Abstract
The mammalian fetus is unique in its dependence during gestation on the supply of maternal nutrients through the placenta. Maternal supply and fetal demand for nutrients need to be fine tuned for healthy growth and development of the fetus along its genetic trajectory. An altered balance between supply and demand can lead to deviations from this trajectory with long-term consequences for health. We have previously shown that in a knockout lacking the imprinted placental-specific Igf2 transcript (P0), growth of the placenta is compromised from early gestation but fetal growth is normal until late gestation, suggesting functional adaptation of the placenta to meet the fetal demands. Here, we show that placental transport of glucose and amino acids are increased in the Igf2 P0+/- null and that this up-regulation of transport occurs, at least in part, through increased expression of the transporter genes Slc2a3 and Slc38a4, the imprinted member of the System A amino acid transporter gene family. Decreasing fetal demand genetically by removal of fetal Igf2 abolished up-regulation of both transport systems and reduced placental System A amino acid transport activity and expression of Slc38a2 in late gestation. Our results provide direct evidence that the placenta can respond to fetal demand signals through regulation of expression of specific placental transport systems. Thus, crosstalk between an imprinted growth demand gene (Igf2) and placental supply transporter genes (Slc38a4, Slc38a2, and Slc2a3) may be a component of the genetic control of nutrient supply and demand during mammalian development.
Keywords: genomic imprinting, nutrient transporters
Imprinted genes in mammals are expressed from only one of the parental chromosomes and are important regulators of placental and fetal growth (1–5). According to the kinship theory, also known as conflict theory, imprinting is thought to have evolved as a result of a tug-of-war over the allocation of maternal resources to the offspring (6–9). Paternal genomes maximize the extraction of maternal resources for the benefit of offspring (at the expense of offspring with different paternal genomes), whereas the interests of the maternal genome are best served by allocating resources equally among all her offspring (9). This theory predicts that a substantial number of imprinted genes have growth-related functions, with antagonistic properties: paternally expressed genes are growth “promoters,” whereas maternally expressed genes are growth “suppressors.” This prediction has been confirmed by a number of studies, which demonstrate that a key function of imprinting is the control of fetal growth (3–5, 9). The different interests of parental genomes are played out in the placenta, at the level of supply of resources, and in the fetus, at the level of the demand for resources (4, 9). How imprinted genes control supply of nutrients and the allocation of maternal resources through the placenta remains, however, poorly understood (4, 9). Knock-outs of several imprinted genes in mice affect the growth, thickness, and organization of the placental tissues interposed between mother and fetus (4, 5, 9, 10). Deletion of the paternally expressed genes Igf2, Peg1, and Peg3 results in a smaller size of the placenta, whereas the deletion of the maternally expressed genes H19, Igf2r, Cdkn1c, Phlda2, and Grb10 all result in overgrowth of the placenta (3–5).
There are several different transcripts of the Igf2 gene in the mouse, expressed from promoters P0–P3, which all code for the IGF-II peptide (11). The P0 transcript is only expressed by the placenta (11). We found that deletion of the P0 transcript has profound effects on placental and fetal growth (12). In these mice, where placental Igf2 mRNA expression is reduced but circulating fetal IGF-II is normal, placental weight is reduced compared to the wild type (wt) from embryonic day (E) 12 but fetal growth continues normally until E16 (12). We also found that materno-fetal transfer of 14C-methylaminoisobutyric acid (MeAIB), a non-metabolizable amino acid analogue usually transported across the placenta utilizing the System A amino acid transport system, was increased per gram of placenta at E16 but that this increase was blunted at E19 (12). Furthermore, the passive permeability of the placentas from the Igf2 P0+/- knockout mice was reduced at E19 because of decreased surface area and increased thickness of the exchange barrier (13). These data suggested that the growth of the Igf2 P0+/- knockout fetus is initially retained, in the face of reduced placental growth, by up-regulation of placental transporter activity and that fetal growth restriction ensues late in gestation because of a failure to maintain this up-regulation and the decreased permeability. We proposed that IGF-II acts as a controller of nutrient supply by affecting placental development and as a signal of fetal demand (4).
Here, we test the hypothesis that Igf2 regulates the pivotal balance between placental nutrient supply and fetal demand. We compared mice lacking Igf2 expression only from the P0 promoter, where fetal IGF-II production is normal, and mice lacking Igf2 produced from all promoters in which placental and fetal IGF-II are both lacking. The activity and expression of key placental supply genes, the System A amino acid transporter genes Slc38a1/SNAT1, Slc38a2/SNAT2, and Slc38a4/SNAT4 and the glucose transporter genes Slc2a1/GLUT1 and Slc2a3/GLUT3, were measured. The data show that although placental growth is reduced when P0 only or all Igf2 transcripts are deleted, it is only in the Igf2 P0+/- fetuses in which fetal Igf2 expression is normal and nutrient demand is maintained that placental transporter expression and activity is up-regulated. By abolishing fetal IGF-II as a growth demand signal, the up-regulation of both transporter systems is also abolished. These data are a direct demonstration that fetal growth demand can regulate placental supply systems. The System A transporter genes Slc38a4 and Slc38a2, and the glucose transporter Slc2a3, appear to be a central link between placental supply and fetal demand. Cross talk between the imprinted Igf2 demand signal itself or through downstream signals and placental supply genes may play an important role in coordinating mammalian growth.
