST8Sia VI as DNA template, the same antisense primer containing the restriction site XbaI (5′1233 CCTTTGGTGTTTGGAGACATCTAGAATCACCTACTGC 3′) and a sense primer containing the restriction site NotI (5′82 GACGCGCCCGCGGCCGCCAGGATTCTGGTGGAG 3′). Reactions were run for 2 min at 94 °C, 1 min at 62 °C and 2 min at 72 °C for 37 cycles. The resulting 1197 bp fragment was subcloned into the pCR2.1 vector of TOPO TA-cloning kit, was cut out by digestion with NotI and XbaI, and inserted into the NotI and XbaI sites of the pFLAG expression vector. The resulting plasmid encoded a fusion protein with a signal peptide sequence, the FLAG sequence and a truncated form of hST8Sia VI (pFLAG–S-ST8Sia VI). Baculovirus-mediated insect cell expression was used to produce a large amount of a soluble form of hST8Sia VI. For this purpose, a truncated form of hST8Sia VI, lacking the first 24 amino acids, fused to the signal peptide sequence of a viral gene EGT (ecdysone-S-glycosyltransferase), and His6-tagged in N-terminal was expressed into Sf-9 cells. A 1169 bp PCR fragment corresponding to the hST8Sia VI amino acids 25–398 was amplified using TOPO-L-ST8Sia-VI plasmid as DNA template (0.6 ng), a sense primer containing the last codon of the EGT signal, the restriction site HpaI and 6 histidine codons 5′72 GATGTTAACGCTCACCATCACCATCACCATTGCCCGGCAGACGCG 3′ and an antisense primer containing the restriction sites HindIII and BamHI 5′1170 GGATCCAAGCTTTGTTTAGGCGACTTCACATTTGCTAAA 3′. Reactions were run for 45 s at 94 °C, 1 min at 65 °C and 1 min 30 s at 72 °C for 10 cycles, followed by 45 s at 94 °C and 2 min at 72 °C for 25 cycles. The amplified DNA fragment was subcloned into the TOPO pCR2.1 vector for further sequencing, digested with HpaI and BamHI and the resulting digested fragment was inserted into the HpaI-BglII sites of the pUC-PSEGT plasmid [35]. This construction, named pUC-PSEGT-ST8Sia-VI was then digested with BamHI and HindIII and the fragment was inserted at the BglII-HindIII sites of the p119 transfer vector designed for recombination into the p10 locus of the AcMNPV baculovirus (clone 1.2) giving rise to the p119-ST8Sia-VI construct [36].
Production of soluble forms of hST8Sia VI
Qiagen-purified pFlag-S-ST8Sia-VI (5 μg) or pFlag plasmids (5 μg) were transiently transfected into COS-7 cells in 100 mm diameter dishes using LIPOFECTAMINE™ Plus reagent, according to the manufacturer's instructions. The medium was harvested 48 h after transfection and the recombinant protein expressed in the medium was used as the enzyme source for sialyltransferase assays. Sf-9 cells were co-transfected by lipofection using DOTAP with the p119-ST8Sia-VI transfer vector and purified viral DNA [37]. The recombinant baculoviruses were plaque purified and viral clones were tested for sialyltransferase activity as described below. The recombinant protein was used as the enzyme source for sialyltransferase assays.
Sialyltransferase assays and sialylated product characterization
Sialyltransferase assays were performed in 100 mM cacodylate buffer (pH 6.2) containing 10 mM MnCl2, 0.2% Triton CF-54, 40 μM CMP-[14C]Neu5Ac (1.94 KBq) and one of the acceptor substrates (2 mg/ml for glycoproteins, 1 mM for arylglycosides or glycolipids) and 23 μl of the enzyme source in a total volume of 50 μl. Unless stated otherwise, the reactions were performed at 32 °C for 4 h. For glycoproteins, the reactions were stopped by addition of 2.5×SDS/sample buffer and the reaction products were separated by SDS/PAGE. After transfer onto nitro-cellulose membrane (Biotrace, Pall Corporation) the radioactive products were detected and quantified by radio-imaging using a Personal Molecular Imager FX (Bio-Rad, France). For glycolipids and arylglycosides, the incubation reaction was stopped with 1 ml of H2O and products were applied onto C18 Sep-Pak cartridges (Millipore Corp., Milford, MA, U.S.A.), eluted with 30% CH3OH and processed for scintillation counting.
[14C]Neu5Ac-labelled fetuin was produced using soluble recombinant hST8Sia VI in the incubation conditions described above. For linkage analysis of sialic acids, sialylated fetuin was treated with specific sialidases at 37 °C for 1 h, according to the manufacturer's instructions (Glyko Inc.). For analysis of the glycan acceptor, sialylated fetuin was incubated with PGNase F at 37 °C for 30–120 min according to the manufacturer's instructions. In a second experiment, native fetuin was desialylated by Glyco® Sialidase S and was resialylated by the soluble recombinant hST8Sia VI. The resulting products were also resolved by SDS/PAGE and detected by radio-imaging. For analysis of the DP, sialylated fetuin and colominic acid were separately incubated for 2.5 h at 50 °C in 50 μl of DMB reagent (2.7 mM DMB, 9 mM sodium hydrosulphite, 0.5 mM 2-mercaptoethanol in 20 mM trifluoroacetic acid) [38]. A volume (one-fifth) of 0.1 M NaOH was added and incubated for 1 h at room temperature to terminate the reaction and to eventually hydrolyse lactones [39]. DMB-tagged oligosialylated sequences from fetuin and colominic acid were co-injected onto a CarboPac PA-100 column (Dionex). Elution was performed at 0.8 ml/min with a concentration gradient of 0–32% of 1 M KNO3 in water. Elution was monitored by an on-line fluorescence detector set to 372 nm for excitation and 456 nm for emission. The samples were automatically collected and subsequently counted for radioactivity. The concentration of fetuin and asialofetuin were calculated on the basis of the number of O-glycosidically linked Galβ1-3GalNAc residues, determined by gas chromatography (61 nmol/mg).
Stable transfections
MDA-MB-231 cells were transfected with 20 μg of pRc-CMV or pRc-CMV-L-ST8SiaVI plasmids using LIPOFECTAMINE™. After transfections (2 days), the dishes were split at a 1:10 ratio and cells were cultured in MEM containing 5% (v/v) FCS and geneticin G418 (1 mg/ml). MEM and geneticin were replaced every 2 days. After 15 days, the resistant colonies were well established, the cells selected and maintained with 1 mg/ml geneticin and 10% (v/v) FCS and used for flow cytometric analysis experiments.
