Abstract
Bilirubin glucuronidation, catalysed by UGT1A1 [UGT (UDP glucuronosyltransferase) isoform 1A1, EC 2.4.1.17], is critical for biliary elimination of bilirubin. UGT1A1 deficiency causes CN-1 (Crigler–Najjar syndrome type 1), which is characterized by potentially lethal unconjugated hyperbilirubinaemia. Nucleotide sequence analysis of UGT1A1 in two CN-1 patients revealed that patient A was homozygous for a nt 530 G→A (where nt 530 G→A means guanine to adenine transition at nucleotide 530) mutation, predicting a C177Y substitution, and patient B had a nt 466 T→C mutation on one allele and a nt 1070 A→G mutation on the other, predicting a C156R and a Q357R substitution respectively. All 11 cysteine residues of mature human UGT1A1 are highly conserved in other human UGT isoforms and in rat, mouse and Rhesus monkey UGT1A1, suggesting their functional importance. Expression of mutagenized UGT1A1 plasmids showed that substitution of any of the seven cysteine residues located within the endoplasmic reticulum cisternae (including those mutated in patients A and B) abolished UGT1A1 activity or markedly increased its apparent Km for bilirubin. Substitution of the three cysteine residues within the C-terminal cytosolic tail had minimal effect on basal UGT1A1 activity, but prevented UGT1A1 activation by UDP-GlcNAc. N-Ethylmaleimide did not inhibit UGT1A1 activity in native microsomes, but prevented UGT1A1 activation by UDP-GlcNAc and inhibited the activity in digitonin-permeabilized microsomes. Dithiothreitol did not affect UGT1A1 activity in human liver microsomes. Together, the results suggested that free thiol groups, but not disulphide bonding, of seven cysteine residues within the intracisternal region of human UGT1A1 are important for its catalytic activity, while cysteine residues in the cytosolic domain may be involved in its physiological activation by UDP-GlcNAc.
Keywords: alkylation, bilirubin, Crigler–Najjar syndrome type 1, cysteine, UDP-GlcNAc, UDP glucuronosyltransferase isoform 1A1 (UGT1A1)
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CN-1, Crigler–Najjar syndrome type 1; DTT, dithiothreitol; ER, endoplasmic reticulum; NEM, N-ethylmaleimide; PEM, N-phenylmaleimide; UGT, UDP glucuronosyltransferase; UGT1A1, UGT isoform 1A1; hUGT1A1, human UGT1A1
INTRODUCTION
Bilirubin, the end-product of haem catabolism, is water-insoluble and toxic because of internal hydrogen-bonding [1]. Bilirubin is converted into polar glucuronide conjugates by the action of hepatic UGT1A1 [UGT (UDP glucuronosyltransferase) isoform 1A1, EC 2.4.1.17], which catalyses the transfer of the glucuronic acid moiety of UDP-GlcA to bilirubin, generating bilirubin monoglucuronide and diglucuronide [2]. Bilirubin glucuronides are polar and are excreted readily in bile. As UGT1A1 is the only UGT isoform that contributes significantly to bilirubin glucuronidation [3], lack of this enzyme activity due to missense, nonsense or splice site mutations of any of the five exons constituting its coding region results in CN-1 (Crigler–Najjar syndrome type 1) [4–7]. CN-1 is characterized by life-long unconjugated hyperbilirubinaemia, which leads to encephalopathy [8] unless treated vigorously with daily phototherapy [9]. Currently, liver transplantation is the only definitive therapy for CN-1 [10]. UGT1A1 is concentrated in the ER (endoplasmic reticulum) and nuclear envelope of hepatocytes [11]. In addition to bilirubin, UGT1A1 catalyses the glucuronidation of oestradiol and a number of drugs and chemicals [12,13].
UGTs comprise a large gene superfamily that is classified into two major gene families, UGT1 and UGT2, on the basis of the extent of nucleotide sequence identity of the isoforms [14]. hUGT1A1 (human UGT1A1) is expressed from the UGT1A locus on chromosome 2q37, which also expresses eight other UGT isoforms [15]. Four of the five exons that constitute the hUGT1A1 mRNA encode the C-terminal half of the molecule and are shared by all isoforms expressed from the UGT1A locus [14,16]. Thus the C-terminal halves of the UGT1A subfamily of isoforms are identical, while the N-terminal halves are different. Since our initial description of genetic lesions causing CN-1 [17,18], many other mutations, deletions or insertions within the five UGT1A1 exons have been reported to cause CN-1 (reviewed in [4]). Recently, by nucleotide sequence analysis of UGT1A1 exons in two patients with clinically diagnosed CN-1, we found that patient A carried a mutation predicting a C177Y substitution and patient B was predicted to have a C156R substitution. These findings prompted us to examine the role of each cysteine residue of hUGT1A1.
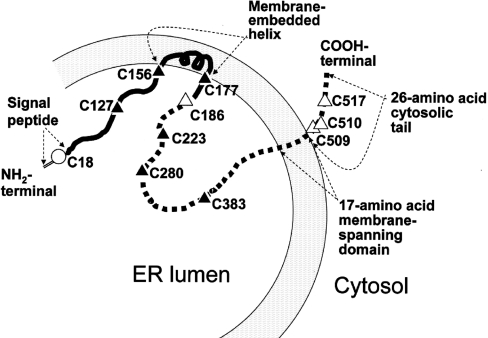
Nascent UGT isoforms have a 20 amino acid signal peptide, which is cleaved during protein maturation. Although the crystallographic structure of UGT1A1 has not been solved, on the basis of hydrophobicity analysis of the primary structure and latency of the enzyme activity, the major portion of mature UGT molecules, including the binding sites for UDP-GlcA and the aglycone substrates, is thought to be located within the ER cisternae. There is a single 17 amino acid membrane-spanning segment and a 26 amino acid cytoplasmic tail at the C-terminal end of the molecule (Figure 1). Within the intraluminal part of the molecule, a short membrane-embedded helix has been identified using the RAOARGOS computer program [19]. Compartmentation of the catalytic domains of UGTs within the ER cisternae is thought to pose a barrier to the access of the polar sugar donor substrate UDP-GlcA, resulting in latency of UGT activities in liver homogenates or sealed microsomal vesicles [20,21]. Full enzyme activity is manifested in vitro by treatment of the microsomes with membrane-permeabilizing agents, such as digitonin or alamethacin. UDP-GlcNAc (UDP-N-acetylglucosamine) stimulates the internalization of UDP-GlcA into intact microsomal vesicles, thereby activating UGT1A1. UDP-GlcNAc activates UGTs at low physiological concentrations and is thought to be the natural activator of UGTs within hepatocytes, although its precise mechanism of action is not known.