Materials and Methods
Mice. The mice used in this study were from Igf2 P0 and Igf2 null deletion lines derived as described in refs. 14 and 15. Both lines have been bred into an inbred C57BL/6J line for >10 generations. All experimental procedures were conducted under licenses issued by the Home Office (U.K.) in accordance with the Animals (Scientific Procedures) Act 1986 and local ethics committee. In all experiments, the mutant alleles were transmitted by a heterozygous father, giving the genotypes +/-, (subsequently called Igf2 P0+/- or Igf2 null+/-) and +/+ (subsequently called wild-type +/+). The mutation transmitted through the father disrupts expression from the active paternal allele. Because of the imprinting regulation of this locus, the maternal allele is silent.
Pregnant females were killed by cervical dislocation, and the fetuses were dissected at E14, E16, and E19 into PBS (E1 was defined as the day of vaginal plug detection). Wet weights are the weights after removal of fluid from around the tissue with absorbent paper. Dry weights were obtained by freeze-drying under vacuum (Edwards Modulyo) until a constant weight was obtained (≥36 h). Placental water content was determined by subtracting the dry from the wet weights and expressed as percentage of wet weights.
Genotyping. The transmission of the Igf2 null+/- allele was followed by lacZ staining of yolk sac as described in ref. 15. Igf2 P0+/- mutants were identified by PCR with DNA extracted from placental tissue. The primer pairs used to amplify a 740-bp fragment across the 5-kb deletion were as follows: delF: 5′-TCCTGTACCTCCTAACTACCAC-3′; delR: 5′-GAGCCAGAAGCAAACT-3′. A third primer (5′-CAATCTGCTCCTGCCTG-3′) was used as a positive control for the PCR reaction by amplifying a 495-bp fragment from wild-type alleles. PCR conditions are available upon request.
Placental Transport Assays of Radiolabeled Solutes. Pregnant mice at E16 or E19 (term is day 20) were anesthetized with an intraperitoneal injection of ≈0.4 ml of fentanyl/fluanisone and midazolam solutions in water (1:1:2 water, Janseen Animal Health). A neck incision was made, and the jugular vein was exposed. A 100-μl bolus of PBS containing 3.5 μCi (1 Ci = 37 GBq) MeAIB (NEN NEC-671; specific activity 50.5 mCi/mmol) or 3.5 μCi 14C-methyl-d-glucose (NEN NEC-377; specific activity 56.4 mCi/mmol) was then injected into the jugular vein via a short length of tubing attached to a 27 gauge needle and connected to a 1-ml syringe. At times up to 4 min after injection of tracer, animals were killed and conceptuses were dissected by hysterectomy. Fetuses were lysed overnight at 55°C in 2 ml (E16 fetuses) or 4 ml (E19 fetuses) of Biosol (National Diagnostics). Fractions of fetal samples were then added to appropriate tubes for β counting (Packard Tri-Carb 1900). Radioactive counts in each fetus were then used to calculate the amount of radioisotope transferred per gram of placenta or per gram of fetus. Average values for wild-type and mutant fetuses within a litter were then calculated and expressed as a ratio of mutant to wild-type for that litter. These values could then be used to calculate a mean for all litters at E16 and E19. The fetal accumulation of radioisotope expressed relative to placental weight and plotted as a ratio of mutant to wild-type gives a relative measure of placental transfer of the solute. The fetal accumulation of radioisotope expressed relative to fetal weight gives a relative measure of the amount of solute received by the fetus. The data for the placental transfer assays performed in Igf2 P0+/- mutants were collected from 11 litters at E16 (Igf2 P0+/-, n = 36; wild-type+/+, n = 48) and 5 litters at E19 (Igf2 P0+/-, n = 19; wild-type+/+, n = 18) for 14C-glucose; from 6 litters at E16 (Igf2 P0+/-, n = 25; wild-type+/+, n = 16) and 4 litters at E19 (Igf2 P0+/-, n = 14; wild-type+/+, n = 18) for 14C-MeAIB. The data for the Igf2 null+/- mutants were collected from 6 litters at E16 (Igf2 null+/-, n = 19; wild-type+/+, n = 26) and 10 litters at E19 (Igf2 null+/-, n = 48; wild-type+/+, n = 44) for 14C-glucose; and from 10 litters at E16 (Igf2 null+/-, n = 36; wild-type+/+, n = 29) and 5 litters at E19 (Igf2 null+/-, n = 16; wild-type+/+, n = 21) for 14C-MeAIB.
mRNA Expression Studies of Nutrient Transporter Genes. mRNA expression levels of nutrient transporter genes were analyzed by Northern blotting at different gestational ages. RNAs were prepared from whole placentas by using RNeasy kits (Qiagen). Northern blots were hybridized with RNA probes produced by in vitro transcription from linearized plasmids of cloned PCR products as described in ref. 16. The Slc38a1 probe was from 63–121 bases in ENSMUST00000023937; the Slc38a2 probe was from 108–633 bases in NM_175121; the Slc38a4 probe that detects all transcripts was from 200–900 bases in NM_027052; the Slc2a3 probe was from 776-1177 bases in NM_011401; and the Slc2a1 probe was from 354–934 bases in NM_011400. The hybridized blots were visualized by phophorimaging (Fuji), and densitometry was performed by using aida v.3.27 software.
