Abstract
Peroxisomal matrix proteins are posttranslationally imported into peroxisomes with the peroxisome-targeting signal 1 receptor, Pex5. The longer isoform of Pex5, Pex5L, also transports Pex7-PTS2 protein complexes. After unloading the cargoes, Pex5 returns to the cytosol. To address molecular mechanisms underlying Pex5 functions, we constructed a cell-free Pex5 translocation system with a postnuclear supernatant fraction from CHO cell lines. In assays using the wild-type CHO-K1 cell fraction, 35S-labeled Pex5 was specifically imported into and exported from peroxisomes with multiple rounds. 35S-Pex5 import was also evident using peroxisomes isolated from rat liver. ATP was not required for 35S-Pex5 import but was indispensable for export. 35S-Pex5 was imported neither to peroxisome remnants from RING peroxin-deficient cell mutants nor to those from pex14 cells lacking a Pex5-docking site. In contrast, 35S-Pex5 was imported into the peroxisome remnants of PEX1-, PEX6-, and PEX26-defective cell mutants, including those from patients with peroxisome biogenesis disorders, from which, however, 35S-Pex5 was not exported, thereby indicating that Pex1 and Pex6 of the AAA ATPase family and their recruiter, Pex26, were essential for Pex5 export. Moreover, we analyzed the 35S-Pex5-associated complexes on peroxisomal membranes by blue-native polyacrylamide gel electrophoresis. 35S-Pex5 was in two distinct, 500- and 800-kDa complexes comprising different sets of peroxins, such as Pex14 and Pex2, implying that Pex5 transited between the subcomplexes. Together, results indicated that Pex5 most likely enters peroxisomes, changes its interacting partners, and then exits using ATP energy.
Most organelle proteins are synthesized on cytoplasmic polyribosomes and are then directed to their destined compartments. Peroxisomal matrix proteins are also synthesized on free polyribosomes and posttranslationally imported into peroxisomes (22), requiring the concerted action of protein import machinery (43, 45). Pex5, the receptor of peroxisome-targeting signal type 1 (PTS1) (16, 27, 28) proteins, plays a central role in the protein import reaction (9, 49, 54, 55). Pex5 shows a dual subcellular localization, mostly in the cytosol and only partly in peroxisomes. Pex5 has been reported to interact with Pex10, Pex12, Pex13 (3, 4, 15, 32), and the potential initial docking protein, Pex14 (2, 36, 44). Pex5 carrying PTS1 proteins initially binds to Pex14 on peroxisomal membranes, translocating to the import machinery comprising Pex13 and the RING finger family peroxins, Pex2, Pex10, and Pex12 (36, 38). The AAA ATPase peroxins, Pex1 (40, 42, 47) and Pex6 (26, 52, 57), and their anchoring peroxin, Pex26 (24, 25), are also involved in peroxisomal matrix protein import, as reported from the studies using fibroblasts from patients with peroxisome biogenesis disorders (PBD) of complementation group 1 (CG1; CG-E in Japan), CG4 (CG-C), and CG8 (CG-A), as well as CHO cell mutants (12, 24, 25, 40, 42, 46, 57). Hence, it is very likely that Pex1, Pex6, and Pex26 are also members of the protein import machinery.
Pex5 is thought to shuttle between the cytosol and peroxisomes based on the findings in in vivo experiments (6). Recently, an in vitro import assay system using a postnuclear supernatant (PNS) fraction from rat liver was reported, addressing the kinetics and energetics of Pex5 (17, 18, 34). This cell-free system appears to be a potential tool for investigating Pex5 translocation involving peroxisomal protein import machinery. Our group has thus far isolated peroxisome-deficient CHO mutant cell lines of 13 CGs (11), including PEX5-defective ZP105 (33, 37) and PEX14-deficient ZP161 (44). In this work, we established an in vitro Pex5 translocation system using PNS from wild-type CHO-K1 and peroxisome-defective CHO cell mutants to shed a mechanistic insight into peroxisomal protein import processes. Here, we show that Pex5 is imported to and exported from peroxisomes in ATP-independent and -dependent manners, respectively. Moreover, Pex1, Pex6, and Pex26 were likely to be dispensable for the Pex5 import but critical for the Pex5 export. Furthermore, we found Pex5-interacting import complexes containing Pex14 and a RING finger peroxin, Pex2, on the peroxisomal membranes. We discuss the Pex5 shuttling mediated by such translocon complexes between peroxisomes and the cytosol.