Detection of α2,6-linked and α2,3-linked sialic acids in MDA-MB-231 transfected cells
Cell surface expression of sialic acids in two different types of linkages was quantified using SNA specific for α2,6-linked sialic acids and MAA specific for α2,3-linked sialic acids. Stably transfected cells were fixed with 4% paraformaldehyde (30 min at 4 °C in the dark), quenched for 30 min with 50 mM NH4Cl in PBS and incubated overnight at 4 °C with SNA or MAA [1:50 in PBS, 1% (v/v) BSA]. After washing with PBS containing 1% BSA the cells were incubated with anti-digoxigenin-fluorescein Fab fragments (1:500 in PBS, 1% BSA) for 2 h on ice. Flow cytometric analysis of the cells stained with lectins was performed using a FACScan fluorospectrometer (Becton Dickinson).
RESULTS
Identification of hST8Sia VI gene
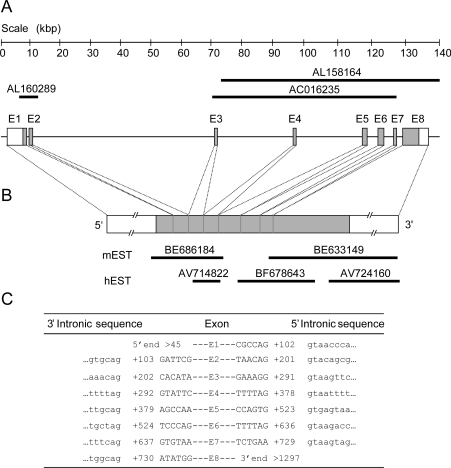
Similarity searches using the BLAST algorithms (BLASTn and tBLASTn) were conducted with the hST8Sia I cDNA (GenBank® accession number: D26360) used as a bioinformatics probe in the human genomic databases (HTG division of NCBI). This study led us to the identification of two genomic clones AC016235 and AL158164 (Figure 1A), which partially aligned with the hST8Sia I cDNA and encompassed the signature motifs of mammalian sialyltransferases. In addition, these nt sequences were localized on human chromosome 10 where no sialyltransferase gene was described. Upon screening the human EST division of the NCBI databases, three ESTs, AV714822, BF678643 and AV724160 were retrieved, which corresponded to the 3′ region of the putative gene whereas no human EST could be found to correspond with the 5′ end of the gene (Figure 1B). However, BLAST analysis conducted in the mouse EST division of NCBI revealed the existence of two ESTs (BE686184 and BE633149) belonging to the same Integrated Molecular Analysis of Genome Expression (I.M.A.G.E.) clone (ID 34128403) that represented the mouse counterpart of the human sequence used for the search (Figure 1B) and which allowed us to identify the missing upstream sequences of the putative sialyltransferase in the human genomic clone, AL160289. The gene identification was completed at the Ensembl site (http://www.ensembl.org). As shown in Figure 1(A) the putative sialyltransferase gene spans over 140 kb of human genomic sequence (NT 008682) on human chromosome 10 (10p12.31). This nt sequence contains an open reading frame of 1197 bp (Figure 1B). In order to gain insights into the evolutional relationship of the hST8Sia VI with other sialyltransferase genes, the hST8Sia VI genomic organization was determined (Figure 1C) through the use of a specialized internet site (http://www.fruitfly.org/seq_tools/splice.html). The sequence of the splice junctions of the hST8Sia VI gene obey the AG/GT rule and the gene splits into eight coding exons in a genomic organization closely related to that of hST8Sia I and hST8Sia V (results not shown, see [40]).
Figure 1. Genomic organization and exon/intron junctions of the hST8Sia VI gene.
(A) The hST8Sia VI gene located on chromosome 10p12.31, spans 140 kb and contains 8 exons (labelled El–E8). Black lines represent the DNA sequences identified in the human genomic databases. The entire genomic sequence of hST8Sia VI is included in the human contig: NT 008682.3. (B) Schematic representation of the hST8Sia VI mRNA. The grey boxes represent the open reading frame and the open boxes the 5′ and 3′ untranslated regions respectively. The black lines represent the mouse (m) and human (h) EST identified in the public databases. (C) The nt sequences at the intron (lowercase letters) and exon (uppercase letters) junctions are shown. Exons are numbered from the 5′ end with the initiator methionine denoted as +1.
Cloning and nt sequencing of hST8Sia VI
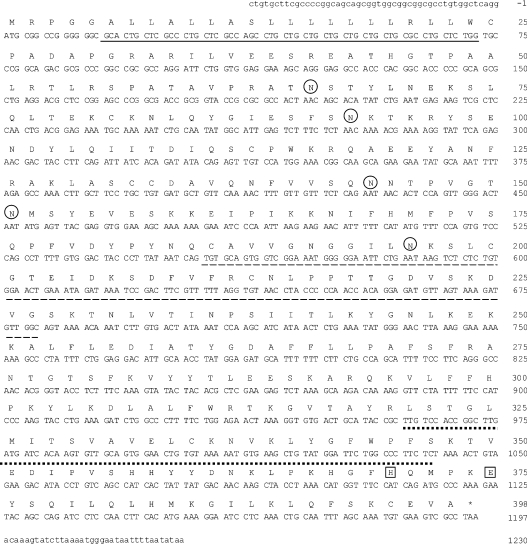
The nt sequences described above were used to design oligo nt primers for PCR amplification with MCF-7 first strand cDNA as template. However, probably because of the low level of expression of a long transcript and strong secondary RNA structures, we could not amplify the target sequence at once, so we amplified a full-length cDNA by nested PCR. Upon DNA sequencing of several of these MCF-7 PCR-amplified cDNA clones, we noticed various splicing variants of hST8Sia VI gene lacking 1 or 2 exons (results not shown). Indeed, theoretically, exons numbered 2, 3, 4 and 7 can be deleted without frame-shift in the open reading frame. The nt and deduced amino acid sequences of the full-length cDNA sequence obtained are shown in Figure 2. Hydropathy analysis of this protein indicated the presence of a hydrophobic sequence of 19 amino acids in the NH2-terminal region, corresponding to the transmembrane domain characteristic of all sialyltransferases cloned to date (results not shown). The predicted protein consisted of 398 amino acids with a calculated molecular mass of 44835 Da, contained in the catalytic domain the four sialylmotifs L, S, III and VS characteristic of all the animal sialyltransferases, and showed five potential N-glycosylation sites (Figure 2). The position of the initiation codon was estimated according to the Kozak consensus sequence [41] (http://www.hri.co.jp/atgpr/). In addition, the protein showed the (I/L)(F/Y)GFW(P/A)F sequence at the 3′ end of the sialylmotifs, which is conserved among the members of the α2,8-sialyltransferase family. Besides, comparison of the amino acid sequence with those of the other human sialyltransferases indicated a phylogenetic linkage with the α2,8-sialyltransferases and particularly the greatest sequence identities with hST8Sia I (35%) and hST8Sia V (32%). Finally, hST8Sia VI is the human counterpart of a mouse sequence (GenBank® accession number AB059554) cloned during the time-course of our study [32]. The two sequences share 82.7% sequence identity. Orthologues of the hST8Sia VI gene are also detected in several other vertebrate genomes such as Rattus norvegicus (AJ699423), Bos taurus (AJ868432), Gallus gallus (AJ699424), Takifugu rubripes (AJ715549, AJ715550) and Danio rerio (AJ715551) [40]. The deduced amino acid sequences of these genes display 81.4%, 82.2%, 59.4%, 35.2%, 34.4% and 36.9% sequence identity respectively with the hST8Sia VI gene.