Statistical Analysis. Differences in mRNA expression levels between group's means were evaluated by the two-tailed unpaired Student t test. All other data were analyzed by means of two-way analyses of variance with “litters” and “genotype” as the two factors. Data are expressed as means ± standard error of the mean (SE). For data representing radioactive counts, a logarithmic transformation was carried out before statistical analysis. The summary data from these experiments were then presented as ratios, together with 95% confidence limits.
Results
Placental and Fetal Growth in Igf2 P0+/- and Igf2 null+/- Mutants. Igf2 P0+/- mutant placentas are as growth-restricted as Igf2+/- null placentas at E14 (Table 1). At later stages of gestation, the growth deficiency is more severe in Igf2 null+/- than in Igf2 P0+/- placentas (Table 1). Igf2 P0+/- and Igf2 null+/- placentas do not differ in water content from their wild-type littermates, showing that the reduction in weight is not due to a loss of water/fluid content (Table 1). As described previously, Igf2 P0+/- fetuses were lighter than their wild-type littermates only after E16, whereas Igf2 null+/- fetuses were growth-restricted from E12 onward (12). Consequently, fetal/placental weight ratios were higher in Igf2 P0+/- than wild-type fetuses, suggesting increased placental transport efficiency in this mutant, but were unaffected in Igf2 null+/- fetuses at E14 and significantly reduced at E19 compared to their wild-type littermates (Table 1).
Table 1. Wet weight, fetal to placental wet weight ratios, and placental water content of Igf2 P0+/–, Igf2 null+/–, and wild-type+/+ conceptuses by comparing mutants with corresponding wild-type littermates in the same litters.
| Gestational age | Mouse strain | n | Fetal wet weight, g | Placental wet weight, g | Fetal-to-placental weight ratios | Placental water content, % (n) |
|---|---|---|---|---|---|---|
| E14 | Igf2 P0+/– | 32 | 0.149 ± 0.003 | 0.075 ± 0.002 | 2.04 ± 0.04 | ND |
| Wild-type+/+ | 29 | 0.150 ± 0.004 | 0.091 ± 0.002 | 1.68 ± 0.04 | ND | |
| (% of wild-type) | 99 NS | 82*** | 121*** | |||
| Igf2 null+/– | 34 | 0.118 ± 0.003 | 0.072 ± 0.002 | 1.68 ± 0.05 | ND | |
| Wild-type+/+ | 46 | 0.151 ± 0.003 | 0.091 ± 0.002 | 1.69 ± 0.04 | ND | |
| (% of wild-type) | 78*** | 79*** | 99 NS | |||
| E16 | Igf2 P0+/– | 111 | 0.414 ± 0.004 | 0.075 ± 0.002 | 5.71 ± 0.07 | 83.3 ± 1.1 (32) |
| Wild-type+/+ | 127 | 0.432 ± 0.003 | 0.105 ± 0.001 | 4.23 ± 0.07 | 83.5 ± 0.5 (32) | |
| (% of wild-type) | 96** | 71*** | 135*** | |||
| Igf2 null+/– | 80 | 0.286 ± 0.004 | 0.065 ± 0.001 | 4.50 ± 0.08 | 83.2 ± 0.6 (41) | |
| Wild-type+/+ | 91 | 0.435 ± 0.004 | 0.104 ± 0.001 | 4.22 ± 0.07 | 83.7 ± 0.6 (33) | |
| (% of wild-type) | 66*** | 63*** | 107** | |||
| E19 | Igf2 P0+/– | 116 | 0.926 ± 0.009 | 0.063 ± 0.001 | 15.25 ± 0.24 | 82.0 ± 0.5 (22) |
| Wild-type+/+ | 117 | 1.228 ± 0.009 | 0.095 ± 0.001 | 13.46 ± 0.22 | 82.5 ± 0.4 (17) | |
| (% of wild-type) | 75*** | 66*** | 113*** | |||
| Igf2 null+/– | 116 | 0.571 ± 0.008 | 0.051 ± 0.001 | 11.60 ± 0.16 | 80.9 ± 1.1 (32) | |
| Wild-type+/+ | 108 | 1.215 ± 0.008 | 0.089 ± 0.001 | 13.88 ± 0.16 | 83.8 ± 0.7 (33) | |
| (% of wild-type) | 47*** | 57*** | 84*** |
ND, not determined; **, P < 0.01; ***, P < 0.001; NS, not significant P > 0.05. Data is mean ± SE.
The Small P0+/- Placenta Up-Regulates Nutrient Supply to Meet Fetal Growth Requirements. To address the hypothesis that the small Igf2 P0+/- placenta has increased nutrient transport efficiency to meet the growth demands of the normally sized fetus, we investigated transport capacity in Igf2 P0+/- and wild-type mice by using unidirectional maternal-fetal transfer of 14C-glucose and 14C-MeAIB at E16 and E19.