Figure 1. Orientation of UGT1A1 in relation to ER cisternae, deduced from amino acid sequence.
Except for a 26 amino acid C-terminal cytosolic tail and a 17 amino acid single membrane-spanning region, the entire molecule is thought to be compartmentalized within the ER lumen. The N-terminal half of the molecule (solid line) contains a 20 amino acid signal peptide, which is cleaved during maturation of the enzyme. This domain confers aglycone substrate specificity to the enzyme and contains a putative membrane-embedded helix. The intraluminal part of the C-terminal half of UGT1A1 includes the UDP-GlcA-binding domain. Locations of cysteine residues are indicated.
hUGT1A1 contains a total of 11 cysteine residues, one of which is within the signal peptide (amino acid 18) that is cleaved during synthesis of the enzyme (Figure 1). Seven cysteine residues (Cys127, Cys156, Cys177, Cys186, Cys223, Cys280 and Cys383) are present within the major segment of the enzyme that is thought to be located within the ER cisternae. Cys509, Cys510 and Cys517 are located within the C-terminal cytoplasmic tail. Sequence alignment revealed that the ten cysteine residues that are present in mature hUGT1A1 are highly conserved in all human UGT1A isoforms. Furthermore, most of these cysteine residues are conserved across species, in rats, mice and Rhesus monkeys, suggesting an important function of these residues. However, the role of the individual cysteine residues in human UGT1A1 has not been delineated systematically. It is also unknown whether function of the cysteine residues is exerted via disulphide bonding or by cysteine residues with free thiol groups. These considerations prompted us to examine the role of the cysteine residues in the catalytic activity of UGT1A1 by combining site-directed mutagenesis studies and experiments utilizing thiol-reactive agents. The results indicated that seven cysteine residues within the intracisternal domain of hUGT1A1 are critical for its catalytic activity, while the three cysteine residues located in the C-terminal cytosolic tail may be required for activation of the enzyme by UDP-GlcNAc. Function of the cysteine residues appears to require free thiol residues, rather than disulphide bonding.
EXPERIMENTAL
Case history
Patient A was a 6-month-old male baby, who was noted to have severe hyperbilirubinaemia since birth that had led to cerebral abnormalities, manifested by chorioathetoid movements and mental retardation. At the time of the present study, the patient was on phototherapy for 12 h a day, which maintained the serum bilirubin concentration at approx. 24.0 mg/dl (408 μmol/l; normal range 0.2–1.0 mg/dl or 3.4–17 μmol/l). Serum bilirubin was mostly unconjugated, since less than 5% of the pigment gave a ‘direct’ van den Bergh reaction. Serological tests for hepatocellular integrity, such as ALT (alanine aminotransferase), AST (aspartate aminotransferase) and alkaline phosphatase activities and protein synthetic functions as determined by serum albumin concentration were within normal limits. There was no evidence of haemolysis. Based on the history and biochemical findings, a clinical diagnosis of CN-1 was made. The UGT1A1 exons and flanking intronic regions were amplified by PCR and nucleotide sequences were determined as described in [22].
Patient B was a 1-year-old male infant who had been noted to be jaundiced since birth. A previous sibling also had jaundice from birth and had died at the age of 3.5 years, apparently from bilirubin encephalopathy. At the time of the present study, patient B was being treated with phototherapy (12 h/day) and phenobarbital (2 mg/kg by mouth daily). The serum bilirubin level was 15.8 mg/dl (268 μmol/l), with a direct van den Bergh reacting fraction of less than 2%. As in patient A, serum albumin levels and ALT, AST and alkaline phosphatase activities were within normal limits for the age of the patient. There was no evidence of haemolysis. CN-1 was diagnosed on the bases of clinical history and serological findings. Nucleotide sequence of the coding region and flanking sequences of UGT1A1 was determined as in the case of patient A.
Site-directed mutagenesis
To determine the effect of substitution of each of the cysteine residues, site-directed mutagenesis was carried out on a plasmid, pSVK3, which contained the entire UGT1A1 coding region, using the Transformed™ Mutagenesis kit (ClonTech Laboratories) by minor modifications of the method reported by Deng and Nickoloff [23] as described in [24]. The mutagenized clones were primarily selected by XbaI digestion. The entire 2.2 kb mutagenized UGT1A1 cDNA was then excised by digestion with NotI and XhoI and subcloned into the pcDNA3.1/Zeo(+) (Invitrogen) expression vector. Introduction of the desired mutation was confirmed by nucleotide sequence analysis using the dideoxy chain termination method [22]. Each cysteine residue was substituted with a tyrosine residue. In addition, for the cysteine residues that were found to be substituted in patients A and B, we also made a TG→GC conversion to substitute cysteine with alanine and a T→C conversion to substitute cysteine with arginine.
Cell culture and transfection
COS7 cells, grown to 50–60% confluency in 100 mm dishes, were transfected with pcDNA3.1/Zeo(+) containing normal or mutagenized UGT1A1 coding region, driven by the cytomegalovirus immediate early promoter, using DEAE-dextran (Amersham Biosciences) as described. UGT1A1 in the cell lysates was identified by Western blot analysis [25] and quantified by ELISA [3,26] as briefly described below.