Figure 2. nt and predicted amino acid sequences of hST8Sia VI.
Numbering of the cDNA begins with the initiation codon. The amino acid sequence is shown in single-letter code. The putative 19 amino acid N-terminal transmembrane domain is underlined. Putative N-glycosylation sites are circled. Dashed and dotted lines underline the sialyl motifs L and S respectively. The conserved His and Glu residues in sialyl motif VS are boxed.
hST8Sia VI gene expression in human tissues and cell lines
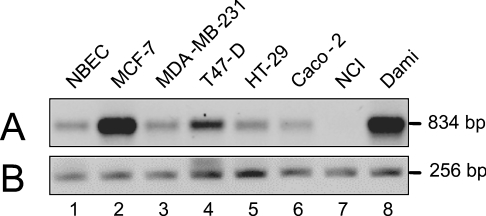
In order to determine the expression pattern of hST8Sia VI gene and the size of hST8Sia VI mRNA, hybridization of a Northern blot of 12 human tissues and of an expression array of 72 different human tissues was performed with a full-length hST8Sia VI cDNA (1.27 kb). However, the expression levels of the ST8Sia VI gene were very low in all the tissues examined (results not shown). Thus we performed semi-quantitative RT-PCR analysis in several human cancer and normal cell lines. As shown in Figure 3, higher levels of expression of the hST8Sia VI gene were observed in breast cancer cell line MCF-7 and in Dami megakaryocyte cell line whereas very low levels of expression were observed in NBEC, in breast cancer cell line MDA-MB-231 and T47-D and in colon cancer cell lines HT-29 and Caco-2. No expression could be detected in the lung cancer cell line NCI.
Figure 3. RT-PCR analysis of the expression of hST8Sia VI gene in various human cell lines.
(A) mRNAs levels of hST8Sia VI were evaluated by RT-PCR, as described in the Experimental section, in various cell lines; lane 1, NBEC; lane 2, MCF-7; lane 3, MDA-MB-231; lane 4, T47-D; lane 5, HT-29; lane 6, Caco-2; lane 7, NCI; lane 8, Dami. (B) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as standard as previously described [50]. The sizes of the amplified fragments are indicated on the right side of the gel.
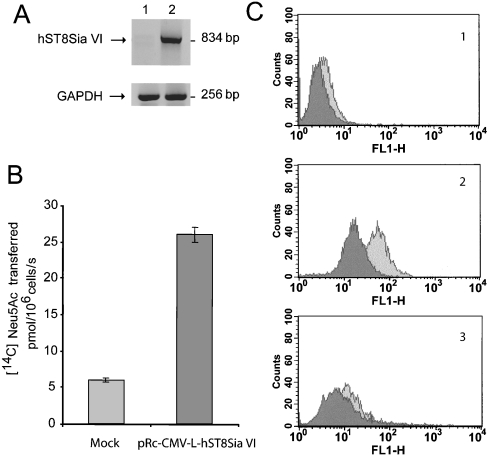
Decreased expression of MAA-binding sites in the MDA-MB-231 cell line stably transfected with full-length hST8Sia VI
In order to assess the function of hST8Sia in vivo, a MDA-MB-231 cell line was stably transfected with the expression vector pRc-CMV-L-hST8Sia VI (full-length) as described in the Experimental section. Cell clones were selected by mRNA expression (Figure 4A) and by ST8Sia activity towards fetuin used as an acceptor substrate for in vitro assays (Figure 4B). Characterization of the sialylated carbohydrate profile of MDA-ST8Sia VI, as well as of the mock-transfected cells was performed by flow cytometry analysis of these cells using MAA and SNA lectins that are specific for α2,3- and α2,6-sialylated oligosaccharides respectively. As shown in Figure 4(C), in vivo expression of hST8Sia VI led to a 4-fold decrease in MAA-binding sites in MDA-MB-231 transfected cells whereas very limited changes were observed for the SNA-binding sites in MDA-MB-231 transfected cells (Figure 4C). Our results suggest that in vivo hST8Sia VI catalyses the transfer of the sialic acid residue onto the disaccharide structure Neu5Acα2-3Gal, but had very limited activity towards the disaccharide structures Neu5Acα2-6GalNAc.
Figure 4. Expression of full-length human ST8Sia VI in MDA-MB-231 breast cancer cells.
(A) The expression of human ST8Sia VI cDNA in MDA-MB-231 breast cancer cells was determined by RT-PCR. Lane 1, mock transfected cells; lane 2, pRc-CMV-L-hST8Sia VI transfected cells. The size of the amplified fragments are indicated on the right side. (B) hST8Sia VI enzymatic activity measured in selected cell clones. Enzymatic activity was measured on fetuin and results are expressed in pmol of transferred Neu5Ac residues per 106 cells/s. (C) Flow cytometry analysis of pRc-CMV-L-hST8Sia VI transfected cells. MDA-MB-231 expressing (pRc-CMV-L-ST8Sia VI transfected cells; dark grey peaks) or not expressing hST8Sia VI (mock transfected cells; light grey peaks), were subjected to MAA and SNA labelling. MAA revealed the presence of a terminal α3-linked sialic acid and SNA the presence of a terminal α6-linked sialic acid. Panel 1, negative control (secondary antibodies only); panel 2, MAA staining; panel 3, SNA staining.