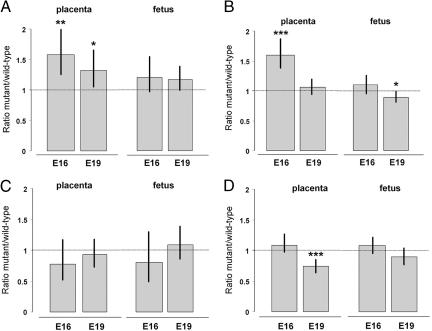
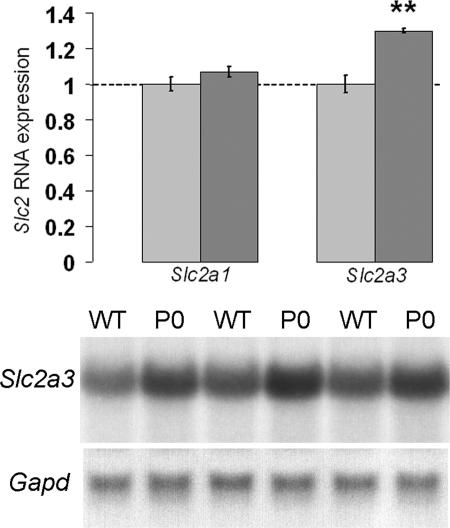
We found that glucose transport was significantly increased per gram of placenta compared to the wild-types at both gestational ages (Fig. 1A). At E16 and E19, Igf2 P0+/- placentas transferred more 14C-glucose per gram compared with wild-type littermates (158% and 132%, respectively; P < 0.05) (Fig. 1A). At both time points, accumulation of 14C-glucose per gram of fetus was the same in Igf2 P0+/- fetuses as the wild-type, i.e., fetuses were receiving the appropriate amount of the radioactive label for their size (Fig. 1A). We then analyzed the placental expression of two main glucose transporters, Slc2a1/GLUT1 and Slc2a3/GLUT3, at E16. We found increased expression of Slc2a3 in Igf2 P0+/- placentas when compared to wild-type controls (ratio P0+/-/WT+/+ ≈ 1.3, P < 0.01; Fig. 2) but not Slc2a1 (Fig. 2). Thus, we hypothesize that the enhanced transport of glucose is due, at least in part, to the increased expression of the Slc2a3 gene.
Fig. 1.
The effects of deletion of either the Igf2 P0 transcript from the labyrinthine trophoblast alone (A and B, Igf2 P0+/-)orthe Igf2 gene from all feto-placental tissues (C and D, Igf2 null+/-) on the placental transfer of 14C-glucose (A and C) and 14C-MeAIB (B and D), calculated as a ratio of mutant to wild-type transfer expressed either per gram of placenta or per gram of fetus at two gestational ages (E16 and E19). Ratios >1 indicate increased transfer by the mutant placenta, whereas ratios <1 indicate reduced transfer by the mutant placenta with respect to either placental or fetal weight. Bars indicate 95% confidence limits. *, P < 0.05; **, P < 0.01; ***, P < 0.001 significantly different from 1.
Fig. 2.
Expression analysis of the glucose transporters Slc2a1 and Slc2a3 by Northern blotting in Igf2 P0+/- placentas at E16. Graphs of the mean expression levels are shown with the wild-type levels normalized to 1, together with representative Slc2a3 Northern blots of total RNA obtained from wild-type (WT) and Igf2 P0+/- placentas (P0). Bars indicate SE. WT+/+, n = 12; P0+/-, n = 12 for Slc2a3; n = 7 for both groups for Slc2a1. **, P < 0.01.
We replicated our previous observations (12) that System A amino acid transport activity was significantly increased per gram of Igf2 P0+/- placentas compared to the wild-types in E16 (Fig. 1B). We now show that this increase in transfer per gram of placenta is no longer observed near term (106%, P > 0.05). This reduction, combined with the smaller size of the placenta, resulted in Igf2 P0+/- fetuses receiving less of the solute per gram of fetus than wild-type littermates at term (89%, P < 0.05) (Fig. 1B). The reduction in up-regulation of System A activity from E16 to E19 (160% to 106%, respectively) correlates with the reduction in fetal weight (from 96% to 75%, respectively).
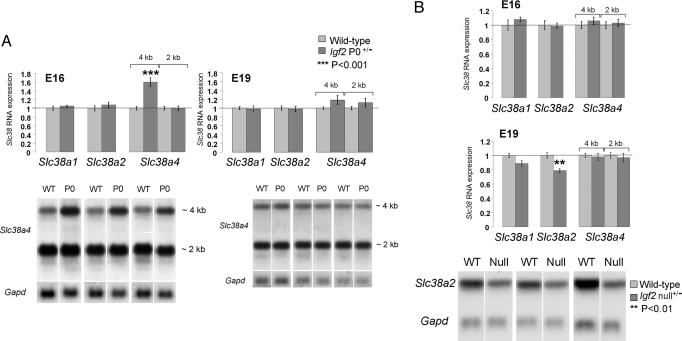
We next investigated the mechanisms at the molecular level responsible for the enhanced System A activity in Igf2 P0+/- mice. We found that the placental expression of the imprinted System A gene (Slc38a4), but not of the nonimprinted members of the System A gene family (Slc38a1 and Slc38a2) (R.S. and G.K., unpublished observations), closely paralleled the in vivo transport of 14C-MeAIB. Increased RNA expression of the imprinted gene Slc38a4 was observed at E16 (1.6-fold increased expression of the 4-kb transcript) compared to wild-type littermates (Fig. 3A), which corresponds to an increase in 14C-MeAIB transport (Fig. 1B). At E19, there was no increase in placental expression of Slc38a4 (Fig. 3A), consistent with the lack of up-regulation of 14C-MeAIB transport observed at this stage of gestation (Fig. 1B). We hypothesize that the enhanced amino acid transport is due, at least in part, to the increased expression of the imprinted Slc38a4 gene.