Western-blot analysis
Lysates of the transfected COS7 cells, containing 20 μg of total protein, were subjected to Western-blot analysis as described in [25], using WP1, a monoclonal antibody directed against the C-terminal domain of hUGT1A isoforms. The immunoreactive bands were visualized using a chemiluminescent substrate (Pierce, Rockford, IL, U.S.A.).
ELISA
Expressed UGT1A1 in each cell lysate was quantified by a sandwich ELISA as described in [3]. In brief, ELISA plates were coated overnight with the WP1 antibody (1:4000 dilution), quenched with PBS containing 3% (w/v) BSA and 5% (v/v) fetal calf serum and then overlaid with lysates of the transfected cells. A rabbit antiserum (Pab 136), raised against a synthetic peptide corresponding to a unique region of UGT1A1, was applied at a 1:500 dilution. The detection system consisted of a goat anti-rabbit IgG, conjugated with horseradish peroxidase. Absorbance at 405 nm was determined using an ELISA plate reader. For absolute quantification, we developed a standard curve using various amounts of hUGT1A1 immunopurified from a stably transduced Gunn rat fibroblast cell line as described in [26,27].
Preparation of human liver microsomal fractions
Fragments of human liver were obtained from portions of normal liver resected during surgical management of trauma (two specimens) or solitary hepatic metastasis. Haematoxylin/eosin staining showed that the liver fragments were histologically normal and there was no history of serological evidence of liver disease. The specimens were left over after clinically indicated pathological examination and were stored anonymously at −80 °C. Fragments of the specimens were minced with a fine razor blade and 10% homogenates were prepared in 25 mM Tris/HCl (pH 7.8) containing 0.25 M sucrose and 1 mM EDTA by five up-and-down strokes of an ice-cooled glass homogenizer fitted with a motor-driven pestle. All subsequent procedures were performed at 4 °C. The homogenate was centrifuged at 800 g for 10 min and the supernatant was centrifuged at 15000 g for 10 min. Microsomal fractions were sedimented by centrifuging the supernatant at 105000 g for 60 min and resuspended gently at 100 mg of microsomal pellet per ml of the homogenization buffer described above. The microsomal suspension could be stored on ice for up to 18 h before use in enzyme assays without loss of latency or reduction of UGT1A1 activity towards bilirubin.
Assay of UGT1A1 activity towards bilirubin
For determination of the UGT1A1 activity expressed in transfected COS7 cells, equal amounts of UGT1A1 from the various cell lysates (based on ELISA quantification as described above) were assayed for UGT activity towards bilirubin by an HPLC-based method as described in [24]. In brief, dioleoyl phosphati-dylcholine was used to activate the enzyme in the cell lysates [3]. The assay was performed at pH 7.8, using 40 μM bilirubin, 4.4 mM UDP-GlcA and 5 mM MgCl2. After incubation at 37 °C for 4 h, the reaction was stopped by adding 0.4 M glycine/HCl (pH 1.4), saturated with NaCl and the pigments were extracted quantitatively in chloroform/ethanol (1:1, v/v) [24]. The solvents were evaporated under a stream of nitrogen. The pigments were dissolved in DMSO, mixed with an equal volume of methanol and analysed by reverse-phase HPLC using a Waters C18 μBondapak column (Waters, Milford, MA, U.S.A.). The rate of formation of bilirubin glucuronides was calculated from electronically integrated areas under the curve, using purified biosynthesized bilirubin diglucuronide and monoglucuronide as the standards. Specific enzyme activity was calculated as the rate of bilirubin glucuronide formation per mg of expressed hUGT1A1. In initial studies, we determined that bilirubin glucuronide formation was proportional to the duration of incubation and the amount of microsomal protein used in the assay.
Alkylation of cysteine thiol groups and treatment with reducing agents
To determine the effect of thiol-reacting agents on UGT1A1 activity towards bilirubin, human liver microsomal preparation was preincubated with the membrane-impermeable alkylating agent NEM (N-ethylmaleimide) or the membrane-permeable alkylating agent PEM (N-phenylmaleimide). NEM was dissolved in water and incubated with the microsomal preparations at 4 mM concentration at pH 7.0 at 37 °C for 30 min before adding the substrates. PEM was dissolved in DMSO and incubated with the microsomal preparations in the same manner. The final DMSO concentration was 1%, and for these experiments, DMSO was added at an equal concentration to the control. To evaluate whether disulphide bonding is required for UGT1A1 activity, the microsomal preparations were preincubated with or without 5 mM DTT (dithiothreitol) before adding the substrates.
Evaluation of the activation of UGT1A1 in human liver microsomal fractions by UDP-GlcNAc and digitonin
UGT1A1 activity towards bilirubin was assayed as above at pH 7.8, using native (sealed) microsomes, or microsomes treated with 4 mM UDP-GlcNAc or digitonin (2 mg/ml). UDP-GlcA and MgCl2 were omitted in negative controls.
Kinetic parameters
UGT activity towards bilirubin was determined at various sub-strate concentrations. Initial glucuronide formation rates were fitted directly to the Michaelis–Menten equation.
RESULTS
UGT1A1 mutations in the CN-1 patients
Patient A was homozygous for an nt 530 G→A mutation, predicting a C177Y substitution within the unique N-terminal domain of UGT1A1. Sequence of the TATAA element within the upstream promoter was normal. Both parents were heterozygous for the mutation. Patient B was a double heterozygote for an nt 466 T→C and an nt 1070 A→G mutation, predicting a C156R and a Q357R substitution respectively. Sequence of the promoter region was normal. Genetic analysis of the parents revealed that the nt 466 T→C mutation was inherited from the mother and the nt 1070 A→G mutation was inherited from the father. Neither parent exhibited hyperbilirubinaemia, which was expected because CN-1 is inherited as an autosomal recessive trait, since one normal allele is generally sufficient to maintain normal serum bilirubin concentrations [2]. Thus our results indicate that the C156R and the Q357R substitutions each result in loss of UGT1A1 activity towards bilirubin. Consistent with this, homozygosity for the Q357R mutation has been reported previously to be associated with CN-1 [7] and site-directed mutagenesis–expression studies in our laboratory showed complete loss of catalytic activity (S. S. Ghosh, J. Roy-Chowdhury and N. Roy-Chowdhury, unpublished work).