Sialyltransferase activity of the hST8Sia VI
In order to conclusively verify that the cloned DNA which we had obtained from MCF-7 cells indeed represented human ST8Sia VI, and to facilitate functional analysis of the enzyme, we produced a soluble form of the enzyme with deleted cytoplasmic and transmembrane domains, using the baculovirus/Sf-9 system. Thus a truncated cDNA lacking the first 24 amino acid residues of the N-terminus region was generated by PCR and used for the construction of a p119-hST8Sia VI vector, as described in the Experimental section. The recombinant hST8Sia VI enzyme produced in insect cells was used for in vitro sialyltransferase assays with various glycoconjugate acceptors (Table 1). This recombinant hST8Sia VI enzyme exhibited highly decreased sialyltransferase activity towards asialoglycoproteins compared with the corresponding sialylated substrates indicating that the enzyme required a sialylated substrate. hST8Sia VI exhibited strong sialyltransferase activity towards BSM (bovine submaxillary mucin) and OSM (ovine submaxillary mucin), which contains only O-glycans and towards fetuin, which contains both N- and O-glycans. On the contrary, it showed very low activity towards α1-acid glycoprotein, which contains only N-glycans. This set of results clearly showed that the recombinant hST8Sia VI preferred sialylated O-glycans such as Neu5Acα2-3Galβ1-3GalNAc-O-Ser/Thr or Neu5Acα2-6GalNAc-O-Ser/Thr found on fetuin, to sialylated N-glycans as acceptor substrates. We next examined hST8Sia VI activity towards various glycolipids such as GM3, GD3, or GD1b containing a sialylated lactosylceramide fraction (Table 1). hST8SiaVI showed almost no activity (<11%) towards these compounds in contrast with the mouse ST8SiaVI enzyme, which showed noticeable activity towards GM3. However, we observed higher sialyltransferase activity of hST8Sia VI towards the gangliosides GT1b and GD1a, (25% and 12% respectively) which both contain a terminal Neu5Acα2-3Galβ1-3GalNAc sequence whereas the mouse enzyme showed almost no activity towards these compounds. Finally, hST8Sia VI exhibited almost no activity towards arylglycosides (<11%) (Table 1). These results suggest that hST8Sia VI prefers Neu5Acα2-3Galβ1-3GalNAc-R (where R is the remainder of the N-linked oligosaccharide chain) or Neu5Acα2-6GalNAc-R found in the O-glycans and also is highly sensitive to the underlying sequences.
Table 1. Comparison of the acceptor substrate specificity of hST8Sia VI and m (mouse) ST8Sia VI.
Various acceptor substrates were incubated in the standard assay mixture using transfected Sf-9 cells as an enzyme source. Each substrate was used at a concentration of 1 mM for arylglycosides, 2 mg/ml for glycoproteins and 1 mM for glycolipids. The specific [14C]Neu5Ac incorporation resulting from recombinant hST8Sia VI activity was estimated by subtracting the background sialyltransferase activities of mock transfected cells from the radioactivity in the presence of exogenous acceptor. Relative rates were calculated as a percentage of the incorporation of sialic acid onto fetuin. A value of 0 indicates <1%; nd, not determined; Cer, ceramide.
| Relative rate (%) | |||
|---|---|---|---|
| Acceptor | Structures | hST8Sia VI | mST8Sia VI * |
| Glycoproteins | |||
| Fetuin | Neu5Acα2-3Galβ1-3GalNAcα1-O-Ser/Thr | ||
| Neu5Acα2-3Galβ1-3[Neu5Acα2-6]GalNAcα1-O-Ser/Thr | |||
| Neu5Acα2-6(3)Galβ1-4GlcNAc-R | 100 (0.15)† | 100 (0.206)† | |
| Asialofetuin | Galβ1-3GalNAcα1-O-Ser/Thr and Galβ1-4GlcNAc-R | 10 | 0 |
| α1-Acid glycoprotein | Neu5Acα2-6Galβ1-4GlcNAc-R | 15 | 0 |
| Asialo-α1-acid glycoprotein | Galβ1-4GlcNAc-R | 6 | 0 |
| BSM | GlcNAcβ1-3[Neu5Acα2-6]GalNAcα1-O-Ser/Thr | ||
| Neu5Acα2-6GalNAcα1-O-Ser/Thr | 350 | 375 | |
| Asialo-BSM | GlcNAcβ1-3GalNAcα1-O-Ser/Thr | ||
| GalNAcα1-O-Ser/Thr | 15 | 0 | |
| OSM | Neu5Acα2-6GalNAcα1-O-Ser/Thr | 334 | nd |
| Asialo-OSM | GalNAcα1-O-Ser/Thr | 12 | nd |
| Glycolipids | |||
| GM3 | Neu5Acα2-3Galβ1-4Glcβ1-1′Cer | 3 | 13 |
| GD3 | Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1-1′Cer | 8 | 0 |
| GD1 | Neu5Acα2-3Galβ1-3GalNAcβ1-4[Neu5Acα2-3]Galβ1-4Glcβ1-1′Cer | 12 | 6 |
| GD1 | Galβ1-3GalNAcβ1-4[Neu5Acα2-8Neu5Acα2-3]Galβ1-4Glcβ1-1′Cer | 11 | 0 |
| GT1 | Neu5Acα2-3Galβ1-3GalNAcβ1-4[Neu5Acα2-8Neu5Acα2-3]Galβ1-4Glcβ1-1′Cer | 25 | 1.1 |
| Arylglycosides | |||
| Galβ1-3GlcNAcβ-octyl | 11 | nd | |
| Neu5Acα2-6Galβ1-4GlcNAcβ1-2Manα-octyl | 3 | nd | |
| Neu5Gcα2-3Galβ1-3GalNAcβ-octyl | 3 | nd | |
*Data are from [32].
†Actual activities are shown in brackets in pmol/h per μl.
In order to confirm that hST8Sia VI preferentially sialylates O-glycans, α2,8-[14C]Neu5Ac-labelled sialylated fetuin was produced using a soluble recombinant hST8Sia VI (see the Experimental section) and was subjected to PNGase F, which hydrolyses N-glycans (Figure 5). After 30 min treatment, we observed a shift of mass corresponding to the loss of N-glycans but the intensity of the signal remained unchanged, suggesting that the sialic acid residue was transferred onto O-glycans of fetuin rather than onto N-glycans.