MATERIALS AND METHODS
Antibodies.
We used rabbit antisera to human catalase (26), Pex14 (44), malate dehydrogenase (MDH) (19), and cytochrome P450 reductase (29), lactate dehydrogenase (LDH) (Rockland, Gilbertsville, Pa.), Lamp-1 (Santa Cruz, Santa Cruz, Calif.), and mouse monoclonal antibody to hemagglutinin A (HA) (Covance, Princeton, N.J.) for Western blotting. We also used rabbit antisera to Pex2 (51) and Pex1 (51) for antibody shift assay (56) and immunoprecipitation, respectively.
Cell culture.
CHO cells were cultured in Ham's F-12 medium supplemented with 10% fetal calf serum under 5% CO2-95% air (53). Human fibroblasts from a normal control patient and PBD patients were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (47).
Synthesis of radiolabeled proteins.
cDNAs encoding full-length rat acyl-coenzyme A oxidase (AOx) (28) and N-terminally His6- and Flag-tagged Chinese hamster Pex5L, the larger isoform (36), were used. His6-Flag tagging did not affect the biological activity of Pex5L (K. Okumoto and Y. Fujiki, unpublished data). These cDNAs were transcribed and translated using TNT Quick Coupled transcription/translation systems (Promega, Madison, Wis.) with [35S]methionine and [35S]cysteine (Amersham Biosciences, Tokyo, Japan) as labels.
In vitro import and export assays.
Assays for in vitro import of AOx and Pex5 were performed as follows. A postnuclear supernatant (PNS) fraction was prepared from CHO-K1 and several CHO pex cell mutants. CHO cells (6 × 107 each) were harvested and homogenized in 0.25 M sucrose, 5 mM HEPES-KOH, pH 7.4, and 0.1% ethanol. The PNS fraction was obtained by centrifuging the homogenate twice at 700 × g for 5 min. A cytosolic fraction was prepared by centrifuging PNS at 100,000 × g for 30 min. PNS from human fibroblasts (4 × 107 each) was also used in Pex5 import assays.
The import reaction was performed using 35S-labeled Pex5 and PNS (1 mg protein) in 200 μl of import buffer, 0.25 M sucrose-5 mM HEPES-KOH (pH 7.4)-0.1% ethanol-5 mM methionine-3 mM MgCl2-50 mM KCl. The import assay was also done with peroxisomes isolated from rat liver (see below). Import of 35S-Pex5 to peroxisomes was verified by its resistance to the treatment with externally added protease in the absence or presence of 1% Triton X-100, as follows. The import reaction mixture was incubated with 90 μg/ml proteinase K (Sigma, St. Louis, Mo.) on ice for 30 min. After terminating the protease digestion with 500 μg/ml of phenylmethylsulfonyl fluoride (PMSF), the assay mixture was centrifuged to separate organelle and cytosolic fractions and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% gel. 35S-Pex5 was detected by a Fujix FLA5000 Autoimaging analyzer (Fuji Film, Tokyo, Japan).
For Pex5 export reaction, PNS incubated with 35S-Pex5 in the import buffer was centrifuged at 20,000 × g for 20 min to isolate the organelles containing the imported 35S-Pex5L in peroxisomes. The organelle fraction was resuspended with the cytosolic fraction in export buffer, 0.25 M sucrose-5 mM HEPES-KOH (pH 7.4)-0.1% ethanol-5 mM methionine-3 mM MgCl2-50 mM KCl-4% rabbit reticulocyte lysate. The reaction mixture was separated into organelle and cytosolic fractions by centrifugation at 20,000 × g for 20 min. In several import and export assays, an ATP regenerating system (ARS) containing 10 mM creatine phosphate (Roche Diagnostics, Indianapolis, Ind.) and 50 μg/ml of creatine kinase (Roche) was added in addition to 1 mM ATP (Sigma, St. Louis, Mo.). For ATP depletion, PNS and cytosol fraction were incubated with 5 U/ml of apyrase (Sigma) at 26°C for 10 min.
Subcellular fractionation of rat liver.