Cysteine residues of hUGT1A1 are highly conserved
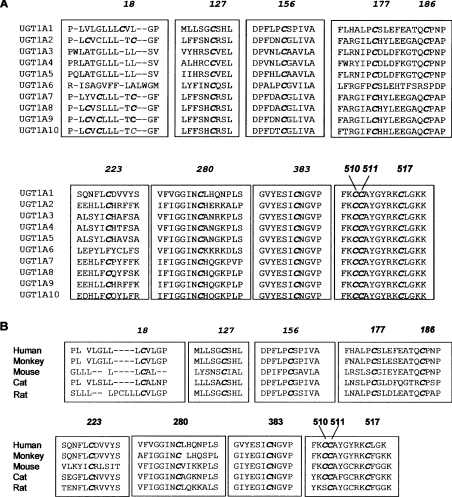
The above findings prompted us to examine systematically the contribution of cysteine residues to the catalytic activity of hUGT1A1. The nine UGT1A isoforms that are expressed from the UGT1A locus have identical C-terminal halves, but their sequences differ considerably in the N-terminal domains that are derived from unique exon 1s (exon 1A1 and exons 1A3–1A10). However, computer alignment of the sequence of all hUGT1A isoforms revealed that the ten cysteine residues that are present in mature hUGT1A1 are fully conserved in all hUGT1A isoforms, except that Cys186 and Cys223 are not present in UGT1A6 (Figure 2A). To examine whether the cysteine residues are conserved across the species, we aligned the amino acid sequences from rats, mice and Rhesus monkeys. The results shown in Figure 2(B) indicate that the cysteine residues are highly conserved in various mammalian species.
Figure 2. Alignment of nucleotide sequences of UGT isoforms.
(A) Alignment of amino acid sequences of ten UGT isoforms expressed from the human UGT1A locus shows that all cysteine residues, except the ones located in the signal peptide, are highly conserved in these isoforms. Note that amino acids derived from exons 2–5 are identical in these isoforms. (B) Alignment of UGT1A1 amino acid sequences from various species, showing a high degree of conservation of these residues in the different mammalian species.
Effect of cysteine substitutions on the enzyme activity of hUGT1A1
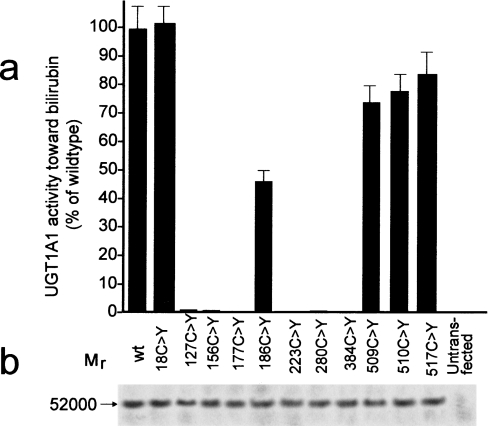
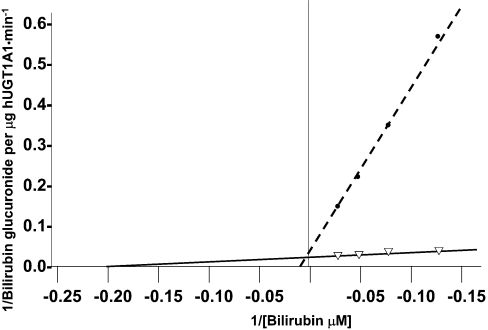
The wild-type UGT1A1 expression vector was mutagenized to express hUGT1A1 with single cysteine substitutions. In initial experiments, we determined by Western-blot analysis that substitution of the cysteine residues did not affect expression of the protein in COS7 cells (Figure 3b). Wild-type and mutagenized hUGT1A1, expressed in COS7 cells, were quantified by ELISA and UGT1A1 activity towards bilirubin was determined. Activities of the mutagenized forms of hUGT1A1 per mg of hUGT1A1 protein are shown in Figure 3(a). Substitution of the cysteine residues within the signal peptide did not affect the enzyme activity. Of the seven cysteine residues located within the intracisternal domain of hUGT1A1 (Figure 1), substitution of any one of those at positions 127, 156, 177, 223, 280 or 383 resulted in complete or near complete loss of UGT1A1 activity (Figure 3a). Substitution of Cys186 reduced UGT1A1 activity by 50%, as determined at 40 μM bilirubin concentration. Since Cys186 is located within the N-terminal half of UGT1A1, which is thought to impart aglycone substrate specificity to the enzyme [28], we took advantage of the residual enzyme activity to perform kinetic analysis to determine the effect of this mutation on the apparent affinity for bilirubin. The apparent Km towards bilirubin was determined for wild-type and C186Y mutagenized UGT1A1 using a Lineweaver–Burk plot (Figure 4). Apparent Km for bilirubin in the case of the C186Y mutant was 80 μM, which was approx. 16-fold higher than that found with wild-type UGT1A1. For Cys177 and Cys156, which were found to be substituted with tyrosine and arginine residues in patients A and B respectively, we also examined the effect of substituting each of these cysteine residues with an alanine or arginine residue. Each substitution completely abolished UGT1A1 activity in the expressed protein.
Figure 3. Expression of wild-type or mutagenized hUGT1A1 proteins and UGT1A1 activity towards bilirubin.
hUGT1A1 expression plasmids were mutagenized to replace individual cysteine residues at the positions indicated in (a). The wild-type and mutagenized plasmids were transfected into COS cells and the transient protein expression was verified by Western blotting (results of a representative experiment are shown in b). Expressed hUGT1A1 was quantified by ELISA, and the UGT1A1 activity towards bilirubin was determined and calculated per μg of the expressed enzyme. Activities are expressed as percentage of the wild-type hUGT1A1 activity. Results shown are the means±S.E.M. for three experiments. Untransfected COS cells were used as a negative control.