Fig. 3.
Expression analysis of System A amino acid transporter genes (Slc38a1, Slc38a2, and Slc38a4) by Northern blotting in Igf2 P0+/- (A) and Igf2 null+/- (B) placentas compared to their wild-types (WT) at two gestational ages (E16 and E19, n = 7 for all WT and mutant groups). Graphs of mean expression levels are shown with the wild-type levels normalized to 1 together with representative Northern blots of total RNA obtained from wild-type (WT) and mutant placentas for Slc38a4 (A), at E16 and E19 and Slc38a2 (B), at E19, compared to Gapd loading. Bars indicate SE. ***, P < 0.001; **, P < 0.01 significantly different from the value in the wild-type. Note increase in signal in the Igf2 P0+/- when compared to WT for the 4-kb transcript only at E16 but not E19.
Up-Regulation of Placental Nutrient Supply Depends on a Fetal Demand Signal. If the increase in nutrient supply activity, per gram of placenta, observed in the Igf2 P0+/- mice is a direct consequence of an Igf2 deficiency in the labyrinthine trophoblast, this increase should also occur in Igf2 null+/- mice that lack Igf2 in all placental and fetal cell types. In fact, we found that Igf2 null+/- placentas, unlike P0, do not transfer 14C-glucose (Fig. 1C) and 14C-MeAIB (Fig. 1D) with greater efficiency when compared to wild-type controls. Indeed, when fetal growth is normally at its maximum in late gestation (Table 1), 14C-MeAIB transfer per gram of placenta is greatly reduced in Igf2 null+/- placentas (Fig. 1D), although this transfer is appropriate for the much smaller fetal body mass (Fig. 1D). This reduction in System A activity was associated with a similar reduction in expression of the nonimprinted Slc38a2 gene, with no effect on the Slc38a4 gene (Fig. 3B). We conclude that adaptation of placental nutrient transfer occurs in response to fetal nutrient demands for growth and involves interaction between the Igf2 gene in feto-placental tissues and nutrient transporter genes in the placenta.
Discussion
This study, using genetic models of altered growth, provides direct evidence that the activity and expression of specific placental nutrient transporters is modulated by the fetal nutrient demands for growth. It also shows that the imprinted Igf2 gene has a key role in this process. We have shown that, in mice that lack IGF-II, specifically in the labyrinthine trophoblast, the small placenta increases its transfer of glucose and amino acids to meet the nutrient demands of the growing fetus in late gestation. These mutant placentas express higher levels of glucose and amino acid transporters encoded by the Slc2a3 and System A Slc38a4 genes and have a greater efficiency measured as grams of fetus produced per gram of placenta. In contrast, when we reduced the fetal demand placed on the small placenta by deleting the Igf2 gene from all feto-placental tissues, activity of the placental nutrient transport systems was either at wild-type levels or reduced in late gestation and was associated with a decrease in placental efficiency. These findings provide a demonstration that the small placenta can respond to the genetic drive for fetal growth by up-regulating its efficiency and nutrient transport capacity.
The growth of the mouse placenta appears to depend on both the paracrine and endocrine actions of IGF-II. In the Igf2 P0+/- knockout, all placental layers are reduced proportionately (12, 13), even though the P0 transcript is expressed only in the labyrinthine trophoblast (12). Consequently, IGF-II-dependent growth of the labyrinthine exchange surface may regulate growth of the placenta as a whole. This effect on growth might be mediated directly by the paracrine action of IGF-II or indirectly by other signals from the labyrinthine placenta to the spongiotrophoblast. Our finding that placental weight is reduced to the same extent in Igf2 P0+/- and complete Igf2 nulls+/- up to E14 shows that local expression of the P0 transcript is essential for normal placental development and that the other Igf2 transcripts are not required to promote early placental growth. After E14, feto-placental expression of the other Igf2 transcripts may be involved in regulating placental growth, as placental growth restriction was less severe in the Igf2 P0+/- than complete Igf2 null+/-. At E16 and E19, the P0 placenta exposed to normal fetal IGF-II concentrations was 14–24% heavier than the placenta of complete Igf2 null with no circulating IGF-II. Similarly, placental weight is reduced by 14% in chimeric embryos with normal IGF-II expression in the placenta but IGF-II deficiency in the fetal tissues (17). Conversely, when fetal IGF-II availability is increased 10-fold by loss of Igf2 imprinting and deletion of the Igf2r gene, which encodes the IGF-II clearance receptor, placental weight is increased to a greater extent than fetal weight at term (18). These observations suggest that, during the later stages of gestation, placental growth can be stimulated by circulating IGF-II produced by the fetal tissues.