The liver of a rat that had been injected with Triton WR-1339 (27) was homogenized in 0.25 M sucrose, 10 mM HEPES-KOH, pH 7.4, 1 mM EDTA, and 0.1% ethanol. Peroxisomes were isolated by equilibrium density gradient centrifugation of a light mitochondrial fraction in a linear sucrose gradient (30 to 60%, wt/wt) in a Beckman VTi-65.2 vertical rotor. Ultracentrifugation was carried out at 230,000 × g (average) for 90 min at 4°C. The gradient was fractionated into 35 tubes.
In vitro binding assay.
The in vitro binding assay was performed using CHO-K1 cells transiently expressing Pex1-HA or Pex6-HA. cDNAs each encoding HA-tagged Pex1 and Pex6 in the pUcD2Hyg vector were transfected into CHO-K1 cells using Lipofectamine (Invitrogene, Carlsbad, Calif.). After a 2-day culture, cells (1 × 107) were lysed on ice for 30 min with lysis buffer consisting of 1% Triton X-100, 50 mM Tris-HCl, pH 7.5, 10% glycerol, 150 mM NaCl, 1 mM dithiothreitol (DTT), 1 mM EDTA, protease inhibitor cocktail (2 μg/ml aprotinin, 25 μg/ml antipain, and 25 μg/ml leupeptin), and 1 mM PMSF and centrifuged at 20,000 × g for 10 min. The supernatant fraction was incubated with glutathione S-transferase (GST)- or GST fused to Pex5 and Pex19 (∼5 μg each)-bound glutathione-Sepharose (30 μl) by rotating for 1 h at 4°C. Sepharose beads were washed three times with the lysis buffer. Bound proteins were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Immunoprecipitation assay.
After the 35S-Pex5 import assays, organelle fractions, including those containing 35S-Pex5-imported peroxisomes, were solubilized with 1% digitonin in 5 mM HEPES-KOH, pH 7.4, 100 mM NaCl, 1 mM DTT, 1 mM EDTA, protease inhibitor cocktail, and 1 mM PMSF for 30 min on ice and centrifuged at 100,000 × g for 15 min. Supernatant fractions were incubated with anti-Pex1 antibody or preimmune serum on ice for 30 min. The antigen-antibody complexes were recovered with the protein A-Sepharose beads (Amersham Biosciences) and were analyzed by SDS-PAGE and a Fujix FLA5000 Autoimaging analyzer.
BN-PAGE.
Blue-native (BN)-PAGE was performed as described previously (7). Briefly, the organelle fraction containing 35S-Pex5-imported peroxisomes was solubilized with 1% digitonin in 5 mM HEPES-KOH, 100 mM NaCl, 1 mM DTT, 1 mM EDTA, protease inhibitor cocktail, and 1 mM PMSF for 30 min on ice and centrifuged at 100,000 × g for 15 min. The supernatant fraction was incubated with antibodies to several peroxins on ice for 30 min for a mobility shift assay (56). One-tenth volume of BN-PAGE sample buffer, 5% Coomassie brilliant blue G-250-0.5 M 6-aminocaproic acid-100 mM BisTris-HCl, pH 7.0, was added to the samples before electrophoresis. Proteins were resolved on a 4.5 to 15% polyacrylamide gradient gel with a 4% polyacrylamide stacking gel for 10 h at 100 V and 5 mA at 4°C. The cathode buffer contained 15 mM BisTris-HCl, pH 7.0, 50 mM Tricine, and 0.02% (wt/vol) Coomassie brilliant blue G-250. The anode buffer was 50 mM BisTris-HCl, pH 7.0.
Western blotting.
Western blotting analysis was done using electrophoretically transferred samplers on polyvinylidene difluoride membranes (Bio-Rad, Hercules, Calif.) with primary antibodies and second antibody, donkey anti-rabbit or mouse immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham Biosciences) (36). Antigen-antibody complexes were visualized with an ECL Western blotting detection reagent (Amersham Biosciences).
RESULTS
In vitro import of Pex5 and AOx into peroxisomes.