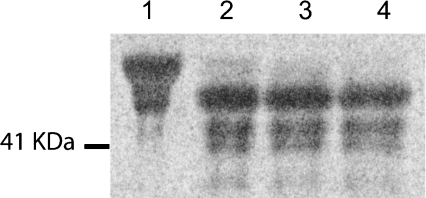
Figure 5. N-deglycosylation of sialylated fetuin.
[14C]Neu5Ac-labelled fetuin was produced using soluble recombinant hST8Sia VI and was subjected to PNGase F treatment for 0 min (lane 1), 30 min (lane 2), 60 min (lane 3) or 120 min (lane 4). The resulting products were separated by SDS/PAGE and detected by phosphorimaging. The molecular mass marker is indicated on the left side of the gel.
To characterize the linkage catalysed by hST8Sia VI, α2,8-[14C]Neu5Ac-labelled fetuin was produced using soluble recombinant hST8Sia VI. It was then subjected to linkage specific sialidases, Glyco® Sialidase S (specific for α2,3-linked sialic acid), Glyco® Sialidase C (specific for α2,3/6-linked sialic acid) and Glyco® Sialidase A™ (specific for α2,3/6/8-linked sialic acid) (Figure 6A). We observed that the treatment with the Glyco® Sialidase S and C, which hydrolyse the α2,3- and α2,6-linked sialic acid is ineffective on the signal. Conversely, this signal disappeared when labelled fetuin was treated with Glyco® Sialidase A™, which is specific for α2,3/6/8-linked sialic acid, indicating that the incorporated sialic acid was α2,8-linked. These results confirmed that the cloned hST8Sia VI belonged to the ST8Sia subfamily.
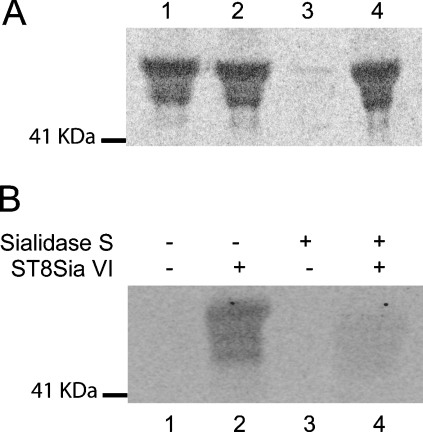
Figure 6. Linkage analysis of incorporated sialic acid.
(A) [14C]Neu5Ac-labelled fetuin was produced using soluble recombinant hST8Sia VI and was subjected to sialidase treatment with Glyco® Sialidase S (specific for α2,3-linked sialic acid, lane 1), Glyco® Sialidase C (specific for α2,3-and α2,6-linked sialic acids, lane 2), Glyco® Sialidase A™ (specific for α2,3- α2,6 and α2,8/9-linked sialic acids, lane 3) or none (lane 4). The resulting products were separated by SDS/PAGE and detected by phosphorimaging. (B) Native fetuin (lanes 1 and 2) or α3-desialylated fetuin (lanes 3 and 4) were [14C]Neu5Ac-sialylated using soluble recombinant hST8Sia VI (lanes 2 and 4) or using mock-transfected cells conditioned medium (lanes 1 and 3). The resulting products were separated by SDS/PAGE and detected by phosphorimaging. The molecular mass marker is indicated on the left side.
We next aimed to determine whether hST8Sia VI preferentially sialylated Neu5Acα2-3Galβ1-3GalNAc-R acceptor substrate or the Neu5Acα2-6GalNAc-R acceptor substrate that are both present on native bovine fetuin. Thus either native fetuin or α2,3-desialylated fetuin was incubated with recombinant hST8Sia VI. Indicative of the efficiency of the desialylation, a shift of mass was observed after Ponceau Red staining of the Western blot, when α2,3-sialic acid was removed before incubation (results not shown). As shown in Figure 6(B), hST8Sia VI did not mediate the efficient transfer of sialic acid residue onto α2,3-desialylated fetuin. This result suggested that the recombinant hST8Sia VI preferentially catalysed the transfer of a sialic acid residue to an α2,3-linked Neu5Ac rather than to an α2,6-linked Neu5Ac and confirmed the flow cytometric analysis of the transfected MDA-ST8Sia VI cells with the MAA lectin.
DP analysis
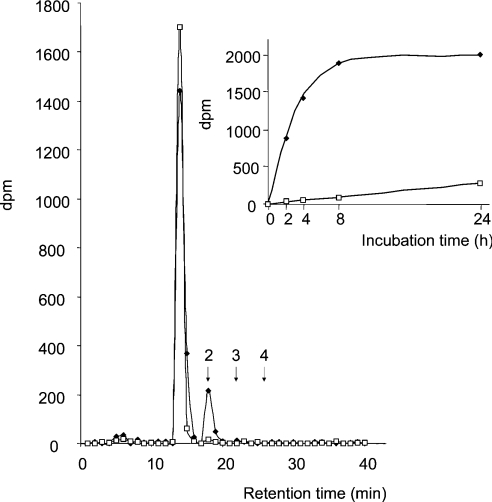
Based on the above results, we next addressed the question of whether hST8Sia VI may synthesize di-, oligo-, or PSA chains onto fetuin. For this purpose, native bovine fetuin was first incubated with hST8Sia VI and CMP-[14C]Neu5Ac. Sialylated sequences were then liberated from glycoproteins by mild acid hydrolysis, tagged with DMB and separated by HPLC on a CarboPac PA-100 column. Retention times for [Neu5Acα2-8]n-Neu5Ac-DMB sequences were determined by injecting tagged total colominic acid standard, as well as di-, tri- and tetra-sialylated sequences purified from a mild hydrolysate of colominic acid. As shown in Figure 7, in the hST8Sia VI incubation mixture, we clearly observed a radioactive peak with a retention time similar to Neu5Acα2-8Neu5Ac-DMB, strongly suggesting that synthesis of the di-sialylated epitope occurred from sialylated fetuin. Accordingly, such a peak was not observed in either the mock-transfected cell incubation mixture or native fetuin, indicating that hST8Sia VI catalysed the transfer of a single sialic acid residue onto native fetuin. Furthermore, a time course analysis of the sialylation of fetuin clearly confirmed the specificity of this enzyme for biosynthesis of diSia motifs.