Our previous study of the Igf2 P0+/- knockout demonstrated that the increased efficiency of the Igf2 P0+/- placenta in supporting fetal growth up to E16 was associated with up-regulation of amino acid transport (12). However, it did not establish the molecular mechanisms involved or provide an explanation for the increased fetal to placental weight ratio at E19. In the current study, we show that the increased efficiency of the Igf2 P0+/- placenta is due, in part, to increased transfer of glucose per gram of placenta at both E16 and E19 and identify potential molecular effectors of the changes in placental glucose and amino acid transfer. Glucose is transported across the placenta by facilitated diffusion down the materno-fetal concentration gradient by using members of the GLUT family of glucose transporters (19). The main glucose transporters in the placenta are GLUT1 and GLUT3, which are expressed from the Slc2a1 and Slc2a3 genes, respectively (20, 21). In rodents, GLUT1 is found on all placental membranes, whereas GLUT3 is localized specifically to the maternal facing surface of the labyrinthine trophoblast (20, 21). During the period of late gestation when the rat fetus is growing most rapidly in absolute terms, there is up-regulation of Slc2a3 expression and down-regulation of Slc2a1 expression in the placenta (20, 21). In the current study, we showed that the increased placental glucose transfer across the Igf2 P0+/- placenta was associated with up-regulation of placental Slc2a3 expression. Taken together, these observations indicate that the placental Slc2a3 gene may have an important role in meeting the nutrient demands of the growing fetus during late gestation. However, despite the continued up-regulation of glucose transfer, fetal growth still declined toward term in the Igf2 P0+/- mutant. Therefore, substrates other than glucose must make a significant contribution to growth of the mouse fetus during late gestation.
In sheep, amino acids account for 50% of the carbon and virtually all of the nitrogen required for fetal growth (22). In this and our previous study of Igf2 P0+/- mutants, transplacental transfer of MeAIB was increased in line with fetal growth requirements at E16 but not at later stages of gestation when the fetus was becoming progressively more growth retarded. MeAIB is transferred across the placenta by the sodium-dependent System A transporters, which normally transport small neutral amino acids (23). In the mouse, there are three System A transporters described so far, SNAT1, SNAT2 and SNAT4, which have different amino acid affinities and are encoded by the Slc38a1, Slc38a2, and Slc38a4 genes, respectively (23). In the current study, we found that, at E16, the small Igf2 P0+/- placenta up-regulates the expression of the imprinted Slc38a4 gene (16) but not the nonimprinted (R.S. and G.K., unpublished observations) Slc38a1 or Slc38a2 genes. All three genes are expressed abundantly in the labyrinthine and spongiotrophoblast of the murine placenta (G. Konfortova and G.K., unpublished observations). The mouse Slc38a4 gene is a complex locus with four promoters (un1-un4), which are regulated differentially by genomic imprinting and produce several transcripts with different 5′ and 3′ untranslated regions (ref. 24; R.S. and G.K., unpublished observations). In the fetus, un1 and un3 transcripts are predominantly expressed from the paternal allele, whereas un4 is expressed predominantly from the maternal allele (R.S. and G.K., unpublished observations). In the placenta, imprinted transcripts are exclusively of paternal origin (R.S. and G.K., unpublished observations). By Northern blotting, two transcripts of 4 kb and 2 kb in size are detected. The distinct transcript lengths are likely to result from alternate polyadenylation usage sites in the 3′ UTR region of the last exon, as described for the rat Slc38a4 gene (24). All 5′ UTRs splice onto the same exon 2 that contains the initiation translation ATG, so that all transcript forms have the potential to encode the same protein. Interestingly, only the longer transcript(s) form (4 kb) of this gene was found to be up-regulated in the Igf2 P0+/- mutants. Whether this up-regulation reflects overexpression of the active allele, loss of imprinting, or changes in mRNA stability remains unknown. Neither is clear whether the increased MeAIB transport is due solely to this transcript or involves other, unidentified System A transporter genes. Up-regulation in Slc38a4 gene expression was not observed in the Igf2 P0+/- placenta at E19. Hence, changes in placental Slc38a4 expression and System A activity occur in parallel and are similar in magnitude in this mutant. This inability of the Igf2 P0+/- placenta to sustain up-regulation of System A amino acid transport, may explain, in part, the growth restriction of the Igf2 P0+/- mutant fetus during late gestation. Certainly, competitive inhibition of System A transport in rats in vivo during pregnancy leads to fetal growth retardation close to term (25). Furthermore, when expression of the Slc38a4 un1 transcript that accounts for the major proportion of placental SNAT4 protein is abolished by deletion of its promoter, birth weight of the mutant mice is reduced by 20% (R.S. and G.K., unpublished observations). In human studies, there is an inverse relationship between the activity of the System A amino acid transporter in the microvillous plasma membrane of the placenta and the size of the baby at birth (26). This scenario, similar to that in the Igf2 P0+/- mice, suggests that in the proportionately small placentas of small normal babies, there is an up-regulation of the expression of the System A amino acid transporter to maintain transfer capacity. However, further studies are required to demonstrate whether placental System A transport and the Slc38a4 gene, in particular, are rate limiting to fetal growth in the Igf2 P0+/- mouse model.