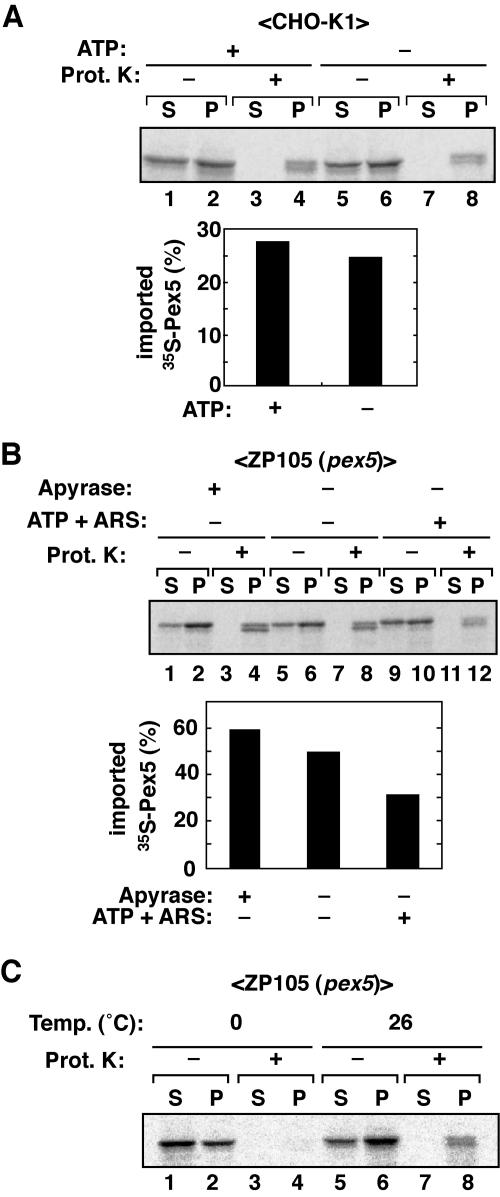
To investigate the peroxisomal protein import machinery, we first attempted to establish a cell-free peroxisomal protein import system. 35S-labeled Pex5 was synthesized using an in vitro transcription/translation system and incubated for 60 min at 26°C with the PNS fraction from CHO cell lines in the presence of 1 mM ATP and ARS. The reaction mixtures were divided into three aliquots, two of which were treated with proteinase K in the absence and presence of 1% Triton X-100. Respective samples were separated into organelle pellet and cytosolic supernatant fractions. With PNS from CHO-K1, 35S-Pex5 was detected in the organelle (P) and cytosolic (S) fractions (Fig. 1A, upper panel, lanes 2 and 3). An organellar marker, Pex14, and a cytosolic one, LDH, were detectable exclusively in P and S fractions, respectively, confirming the adequate separation of organelles and the cytosol (Fig. 1A, middle and lower panels, lanes 2 and 3). About half of 35S-Pex5 of the organelle fraction was resistant to proteinase K treatment (Fig. 1A, upper panel, lane 5, solid arrowhead), while 35S-Pex5 of the cytosolic fraction was sensitive to the digestion (lane 4). Pex14 exposing both N- and C-terminal parts to the cytosol was likewise digested by the protease, suggesting that the protease treatment was properly performed, although LDH was highly resistant to the protease (middle and lower panels, lanes 4 and 5). A partially cleaved form of protease-resistant 35S-Pex5 was detected in the organelle fraction (upper panel, lane 5, open arrowhead), presumably representing 35S-Pex5 locating in the membrane and partly accessible to the protease (36). Digestion in the presence of Triton X-100 abolished the resistance of 35S-Pex5. Together, the results indicated that 35S-Pex5 was imported to organelles, presumably peroxisomes.
FIG. 1.