Figure 7. HPLC/DMB analysis of α2,8-linked-[14C]-Neu5Ac on fetuin.
Native fetuin was [14C]Neu5Ac-sialylated for 4 h using soluble recombinant hST8Sia VI or using mock-transfected cells conditioned medium and subjected to mild acid hydrolysis followed by DMB derivation. Oligosialylated derivatives were fractionated by HLPC on CarboPac PA-100 column according to the DP. Arrows indicate the retention time of standard di-, tri- and tetrasialo-DMB derivatives prepared from partially hydrolysed colominic acid. Inset: time course analysis of [14C]-labelled disialo-DMB derivatives synthesis. (◆) hST8Sia VI; (□) mock.
DISCUSSION
In the present study, we report on the molecular cloning and enzymatic characterization of a human sialyltransferase gene that encodes an ST8Sia with preference for O-glycans. This gene spans over 140 kb of human chromosome 10 (10p12.31) and splits into eight coding exons with an organization closely related to that of the hST8Sia I and hST8Sia V genes. Phylogenetic analyses have shown that the ST8Sia gene family can be divided into two groups of genes, which evolved from a common ancestor and it was observed that the human sialyltransferases of each group showed distinct enzymatic properties [40]. In the first group, ST8Sia II, III and IV are known as oligo- or poly-sialyltransferases involved in the elongation of linear chains of sialic acids found mainly in glycoproteins. ST8Sia II and IV were clearly shown to be responsible for the elongation of polysialylated chains of NCAM [26] and even if the substrate specificity of ST8Sia III is not clearly demonstrated, this latter enzyme is capable of autopolysialylation in vitro [29]. In the second group encompassing ST8Sia I, ST8Sia V and ST8Sia VI, ST8Sia I (GD3 synthase) and ST8Sia V (GT3 synthase) are acting as di-sialyltransferases, transferring only one sialic acid residue onto GM3 or GD3 to convert these gangliosides into GD3 or GT3 respectively. It could be predicted that the enzymatic properties of hST8Sia VI would be those of a di-sialyltransferase. As hypothesized, we have clearly demonstrated that the human ST8Sia VI transferred only one sialic acid residue in α2,8-linkage. However, in contrast with its recently characterized mouse counterpart [32], which also acts on the Neu5Acα2-3Galβ1-4Glc sequence of GM3 and on the Neu5Acα2-6Galβ sequences such as are found in N-glycosylproteins, the hST8Sia VI works almost exclusively on O-glycans. Stable transfections of hST8Sia VI in the MDA-MB-231 cell line have shown that hST8Sia VI works preferentially on cell surface α2,3-sialylated structures. Our in vitro assays using a soluble form of the enzyme have demonstrated that hST8Sia VI preferentially uses the trisaccharide Neu5Acα2-3Galβ1-3GalNAcα1-O-Ser/Thr found in bovine fetuin, and to a lesser extent, to that found in gangliosides (GD1a). It shows also an activity towards the disaccharide Neu5Acα2-6GalNAcα1-O-Ser/Thr found on O-glycans of BSM and OSM. This discrepancy between the specific enzymatic activity of the mouse and the human enzymes could be related to inter-species enzymatic differences which indeed lead to species-specific glycosylation. Currently work is in progress to determine which O-glycosylprotein(s) might be disialylated in these stably-transfected MDA cells using specific monoclonal antibodies towards diSia, oligoSia or PSA [42].
The occurrence of diSia motifs in animal tissues was suggested a long time ago by Finne et al. [43] but indeed, Neu5Acα2-8Neu5Acα2-3Galβ1-3GalNAc-α1-O-Ser/Thr or Galβ1-3[Neu5-Acα2-8Neu5Acα2-6]GalNAc-α1-O-Ser/Thr oligosaccharides are not frequently described in mammalian glycoproteins. However, these diSia motifs were shown previously to occur in several embryonic and adult pig brain glycoproteins [44], in the murine CD-166 [18], on the N-glycans of Band 3 of human erythrocytes [14], on the N-glycans of fetuin and α2-macroglobulin of calf serum [17], on the O-glycans of human erythrocyte glycophorin [15], bovine adipo-Q [19] and chromogranin [16]. It has been suggested that the biosynthesis of these diSia residues on glycoproteins might be catalysed by an α2,8-sialyltransferase distinct from the known polysialyltransferases ST8Sia II and ST8Sia IV and it was shown that a recombinant ST8Sia III could sialylate purified bovine adipo-Q [19]. We have clearly demonstrated in in vitro assays that hST8Sia VI is highly specific for the synthesis of the diSia epitope found on O-glycosylproteins although the presence of this structure remains to be demonstrated in vivo. However, diSia epitopes on O-glycosylproteins might represent biosynthetic intermediates, thus generating a multiplicity of di- oligo- and poly-Sia structures that have recently been described in mammalian cell lines and tissues [44,45]. The development of chemical detection tools [46] or biochemical tools [42] should help us to solve these biosynthetic pathways.
Whereas the importance of the diSia epitope on glycolipids is well documented, the role of the diSia epitopes found on glycoproteins is not known. As suggested by the fact that the anti-diSia antibody specifically blocked neurite extension in the Neuro2A mouse neuroblastoma cell line, it has been proposed that the diSia epitope on glycoproteins could be involved in neurite formation [18]. During the adipocyte differentiation of murine fibroblastic 3T3-L1 preadipocytes, significant increases in diSia structures on glycoproteins were observed in fully differentiated adipocyte cells and among them the serum adipo-Q glycoprotein has been shown to have the diSia epitope [19]. Adipo-Q is considered to play an important role in energy homoeostasis and it is believed that the diSia epitope could be functionally involved in these physiological phenomena in a way that remains to be determined [19]. Of the siglecs cloned so far, it is known that siglec-7 and siglec-11 have a high affinity for the diSia epitopes found on glycoproteins [47]. Siglec-11 shows a unique expression pattern on tissue macrophages and infiltrating mononuclear leucocytes in inflammatory tissues suggesting that this molecule and the diSia epitopes might be involved in the regulation of innate immune responses. Siglec-7 is the major siglec expressed by natural killer cells [48] and displays unique ligand binding properties, different from other members of the siglec family, towards α2,8-linked sialic acids over α2,6- and α2,3-linked sialic acids [49]. One can assume that glycan recognition by siglec-7 is likely to be directly linked to its function in modulating the activation of natural killer cells. The characterization of this new ST8Sia VI might help to determine the function of the diSia epitopes found on O-glycosylated proteins, although the presence of this structure remains to be demonstrated in vivo.