Up-regulation of placental glucose and System A amino acid transport was not seen in the complete Igf2 null+/- mice. Indeed, 14C-MeAIB transfer per gram of placenta and placental expression of Slc38a2 were decreased at E19 in these mutants. Because the P0 transcript is absent in the labyrinthine trophoblast of both Igf2 P0+/- and complete Igf2 nulls+/-, IGF-II derived from the P0 promoter is unlikely to suppress expression of the Slc38a4 and Slc2a3 genes during normal development of the placenta. It is more likely that the small Igf2 P0+/--deficient placenta is responding to nutrient demand signals produced by tissues still expressing IGF-II. These fetal demand signals appear to stimulate placental growth and up-regulate placental expression of the nutrient supply genes, Slc38a4, Slc2a3 and, possibly, also Slc38a2. Over the gestational ages at which we measured nutrient transfer, there is little Igf2 gene expression in the junctional zone because spongiotrophoblast cells no longer express IGF-II, and the numbers of IGF-II expressing glycogen cells decline dramatically (27, 28; unpublished observations). In addition, because there is no evidence that IGF-II is released from these cell types into the maternal arteries supplying the labyrinthine trophoblast, up-regulation of the nutrient supply genes is unlikely to be due to IGF-II derived from the junctional zone of the placenta. Furthermore, we have not found any evidence for up-regulation of Igf2 gene expression from the P1–P3 promoters in the labyrinthine trophoblast after deletion of the P0 transcript (data not shown). The nutrient demand signals, therefore, appear to originate in the fetus. Certainly, when the drive for fetal growth is reduced in the complete Igf2 null+/-, a smaller placental supply of nutrients is required to support the nutrient demand of the lower growth rate and placental System A activity and expression of Slc38a2 are reduced accordingly. The nutrient transfer capacity of the mouse placenta is regulated ultimately by the interplay between Igf2 gene expression in the placenta and fetus.
There are several possible mechanisms that could signal the fetal nutrient requirement to the placenta. The current study suggests that circulating fetal IGF-II is a likely signal, at least, when the fetal nutrient demand for growth exceeds the nutrient transport capacity of the small Igf2-deficient placenta. Circulating IGF-II appears to stimulate placental growth (see above), and there is evidence that IGF-II increases glucose transport in human chorionic villi in vitro (29). However, if IGF-II is acting as the fetal demand signal in the Igf2 P0+/- mice, its influence on the placental Slc38a4 gene must wane toward term as up-regulation of System A activity was not maintained throughout late gestation. Because the increase in glucose transport was sustained, facilitated and active transport processes may be responding to different demand signals during late gestation. Our observation that System A amino acid transporter genes were expressed differentially in the placentas of Igf2 P0+/- and Igf2 null+/- mutants also suggests that fetal IGF-II is not the only demand signal. Fetal nutrient requirements could be signaled to the placenta by other fetally derived growth factors or by fetally induced changes in the nutritional or endocrine environments of the mother. Alternatively, fetal stress hormones may act as signals of nutrient insufficiency and alter placental nutrient delivery in the Igf2 P0+/-, particularly during late gestation as fetal growth begins to deviate from its early trajectory. Whatever the specific signals involved, it is the mismatch between the supply and the demand for nutrients generated by growing fetal tissue still expressing IGF-II that up-regulates the nutrient supply genes in the small IGF-II-deficient placenta. When there is no mismatch between supply and demand in the complete IGF-II null, nutrient transport across the small IGF-II-deficient placenta is not up-regulated. The role of fetal/circulating IGF-II as a demand signal can be unequivocally demonstrated only with a fetal IGF-II conditional knock-out.
The crosstalk between nutrient demand and supply genes that we have identified here has important implications for the genetic conflict theory and for placental physiology. Several studies have suggested that the placenta has a considerable functional reserve capacity, so that a reduction in placental size will not, unless extreme, limit fetal growth (30, 31). Our work indicates that increased placental efficiency can compensate for the loss of placental mass and sustain fetal growth by up-regulating nutrient transfer across the placenta. Because the placenta is the major source of nutrients during intrauterine development, it is a primary site for conflict between paternal and maternal genes in allocating maternal resources to the fetus. The interaction between a paternally expressed demand gene, Igf2, in the fetus and a paternally expressed supply gene, Slc38a4, in the placenta observed in our study is consistent with the concept that paternally inherited genes maximize extraction of maternal resources and is evidence of two paternally expressed genes acting cooperatively to influence allocation of maternal nutrients. The mechanisms underlying this interaction remain unknown but may involve signals downstream of the yet-unidentified IGF-II receptor X in the placenta (32) and specific promoter usage.
Acknowledgments
We thank E. Walters for help with the statistical analyses and Myriam Hemberger and Graham Burton for discussions and critical reading of the manuscript. This study was supported by grants from the Biotechnology and Biological Sciences (BBSRC) Research Council and Medical Research Council. M.C. is a David Phillips Fellow (BBSRC).
Author contributions: M.C., C.P.S., W.R., and A.F. designed research; M.C., E.A., I.S., P.S., W.D., A.F.-S., C.P.S., W.R., and A.F. performed research; R.S. and G.K. contributed new reagents/analytic tools; M.C., E.A., I.S., C.P.S., W.R., and A.F. analyzed data; and M.C., C.P.S., W.R., and A.F. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: En, embryonic day n; MeAIB, 14C-methylaminoisobutyric acid.