Figure 4. Lineweaver–Burk plot of UGT1A1 activity at various bilirubin concentrations.
Wild-type (solid line) and C186Y substituted (broken line) hUGT1A1 were expressed in COS cells and quantified by ELISA as in Figure 3. UGT1A1 activity was determined at bilirubin concentrations as indicated and expressed per μg of the expressed enzyme.
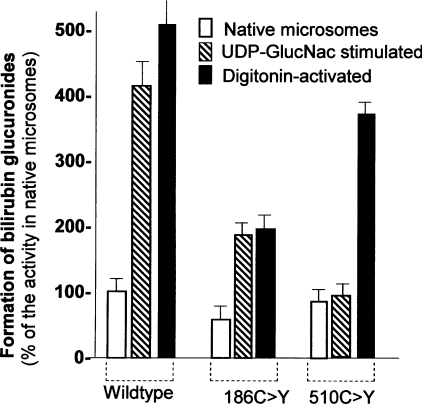
The effect of substitution of cysteine residues located in the cytoplasmic C-terminal tail was quite distinct from that of substituting cysteine residues within the intracisternal domain. Substitution of the cytoplasmic tail cysteines resulted in only a 15–25% reduction of the enzyme activity as determined by our standard UGT1A1 assay, in which the enzyme is activated by permeabilization of the microsomal vesicles by digitonin treatment. However, these substitutions abolished UGT1A1 activation by UDP-GlcNAc (Figure 5). In contrast, although the activity of the C186Y mutant UGT1A1 was reduced to 50% of normal, the enzyme activity increased proportionately in the presence of UDP-GlcNAc.
Figure 5. Effect of cysteine residue substitutions on activation of UGT1A1 activity by UDP-GlcNAc.
COS cells were transfected with plasmids expressing wild-type UGT1A1, or UGT1A1 containing C186Y or C510Y mutations. UGT1A1 activity was determined at 40 μM bilirubin. Assays were performed on native cell homogenates or in the presence of digitonin (2 mg/ml) or UDP-GlcNAc (UDP-GlucNAc) (4 mM). Results shown are the means±S.E.M. for three experiments.
DTT did not affect UGT1A1 activity towards bilirubin
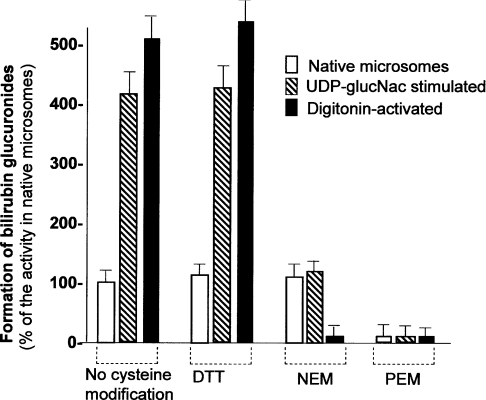
Treatment with 5 mM DTT did not affect UGT activity towards bilirubin significantly in native, digitonin-permeabilized or UDP-GlcNAc-treated human liver microsomes (Figure 6). These findings indicated that intra- or inter-molecular disulphide bonding is not required for UGT1A1 activity towards bilirubin.
Figure 6. Effect of cysteine-modifying agents on UGT1A1 activity in human liver microsomal fractions.
Human liver microsomes were prepared as described in the Experimental section. UGT1A1 activity was assayed in native (sealed), digitonin (2 mg/ml)-treated or UDP-GlcNAc (UDP-glucNac) (4 mM)-treated microsomes, without treatment with cysteine-modifying agents or after treatment with DTT, NEM or PEM. Results are the means±S.E.M. for three experiments.
Effect of alkylation of cysteine thiols on UGT1A1 activity
NEM, a membrane-impermeable cysteine alkylating agent, did not inhibit significantly UGT activity towards bilirubin in native human liver microsomes (Figure 6). However, NEM markedly inhibited the activation of UGT1A1 by UDP-GlcNAc. When the microsomal vesicles were permeabilized by pretreatment with digitonin, before the addition of NEM, UGT1A1 activity was markedly inhibited. PEM, a membrane-permeable alkylating agent, inhibited the enzyme activity in both native and digitonin-treated microsomes.
DISCUSSION
Our finding that missense mutations resulting in substitution of Cys177 and Cys156 caused CN-1 in patients A and B respectively prompted us to determine which cysteine residues in UGT1A1 are important for its catalytic activity. In a previously published report, we noted that substitution of Cys177 by a cationic amino acid, arginine, resulted in abolition of UGT1A1 activity [28]. Our present findings show that substitution of this cysteine residue by tyrosine or alanine also has a similar effect. Importance of the cysteine residues was also suggested by the fact that these residues are highly conserved not only in different human UGT isoforms, but also in UGT1A1 isoforms from various species. Although cysteine residues are often critical in the catalytic activity of enzymes, their substitution does not affect enzyme activities to the same extent. Some cysteine residues contribute to enzyme secondary or tertiary structure by forming intra- or inter-molecular disulphide bridges. For example, in a recent report on the biochemical characterization of cysteine residues of human UDP-xylosyltransferase-I, another transmembrane enzyme located inside the ER cisternae, it was observed that disulphide-bonded cysteine residues were important in the catalytic activity, but free thiol groups were not needed [29]. Therefore we decided to determine the functional importance of each cysteine residue and whether their function is mediated by disulphide bonding. One novel finding of our study was that six out of the seven cysteine residues located within the intracisternal domain of hUGT1A1 were critical for the catalytic activity of the enzyme. However, substitution of Cys186 significantly reduced the apparent affinity of the enzyme for bilirubin, but did not abolish the enzyme activity. Another new finding was that substitution of any one of the three cysteine residues in the cytoplasmic tail appears to interfere with the activation of the enzyme by its physiological activator UDP-GlcNAc, which may indirectly affect UGT1A1 activity in vivo. In contrast with the finding with UDP-xylosyltransferase-I [29], cysteine residues with free thiol groups, rather than disulphide bonding, was found to be important for the function of the cysteine residues in hUGT1A1.