Acknowledgments
We are grateful to Anne-Marie Mir for technical assistance with cell culture, to Annick Ozyl for large scale production of the recombinant enzyme in insect cells and to Dr Carmen Odberg (CNRS UMR 8576) and Dr Ken Kitajima (Bioscience and Biotechnology Center, Nagoya University, Japan) for helpful discussions.
References
- 1.Tsuji S., Datta A. K., Paulson J. C. Systematic nomenclature for sialyltransferases. Glycobiology. 1996;6:5–7. doi: 10.1093/glycob/6.7.647. [DOI] [PubMed] [Google Scholar]
- 2.Troy F. A. I. Sialobiology and the polysialic acid glycotope. In: Rosenberg A., editor. Biology of the Sialic Acids. New York and London: Plenum Press; 1996. pp. 95–144. [Google Scholar]
- 3.Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J. Biol. Chem. 1982;257:11966–11970. [PubMed] [Google Scholar]
- 4.Livingston B. D., Jacobs J. L., Glick M. C., Troy F. A. Extended polysialic acid chains (n>55) in glycoproteins from human neuroblastoma cells. J. Biol. Chem. 1988;263:9443–9448. [PubMed] [Google Scholar]
- 5.Yabe U., Sato C., Matsuda T., Kitajima K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J. Biol. Chem. 2003;278:13875–13880. doi: 10.1074/jbc.M300458200. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K., Rutishauser U., Crandall J. E., Schwarting G. A. Polysialic acid facilitates migration of luteinizing hormone-releasing hormone neurons on vomeronasal axons. J. Neurosci. 1999;19:794–801. doi: 10.1523/JNEUROSCI.19-02-00794.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuber C., Lackie P. M., Catterall W. A., Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J. Biol. Chem. 1992;267:9965–9971. [PubMed] [Google Scholar]
- 8.Rutishauser U. Polysialic acid and the regulation of cell interactions. Curr. Opin. Cell Biol. 1996;8:679–684. doi: 10.1016/s0955-0674(96)80109-8. [DOI] [PubMed] [Google Scholar]
- 9.Cremer H., Chazal G., Goridis C., Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- 10.Muller D., Wang C., Skibo G., Toni N., Cremer H., Calaora V., Rougon G., Kiss J. Z. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J. Cell Biol. 1986;102:688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasahara K., Watanabe Y., Yamamoto T., Sanai Y. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J. Biol. Chem. 1997;272:29947–29953. doi: 10.1074/jbc.272.47.29947. [DOI] [PubMed] [Google Scholar]
- 13.Liu H., Kojima N., Kurosawa N., Tsuji S. Regulated expression system for GD3 synthase cDNA and induction of differentiation in Neuro2a cells. Glycobiology. 1997;7:1067–1076. doi: 10.1093/glycob/7.8.1067. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J. Biol. Chem. 1984;259:4782–4791. [PubMed] [Google Scholar]
- 15.Fukuda M., Lauffenburger M., Sasaki H., Rogers M. E., Dell A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J. Biol. Chem. 1987;262:11952–11957. [PubMed] [Google Scholar]
- 16.Kiang W. L., Krusius T., Finne J., Margolis R. U., Margolis R. K. Glycoproteins and proteoglycans of the chromaffin granule matrix. J. Biol. Chem. 1982;257:1651–1659. [PubMed] [Google Scholar]
- 17.Kitajima K., Sato C., Honda N., Matsuda T., Yokoyama M.-H., Close B., Colley K. Occurence of α2,8-linked oligosialic acid residues in mammalian glycoproteins. In: Inoue Y., Lee Y. C., II F. A. T., editors. Sialobiology and Other Novel Forms of Glycosylation. Osaka, Japan: Gakushin Publishing Co.; 1999. pp. 69–76. [Google Scholar]
- 18.Sato C., Matsuda T., Kitajima K. Neuronal differentiation-dependent expression of the disialic acid epitope on CD166 and its involvement in neurite formation in Neuro2A cells. J. Biol. Chem. 2002;277:45299–45305. doi: 10.1074/jbc.M206046200. [DOI] [PubMed] [Google Scholar]
- 19.Sato C., Yasukawa Z., Honda N., Matsuda T., Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J. Biol. Chem. 2001;276:28849–28856. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 20.Nadanaka S., Sato C., Kitajima K., Katagiri K., Irie S., Yamagata T. Occurrence of oligosialic acids on integrin α5 subunit and their involvement in cell adhesion to fibronectin. J. Biol. Chem. 2001;276:33657–33664. doi: 10.1074/jbc.M011100200. [DOI] [PubMed] [Google Scholar]
- 21.Ziak M., Meier M., Novak-Hofer I., Roth J. Ceruloplasmin carries the anionic glycan oligo/poly α2,8 deaminoneuraminic acid. Biochem. Biophys. Res. Commun. 2002;295:597–602. doi: 10.1016/s0006-291x(02)00718-0. [DOI] [PubMed] [Google Scholar]
- 22.Ziak M., Kerjaschki D., Farquhar M. G., Roth J. Identification of megalin as the sole rat kidney sialoglycoprotein containing poly α2,8 deaminoneuraminic acid. J. Am. Soc. Nephrol. 1999;10:203–209. doi: 10.1681/ASN.V102203. [DOI] [PubMed] [Google Scholar]
- 23.Ziak M., Meier M., Roth J. Megalin in normal tissues and carcinoma cells carries oligo/poly α2,8 deaminoneuraminic acid as a unique posttranslational modification. Glycoconj. J. 1999;16:185–188. doi: 10.1023/a:1007068102436. [DOI] [PubMed] [Google Scholar]
- 24.Kono M., Yoshida Y., Kojima N., Tsuji S. Molecular cloning and expression of a fifth type of α2,8-sialyltransferase (ST8Sia V). Its substrate specificity is similar to that of SAT-V/III, which synthesize GD1c, GT1a, GQ1b and GT3. J. Biol. Chem. 1996;271:29366–29371. doi: 10.1074/jbc.271.46.29366. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki K., Kurata K., Kojima N., Kurosawa N., Ohta S., Hanai N., Tsuji S., Nishi T. Expression cloning of a GM3-specific α-2,8-sialyltransferase (GD3 synthase) J. Biol. Chem. 1994;269:15950–15956. [PubMed] [Google Scholar]