In vitro Pex5 import assay. (A) An in vitro Pex5 import assay was performed for 60 min at 26°C using cell-free synthesized 35S-Pex5 and PNS fraction from wild-type CHO-K1 cells in import buffer containing 1 mM ATP plus ARS. After incubation, the reaction mixtures were mock treated (lanes 2 and 3) or treated with 90 μg/ml of proteinase K for 30 min at 0°C (lanes 4 to 7) in the absence (−) or presence (+) of 1% Triton X-100 and were separated to organelle (P) and cytosolic (S) fractions by centrifugation. Total reaction mixture (T, lane 1) and equal aliquots of S and P fractions were analyzed by SDS-PAGE using a 10% gel. 35S-Pex5 was detected by a Fujix FLA5000 autoimaging analyzer. Solid and open arrowheads indicate full-length and partially proteinase K-cleaved 35S-Pex5, respectively. Endogenous Pex14 and LDH were detected with respective antibodies. (B) In vitro import assay of 35S-Pex5 and 35S-AOx (lane 1, solid and open arrows, respectively) was performed as in panel A, using PNS fractions from wild-type (lanes 2 to 7) and mutant pex2 Z65 (lanes 8 to 13) CHO cells. Solid and open arrowheads were as in panel A. The downward open arrowhead indicates the 35S-AOx-B chain proteolytically processed 35S-AOx in peroxisomes. (C) In vitro 35S-Pex5 import assay using CHO-K1-derived PNS was performed as for panel A. The reaction mixtures were centrifuged. Organelle fractions (input lanes) were suspended with a hypotonic buffer containing 5 mM HEPES-KOH, pH 7.4, and 50 mM NaCl, and centrifuged. Pex14 and catalase were detected by immunoblotting. (D) An in vitro 35S-Pex5 import assay was likewise done with PNS fractions from CHO-K1, pex12 ZP109, pex14 ZP161, and pex7 ZPG207 cells. The input 35S-Pex5 used for the import assay was loaded in lane 1. Solid and open arrowheads are as in panel A. (E) Subcellular fractionation of rat liver. A light mitochondrial fraction from rat liver was fractionated by sucrose density gradient ultracentrifugation. Activity of catalase, a marker enzyme for peroxisome, for each fraction was determined (upper panel). Distributions of peroxisomes, mitochondria, ER, and lysosomes were also verified by Western blotting using antibodies against marker proteins: Pex14, MDH, P450 reductase (P450r), and Lamp1, respectively. The Lamp1 band is indicated by a dot. Solid arrowheads indicate peak fractions of peroxisomes (Ps) (fraction no. 4) and mitochondria (Mt) (fraction no. 17), respectively. (F) Pex5 was specifically imported into peroxisomes. 35S-Pex5 was incubated with fractions 4 (100 μg peroxisomes) and 17 (100 μg mitochondria), each supplemented with rat liver cytosol (600 μg) in the import buffer (200 μl) containing 1 mM ATP and ARS. 35S-Pex5 import was verified as for panel A.
We assessed whether the 35S-Pex5 import shown in Fig. 1A reflects the import of matrix protein by verifying the import of PTS1-type AOx, the first enzyme of the peroxisomal fatty acid β-oxidation system (28, 53). The import reaction of 35S-labeled Pex5 and AOx was performed with a CHO-K1-derived PNS fraction as for Fig. 1A. 35S-Pex5 protected from the protease digestion was in the organelle fraction, not in the cytosolic fraction, and such protease resistance of 35S-Pex5 was abolished by pretreatment with the detergent (Fig. 1B, lanes 1 to 7), thereby reproducing the results shown in Fig. 1A. 35S-AOx was similarly detected in the organelle and cytosolic fractions (Fig. 1B, lanes 2 and 3). Most of 35S-AOx in the organelle fraction was resistant to proteinase K treatment, while 35S-AOx of the cytosolic fraction was digested (lanes 4 and 5). The 53-kDa 35S-AOx-B chain, a cleaved product of AOx-A, was discernible in the organelle pellet before and after the proteinase K treatment (lanes 3 and 5, downward open arrowheads), reflecting the intraperoxisomal conversion of AOx (27, 28). Treatment of the reaction mixture with Triton X-100 prior to protease digestion abolished the resistance of 35S-AOx (lanes 6 and 7). Together, the results indicated that 35S-Pex5 and 35S-AOx were imported to organelles, more likely to peroxisomes as in the 35S-AOx import reported earlier (27, 28). 35S-Pex5 and 35S-AOx were likewise detected in the organelle fraction of PNS from pex2 Z65 cells (Fig. 1B, lanes 8 and 9) but were not resistant to proteinase K treatment (lanes 10 to 13), where both proteins in the cytosol fraction were digested (lanes 8 to 13), demonstrating that 35S-Pex5 and 35S-AOx were not imported to peroxisome membrane remnant so-called ghosts. These results were consistent with the phenotype with respect to the PTS1 protein import of wild-type CHO-K1 and the peroxisome-deficient cell mutants, including Z65 impaired in matrix protein import (53).