References
- 1.Reik, W. & Walter, J. (2001) Nat. Rev. Genet. 2, 21-32. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson-Smith, A. C. & Surani, M. A. (2001) Science 293, 1086-1089. [DOI] [PubMed] [Google Scholar]
- 3.Tycko, B. & Morison, I. M. (2002) J. Cell Physiol. 192, 245-258. [DOI] [PubMed] [Google Scholar]
- 4.Reik, W., Constância, M., Fowden, A. L., Anderson, N., Dean, W., Ferguson-Smith, A. C., Tycko, B. & Sibley, C. P. (2003) J. Physiol. 547.1, 35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coan, P. M., Burton, G. J. & Ferguson-Smith, A. C. (2005) Placenta 26, Suppl. A, S10-S20. [DOI] [PubMed] [Google Scholar]
- 6.Moore, T. & Haig, D. (1991) Trends Genet. 7, 45-49. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins, J. F. & Haig, D. (2003) Nat. Rev. Genet. 4, 359-368. [DOI] [PubMed] [Google Scholar]
- 8.Haig, D. (2004) Annu. Rev. Genet. 38, 553-585. [DOI] [PubMed] [Google Scholar]
- 9.Constância, M., Kelsey, G. & Reik, W. (2004) Nature 432, 53-57. [DOI] [PubMed] [Google Scholar]
- 10.Georgiades, P., Watkins, M., Burton, G. J. & Ferguson-Smith, A. C. (2001) Proc. Natl. Acad. Sci. USA 98, 4522-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore, T., Constância, M., Zubair, M., Bailleul, B., Feil, R., Sasaki, H. & Reik, W. (1997) Proc. Natl. Acad. Sci. USA 94, 12509-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constância, M., Hemberger, M., Hughes, J., Dean, W., Ferguson-Smith, A. C., Fundele, R., Stewart, F., Kelsey, G., Fowden, A. L., Sibley, C. P. & Reik, W. (2002) Nature 417, 945-948. [DOI] [PubMed] [Google Scholar]
- 13.Sibley, C. P., Coan, P. M., Ferguson-Smith, A. C., Dean, W., Hughes, J., Smith, P., Reik, W., Burton, G. J., Fowden, A. L. & Constância, M. (2004) Proc. Natl. Acad. Sci. USA 101, 8204-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constância, M., Dean, W., Lopes, S., Moore, T., Kelsey, G. & Reik, W. (2000) Nat. Genet. 26, 203-206. [DOI] [PubMed] [Google Scholar]
- 15.Murrell, A., Heeson, S., Bowden, L., Constância, M., Dean, W., Kelsey, G. & Reik, W. (2001) EMBO Rep. 2, 1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, R. J., Dean, W., Konfortova, G. & Kelsey, G. (2003) Genome Res. 13, 558-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner, R. L., Squire, S., Zaina, S., Hills, S. & Graham, C. F. (1999) Biol. Reprod. 60, 190-195. [DOI] [PubMed] [Google Scholar]
- 18.Eggenschwiler, J., Ludwig, T., Fisher, P., Leighton, P. A., Tilghman, S. M. & Efstratiadis, A. (1997) Genes Dev. 11, 3128-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uldry, M. & Thorens, B. (2004) Pflugers Arch. 447, 480-489. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, J. & Bondy, C. A. (1993) J. Clin. Invest. 91, 845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin, B. C., Fujikura, K., Suzuki, T., Tanaka, S. & Tanata, K. (1997) Endocrinology 138, 3997-4004. [DOI] [PubMed] [Google Scholar]
- 22.Fowden, A. L. & Forhead, A. J. (2004) Reproduction 127, 515-526. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie, B. & Erickson, J. D. (2004) Pflugers Arch. 447, 784-795. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara, M., Nakanishi, T., Fei, Y.-J., Martindale, R.G., Ganapathy, M. E., Leibach, F. H. & Ganapathy, V. (2000) Biochem. Biophys. Acta 1509, 7-13. [DOI] [PubMed] [Google Scholar]
- 25.Cramer, S., Beveridge, M., Kilberg, M. & Novak, D. (2002) Am. J. Physiol. 282, C153-C160. [DOI] [PubMed] [Google Scholar]
- 26.Godfrey, K. M., Matthews, N., Glazier, J., Jackson, A., Wilman, C. & Sibley, C. P. (1998) J. Clin. Endocrinol. Metab. 83, 3320-3326. [DOI] [PubMed] [Google Scholar]
- 27.Redline, R. W., Chernicky, C. L., Tan, H. Q., Ilan, J. & Ilan, J. (1993) Mol. Reprod. Dev. 36, 121-129. [DOI] [PubMed] [Google Scholar]
- 28.Lopez, M. F., Dikkes, P., Zurakowski, D. & Villa-Komaroff, L. (1996) Endocrinology 137, 2100-2108. [DOI] [PubMed] [Google Scholar]
- 29.Kniss, D. A., Shubert, P. J., Zimmerman, P. D., Landon, M. B. & Gabbe, S. G. (1994) J. Reprod. Med. 39, 249-256. [PubMed] [Google Scholar]
- 30.Fox, H. (1981) in Fetal Growth Retardation, eds. Van Assche, F. A. & Robertson, W. B. (Churchill Livingstone, London), pp. 117-125.
- 31.Robinson, J. S., Kingston, E. J., Jones, C. T. & Thorburn, G. D. (1979) J. Dev. Physiol. 1, 379-398. [PubMed] [Google Scholar]
- 32.Efstratiadis, A. (1998) Int. J. Dev. Biol. 42, 955-976. [PubMed] [Google Scholar]