- 26.Angata K., Suzuki M., Fukuda M. Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J. Biol. Chem. 1998;273:28524–28532. doi: 10.1074/jbc.273.43.28524. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y. C., Kim Y. J., Lee K. Y., Kim K. S., Kim B. U., Kim H. N., Kim C. H., Do S. I. Cloning and expression of cDNA for a human Sia α2,3Galβ1, 4GlcNA:α2,8-sialyltransferase (hST8Sia III) Arch. Biochem. Biophys. 1998;360:41–46. doi: 10.1006/abbi.1998.0909. [DOI] [PubMed] [Google Scholar]
- 28.Angata K., Suzuki M., McAuliffe J., Ding Y., Hindsgaul O., Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct α2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J. Biol. Chem. 2000;275:18594–18601. doi: 10.1074/jbc.M910204199. [DOI] [PubMed] [Google Scholar]
- 29.Muhlenhoff M., Eckhardt M., Bethe A., Frosch M., Gerardy-Schahn R. Autocatalytic polysialylation of polysialyltransferase-1. EMBO. 1996;15:6943–6950. [PMC free article] [PubMed] [Google Scholar]
- 30.Close B. E., Colley K. J. In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J. Biol. Chem. 1998;273:34586–34593. doi: 10.1074/jbc.273.51.34586. [DOI] [PubMed] [Google Scholar]
- 31.Muhlenhoff M., Manegold A., Windfuhr M., Gotza B., Gerardy-Schahn R. The impact of N-glycosylation on the functions of polysialyltransferases. J. Biol. Chem. 2001;276:34066–34073. doi: 10.1074/jbc.M101022200. [DOI] [PubMed] [Google Scholar]
- 32.Takashima S., Ishida H. K., Inazu T., Ando T., Ishida H., Kiso M., Tsuji S., Tsujimoto M. Molecular cloning and expression of a sixth type of α2,8-sialyltransferase (ST8Sia VI) that sialylates O-glycans. J. Biol. Chem. 2002;277:24030–24038. doi: 10.1074/jbc.M112367200. [DOI] [PubMed] [Google Scholar]
- 33.Toillon R. A., Chopin V., Jouy N., Fauquette W., Boilly B., Le Bourhis X. Normal breast epithelial cells induce p53-dependent apoptosis and p53-independent cell cycle arrest of breast cancer cells. Breast Cancer Res. Treat. 2002;71:269–280. doi: 10.1023/a:1014422101452. [DOI] [PubMed] [Google Scholar]
- 34.Altschul S., Madden T., Schaffer A., Zhang J., Zhang Z., Miller W., Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly D. R., Miller L. K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science (Washington, DC) 1989;245:1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- 36.Croizier G., Quiot J. M. [Electron microscopic study of the reticulated cellular structures induced in Lepidoptera by baculovirus] Acad. Sci. Hebd. Seances Acad. Sci. D. 1975;281:1055–1057. [PubMed] [Google Scholar]
- 37.Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature (London) 1989;337:387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 38.Sato C., Inoue S., Matsuda T., Kitajima K. Fluorescent-assisted detection of oligosialyl units in glycoconjugates. Anal. Biochem. 1999;266:102–109. doi: 10.1006/abio.1998.2921. [DOI] [PubMed] [Google Scholar]
- 39.Cheng M. C., Lin S. L., Wu S. H., Inoue S., Inoue Y. High-performance capillary electrophoretic characterization of different types of oligo- and polysialic acid chains. Anal. Biochem. 1998;260:154–159. doi: 10.1006/abio.1998.2701. [DOI] [PubMed] [Google Scholar]
- 40.Harduin-Lepers A., Mollicone R., Delannoy P., Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology. 2005;15:805–817. doi: 10.1093/glycob/cwi063. [DOI] [PubMed] [Google Scholar]
- 41.Kozak M. Regulation of translation in eukaryotic systems. Annu. Rev. Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- 42.Sato C., Kitajima K., Inoue S., Seki T., Troy F. A., 2nd, Inoue Y. Characterization of the antigenic specificity of four different anti-(α2,8-linked polysialic acid) antibodies using lipid-conjugated oligo/polysialic acids. J. Biol. Chem. 1995;270:18923–18928. doi: 10.1074/jbc.270.32.18923. [DOI] [PubMed] [Google Scholar]
- 43.Finne J., Krusius T., Rauvala H., Hemminki K. The disialosyl group of glycoproteins. Occurrence in different tissues and cellular membranes. Eur. J. Biochem. 1977;77:319–323. doi: 10.1111/j.1432-1033.1977.tb11670.x. [DOI] [PubMed] [Google Scholar]
- 44.Sato C., Fukuoka H., Ohta K., Matsuda T., Koshino R., Kobayashi K., Troy F. A., 2nd, Kitajima K. Frequent occurrence of pre-existing α2,8-linked disialic and oligosialic acids with chain lengths up to 7 Sia residues in mammalian brain glycoproteins. Prevalence revealed by highly sensitive chemical methods and anti-di-, oligo-, and poly-Sia antibodies specific for defined chain lengths. J. Biol. Chem. 2000;275:15422–15431. doi: 10.1074/jbc.275.20.15422. [DOI] [PubMed] [Google Scholar]
- 45.Martersteck C. M., Kedersha N. L., Drapp D. A., Tsui T. G., Colley K. J. Unique α2,8-polysialylated glycoproteins in breast cancer and leukemia cells. Glycobiology. 1996;6:289–301. doi: 10.1093/glycob/6.3.289. [DOI] [PubMed] [Google Scholar]
- 46.Sato C., Inoue S., Matsuda T., Kitajima K. Development of a highly sensitive chemical method for detecting α2,8-linked oligo/polysialic acid residues in glycoproteins blotted on the membrane. Anal. Biochem. 1998;261:191–197. doi: 10.1006/abio.1998.2718. [DOI] [PubMed] [Google Scholar]
- 47.Angata T., Kerr S. C., Greaves D. R., Varki N. M., Crocker P. R., Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J. Biol. Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 48.Nicoll G., Ni J., Liu D., Klenerman P., Munday J., Dubock S., Mattei M. G., Crocker P. R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 49.Yamaji T., Teranishi T., Alphey M. S., Crocker P. R., Hashimoto Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to α2,8-disialyl and branched α2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 2002;277:6324–6332. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 50.Recchi M. A., Hebbar M., Hornez L., Harduin-Lepers A., Peyrat J. P., Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58:4066–4070. [PubMed] [Google Scholar]