We routinely substituted cysteine residues with tyrosine because this substitution does not alter the charge or polarity of the residue and can be effected by a single G→A transition. Notably, this was also the natural mutation found in patient A. In patient B, the observed mutation predicted the substitution of Cys156 with an arginine residue. Therefore, for both Cys156 and Cys177, we also examined the effect of substituting cysteine with arginine. In addition, for these cysteine residues, we also studied the effect of substitution with alanine, which, like cysteine, does not contain bulky side chains, but differs from cysteine in polarity. Each of these substitutions abolished expressed UGT1A1 activity, suggesting that these cysteine residues are irreplaceable.
The 267 amino acids that comprise the N-terminal half of hUGT1A1 are derived from the unique exon 1A1, which is used only in hUGT1A1 and not in other isoforms expressed from the UGT1A locus. As the N-terminal region imparts aglycone specificity to UGT isoforms [30], it was not surprising that a substitution in this region could alter the affinity of the enzyme for bilirubin. Although the substitution of Cys186 did not completely abolish the enzyme activity, it markedly increased the apparent Km, implying reduction of the affinity of bilirubin for the enzyme.
Cys18 is located in the signal peptide of hUGT1A1, which is cleaved during enzyme synthesis. This cysteine residue is not conserved in various UGT isoforms. As expected, its substitution did not affect UGT1A1 enzyme activity. All other cysteine residues are highly conserved in UGT1A1 from various mammalian species. These are also conserved in all human UGT1A subfamily of proteins, with the exception of Cys186 and Cys223 (Figures 2A and 2B), which are not present in UGT1A6.
The three cysteine residues (Cys509, Cys510 and Cys517) within the C-terminal tail are also highly conserved in UGT isoforms, including those that belong to other UGT subfamilies, such as UGT2B, and are expressed from loci on other chromosomes, suggesting that these residues are also important in enzyme function. However, substitution of these amino acids had only a minor effect on the UGT1A1 activity as determined by the standard UGT1A1 assay method using digitonin activation. Therefore we proceeded to examine the effect of these substitutions on the activation of the enzyme activity by the physiological activator, UDP-GlcNAc. Interestingly, each of these substitutions abolished stimulation of the enzyme activity by UDP-GlcNAc. UDP-GlcNAc is thought to activate UGT by stimulating the import of the polar substrate UDP-GlcA through the lipid bilayer of the ER to intraluminal sites, where the substrate-binding sites of UGTs are located. A putative ‘permease’ has been hypothesized to be involved in mediating the transport of UDP-GlcA by UDP-GlcNAc [21]; however, no such molecule has been identified. To examine further the role of the three cysteine residues that are accessible from the cytosolic aspect, we used NEM and PEM, two cysteine thiol alkylating agents. Because of its polarity, NEM does not permeate efficiently into sealed microsomal vesicles, whereas PEM, which is relatively non-polar, diffuses through the microsomal membranes. Preincubation of native microsomal vesicles with NEM did not affect UGT1A1 activity significantly, but abolished the activation of the enzyme by UDP-GlcNAc. This finding, in combination with the results of our mutagenesis studies, suggests that the three cysteine residues within the cytosolic tail of UGT1A1 are required for UDP-GlcNAc-stimulated internalization of UDP-GlcA. On the other hand, after permeabilization of the microsomal membranes by incubation with digitonin, NEM markedly inhibited the UGT1A1 activity, which was consistent with a critical role of the free thiol groups of the cysteine residues located in the region of the molecule that is compartmentalized within the ER lumen. As expected, PEM markedly inhibited UGT1A1 activity with or without digitonin treatment. We have shown previously that hUGT1A1 forms dimers [24]. Dimerization of rat liver UGT1A1 with another UGT isoform, UGT2B, has been reported to be important in the stimulation of UDP-GlcA import by UDP-GlcNAc [31]. It is possible that the cysteine residues in the cytosolic tail of UGTs facilitate intermolecular dimerization, but such a dimerization does not seem to be mediated by disulphide bridging [24].
Finally, we wanted to determine whether disulphide bridges between cysteine residues of hUGT1A1, or between these cysteines and cysteine residues of other proteins, are necessary for UGT1A1 activity. PAGE of microsomes with or without treatment with reducing agents followed by Western-blot analysis of hUGT1A1 or rat UGT1A1 showed immunoreactive bands of monomeric size, indicating the lack of intermolecular disulphide bonding [24]. However, this did not exclude a role for intramolecular disulphide bridges in the catalytic activity of the enzyme. Therefore we examined the effect of DTT on UGT1A1 activity of human liver microsomal preparations. DTT did not affect UGT1A1 activity with or without permeabilization of the microsomal membranes by preincubation with digitonin, suggesting that intramolecular disulphide bridging of the cysteine residues is not required for UGT1A1 activity towards bilirubin. A previous study on a different human UGT isoform, UGT1A6, showed that substitution of Cys126 in that isoform with valine abolished the enzyme activity towards 4-methylumbelliferone, and its substitution with serine reduced the apparent affinity of UGT1A6 for the substrate by an order of magnitude [32]. The results indicated that although Cys126 is important in UGT1A6 activity, its role is not mediated by disulphide bonding. These results are consistent with our conclusion in the context of hUGT1A1 that the critical role of cysteine residues is not mediated by disulphide bonding. In a different study on rat liver UGT1A6, Ikushiro et al. [33] found that disruption of the disulphide bond between Cys121 and Cys125 activated the enzyme activity towards 4-nitrophenol. However, these findings cannot be compared with our results with hUGT1A1, because there is no equivalent of Cys121 in hUGT1A1 or hUGT1A6 (Figure 1).
In summary, the present study is the first demonstration that six of the seven cysteine residues within the intracisternal domain of hUGT1A1 are critically needed for bilirubin glucuronidation, while the substitution of Cys186 markedly reduces the apparent affinity of the enzyme for bilirubin. Our results suggest that the three cysteine residues located in the C-terminal cytoplasmic tail of hUGT1A1 are not directly needed for the enzyme activity, but may be required for activation of the enzyme by the physiological activator UDP-GlcNAc, which stimulates the import of the donor substrate UDP-GlcA. The role of the cysteine residues appears to be mediated by their free thiol groups, rather than by disulphide bridging. Importance of the cysteine residues is underscored by the finding of cysteine substitution as the cause of severe hyperbilirubinaemia in two patients with CN-1. Our mutagenesis studies predict that inherited substitution of any other of the seven intracisternal cysteine residues would also lead to severe non-haemolytic unconjugated hyperbilirubinaemia.
Acknowledgments
We thank Professor Irving Listowsky (Department of Biochemistry, Albert Einstein College of Medicine) and Professor Richard J. Stockert (Department of Medicine, Albert Einstein College of Medicine) for carefully reading this paper and offering valuable suggestions. This work was supported in part by the following: National Institutes of Health grants DK 39137 (to N. R.-C.) and DK 46057 (to J. R.-C.), Liver Research Core Center (P30-DK 41296) and the General Clinical Research Center (MO1-PP12248; Principal Investigator: Dominick P. Purpura).
References
- 1.Bonnett R., Davies J. E., Hursthouse M. B. Structure of bilirubin. Nature (London) 1976;262:327–328. doi: 10.1038/262326a0. [DOI] [PubMed] [Google Scholar]
- 2.Roy Chowdhury J., Wolkoff A. W., Roy Chowdhury N., Arias I. M. Hereditary jaundice and disorders of bilirubin metabolism. In: Scriver C. R., Boudet A. L., Sly W. S., Valle D., editors. The Metabolic Basis of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 3063–3101. [Google Scholar]
- 3.Bosma P. J., Seppen J., Goldhoorn B., Bakker C., Oude Elferink R. P. J., Roy Chowdhury J., Roy Chowdhury N., Jansen P. L. M. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- 4.Kadakol A., Ghosh S. S., Sappal B. S., Sharma G., Roy-Chowdhury J., Roy-Chowdhury N. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum. Mutat. 2000;16:297–306. doi: 10.1002/1098-1004(200010)16:4<297::AID-HUMU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Kadakol A., Sappal B., Ghosh S. S., Lowenheim M., Chowdhury A., Chowdhury S., Santra A., Arias I. M., Roy-Chowdhury J., Roy-Chowdhury N. Interaction of coding region mutations and the Gilbert-type promoter abnormality of the UGT1A1 gene causes moderate degrees of unconjugated hyperbilirubinaemia and may lead to neonatal kernicterus. J. Med. Genet. 2001;38:244–248. doi: 10.1136/jmg.38.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantla S., Bakker C. T. M., Deocharan B., Thummala N. R., Zweiner J., Sinaasappel M., Roy-Chowdhury J., Bosma P. J., Roy-Chowdhury N. Splice-site mutations: a novel genetic mechanism of Crigler-Najjar syndrome type 1. Am. J. Hum. Genet. 1998;62:585–592. doi: 10.1086/301756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrune P. H., Myara A., Hadchouel M., Ronchi F., Bernard O., Trivin F., Roy-Chowdhury N., Roy-Chowdhury J., Munnich A., Odièvre M. Genetic heterogeneity of Crigler-Najjar syndrome type I: a study of 14 cases. Hum. Genet. 1994;94:693–700. doi: 10.1007/BF00206965. [DOI] [PubMed] [Google Scholar]
- 8.Crigler J. F., Jr, Najjar V. A. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169–180. [PubMed] [Google Scholar]
- 9.Agati G., Fusi F., Pratesi S., Galvan P., Donzelli G. P. Bilirubin photoisomerization products in serum and urine from a Crigler-Najjar type I patient treated by phototherapy. J. Photochem. Photobiol. B. 1998;47:181–189. doi: 10.1016/s1011-1344(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 10.Schauer R., Stangl M., Lang T., Zimmermann A., Chouker A., Gerbes A. L., Schildberg F. W., Rau H. G. Treatment of Crigler-Najjar type 1 disease: relevance of early liver transplantation. J. Pediatr. Surg. 2003;38:1227–1231. doi: 10.1016/s0022-3468(03)00273-2. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Chowdhury J., Novikoff P. M., Roy-Chowdhury N., Novikoff A. B. Distribution of UDP glucuronosyltransferase in rat tissue. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2990–2994. doi: 10.1073/pnas.82.9.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuff R. L., Wade L. T., Rychlik B., Jedlitschky G. A., Burchell B. Characterisation of glucuronidation and transport in V79 cells co-expressing UGT1A1 and MRP1. Toxicol. Lett. 2001;120:43–49. doi: 10.1016/s0378-4274(01)00305-8. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman E., Myara A., Lokiec F., Goldwasser F., Trivin F., Mahjoubi M., Misset J. L., Cvitkovic E. Severe CPT-11 toxicity in patients with Gilbert's syndrome: two case reports. Ann. Oncol. 1997;8:1049–1051. doi: 10.1023/a:1008261821434. [DOI] [PubMed] [Google Scholar]
- 14.Van Es H. H. G., Bout A., Liu J., Anderson I., Duncan A. M. V., Bosma P. J., Oude Elferink R. P. J., Jansen P. L. M., Roy-Chowdhury J., Schurr E. Assignment of the human UDP glucuronosyltransferase gene (UGT1A1) to chromosome region 2q37. Cytogenet. Cell Genet. 1993;63:114–116. doi: 10.1159/000133513. [DOI] [PubMed] [Google Scholar]
- 15.Ritter J. K., Chen F., Sheen Y. Y., Tran H. M., Kimura S., Yeatman M. T., Owens I. S. A novel complex locus UGT1 encodes human bilirubin, phenol, and other UDP-glucuronosyltransferase isozymes with identical carboxyl termini. J. Biol. Chem. 1992;267:3257–3261. [PubMed] [Google Scholar]
- 16.Mackenzie P. I., Owen I. S., Burchell B., Bock K. W., Bairoch A., Belange A., Fournel-Gigleux S., Green M., Jum D. W., Iyanagi T., et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics. 1997;7:255–269. doi: 10.1097/00008571-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bosma P. J., Roy-Chowdhury N., Goldhoorn B. G., Hofker M. H., Oude Elferink R. P. J., Jansen P. L. M., Roy-Chowdhury J. Sequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type I. Hepatology. 1992;15:941–947. doi: 10.1002/hep.1840150531. [DOI] [PubMed] [Google Scholar]
- 18.Bosma P. J., Roy-Chowdhury J., Huang T. J., Lahiri P., Oude Elferink R. P. J., Van Es H. H. G., Lederstein M., Whitington P. F., Jansen P. L. M., Roy-Chowdhury N. Mechanisms of inherited deficiencies of multiple UDP-glucuronosyltransferase isoforms in two patients with Crigler-Najjar syndrome, type I. FASEB J. 1992;6:2859–2863. doi: 10.1096/fasebj.6.10.1634050. [DOI] [PubMed] [Google Scholar]
- 19.Ciotti M., Cho J. W., George J., Owens I. S. Required buried alpha-helical structure in the bilirubin UDP-glucuronosyltransferase, UGT1A1, contains a nonreplaceable phenylalanine. Biochemistry. 1998;37:11018–11025. doi: 10.1021/bi980747q. [DOI] [PubMed] [Google Scholar]
- 20.Dutton G. J. Commentary: control of UDP-glucuronyltransferase activity. Biochem. Pharmacol. 1975;24:1835–1841. doi: 10.1016/0006-2952(75)90399-8. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt X., Blanckaert N. Carrier-mediated transport of uridine diphosphoglucuronic acid across the endoplasmic reticulum membrane is a prerequisite for UDP-glucuronosyltransferase activity in rat liver. Biochem. J. 1997;323:645–648. doi: 10.1042/bj3230645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal. Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S. S., Sappal B. S., Ganjam V. K., Lee S. W., Roy-Chowdhury J., Roy-Chowdhury N. Homodimerization of human bilirubin-uridine-diphosphoglucuronate glucuronosyltransferase-1 (UGT1A1) and its functional implications. J. Biol. Chem. 2001;276:42108–42115. doi: 10.1074/jbc.M106742200. [DOI] [PubMed] [Google Scholar]
- 25.Van Es H. H., Goldhoorn B. G., Paul-Abrahamse M., Oude Elferink R. P. J., Jansen P. L. Immunochemical analysis of uridine diphosphate-glucuronosyltransferase in four patients with the Crigler-Najjar syndrome type I. J. Clin. Invest. 1990;85:1199–1205. doi: 10.1172/JCI114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seppen J., Tada K., Hellwig S., Bakker C. T. M., Prasad V. R., Roy-Chowdhury N., Roy-Chowdhury J. R., Bosma P. J., Oude Elferink R. P. J. Bilirubin glucuronidation by intact Gunn rat fibroblasts expressing bilirubin UDP-glucuronosyltransferase. Biochem. J. 1996;314:477–483. doi: 10.1042/bj3140477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppen J., Jansen P. L. M., Oude Elferink R. P. J. Immunoaffinity purification and reconstitution of the human bilirubin/phenol UDP-glucuronosyltransferase family. Protein Expr. Purif. 1995;6:149–154. doi: 10.1006/prep.1995.1018. [DOI] [PubMed] [Google Scholar]
- 28.Seppen J., Bosma P. J., Goldhoorn B. J., Bakker C. T., Roy-Chowdhury J., Roy-Chowdhury N., Jansen P. L., Oude Elferink R. P. Discrimination between Crigler-Najjar type I and II by expression of mutant bilirubin uridinediphosphate-glucuronosyltransferase. J. Clin. Invest. 1994;94:2385–2391. doi: 10.1172/JCI117604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller S., Schöttler M., Schön S., Prante C., Brinkmann T., Kuhn J., Götting C., Kleesiek K. Human xylosyltransferase I: functional and biochemical characterization of cysteine residues required for enzymic activity. Biochem. J. 2005;386:227–236. doi: 10.1042/BJ20041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meech R., Mackenzie P. I. Structure and function of uridine diphosphate glucuronosyltransferases. Clin. Exp. Pharmacol. Physiol. 1997;24:907–915. doi: 10.1111/j.1440-1681.1997.tb02718.x. [DOI] [PubMed] [Google Scholar]
- 31.Ikushiro S., Emi Y., Iyanagi T. Activation of glucuronidation through reduction of a disulfide bond in rat UDP-glucuronosyltransferase 1A6. Biochemistry. 2002;41:12813–12820. doi: 10.1021/bi0262451. [DOI] [PubMed] [Google Scholar]
- 32.Senay C., Jedlitschky G., Terrier N., Burchell B., Magdalou J., Fournel-Gigleux S. The importance of cysteine 126 in the human liver UDP-glucuronosyltransferase UGT1A6. Biochim. Biophys. Acta. 2002;1597:90–96. doi: 10.1016/s0167-4838(02)00266-2. [DOI] [PubMed] [Google Scholar]
- 33.Ikushiro S., Emi Y., Iyanagi T. Protein-protein interactions between UDP-glucuronosyltransferase isozymes in rat hepatic microsomes. Biochemistry. 1997;36:7154–7161. doi: 10.1021/bi9702344. [DOI] [PubMed] [Google Scholar]