Furthermore, upon treatment of 35S-Pex5-imported organelle fractions with a hypotonic buffer, protease-resistant 35S-Pex5 remained in the organelle fraction as Pex14 (Fig. 1C), while catalase, a matrix enzyme, was fully released from the organelles. These data suggested that imported 35S-Pex5 associated with peroxisomal membranes. With the PNS fraction from CHO pex12 ZP109 cells, no 35S-Pex5 was detectable after proteinase K digestion (Fig. 1D, lanes 2 to 5), while two apparently full-length forms as well as partially cleaved forms of protease-resistant 35S-Pex5 were more distinct in the organelle fraction of CHO-K1 (lane 5, solid and open arrowheads, respectively). Furthermore, in contrast to CHO-K1 and these pex2 and pex12 mutants, most 35S-Pex5 was found in the cytosolic fraction and only a little was detected in the organelle fraction from CHO pex14 ZP161 (lanes 2 and 3), in good agreement with the morphological results previously reported (36). 35S-Pex5 in the assay mixture was completely digested with proteinase K (lanes 4 and 5). Together, 35S-Pex5 was associated with peroxisome membrane ghosts harboring Pex14 of mutants such as pex2 Z65 and pex12 ZP109 but barely with those from Pex14-deficient ZP161. In the PEX7-defective CHO mutant ZPG207 impaired only in PTS2 protein import, 35S-Pex5 was imported as efficiently as in CHO-K1 (Fig. 1D, lanes 2 to 5), consistent with the normal import of PTS1 proteins in such mutants (14, 30).
Next, to corroborate peroxisome-specific import of 35S-Pex5, we performed a Pex5 in vitro import assay using subcellular fractions of rat liver. The light-mitochondrial fraction was subjected to sucrose density gradient centrifugation. The gradient was then fractionated into 35 tubes and assayed for the distributions of Pex14 and catalase (peroxisomes), MDH (mitochondria), cytochrome P450 reductase (endoplasmic reticulum [ER]), and Lamp1 (lysosomes). Pex14 and catalase activities were detected with a peak in fraction 4, nearly free from mitochondria, ER, and lysosomes, which were highly enriched in fractions 16 to 18, 24 to 26, and 17 to 19, respectively (Fig. 1E). The 35S-Pex5 import assay was done using fractions 4 and 17, as in Fig. 1A. Protease-resistant 35S-Pex5 was detected in fraction 4 but not in fraction 17 (Fig. 1F), thereby demonstrating that 35S-Pex5 was imported specifically to peroxisomes.
Pex5 is imported into peroxisomes in an ATP-independent manner.
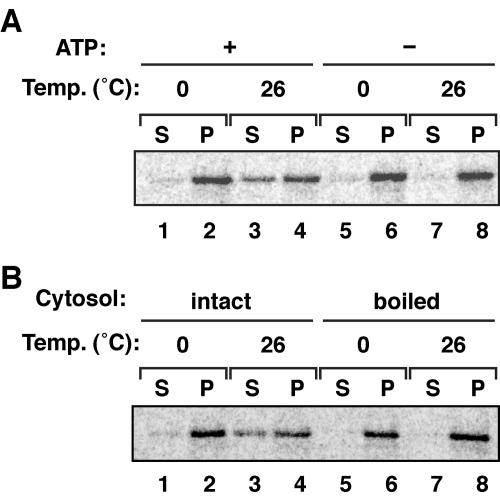
To assess whether ATP is required for Pex5 import, we performed 35S-Pex5 import assays in the presence or absence of ATP. 35S-Pex5 was incubated with CHO-K1-derived PNS that had been supplemented with 1 mM ATP and ARS as in Fig. 1A or pretreated with apyrase to deplete the endogenous ATP. Under both conditions, nearly the same amount of 35S-Pex5 was detected in the organelle fraction (Fig. 2A, upper panel, lanes 2 and 6), most of which was protected from proteinase K treatment, as two distinct bands in slightly smaller amounts with the apyrase-treated PNS (Fig. 2A, upper panel, lanes 4 and 8, and lower panel). Thus, it is likely that ATP is not required for Pex5 import. To further verify this finding, we performed the Pex5 import assay using a PEX5-defective CHO cell mutant. To avoid any effect of endogenous Pex5 in CHO-K1, we used a CHO pex5 mutant, ZP105, showing a barely detectable level of Pex5 (36). 35S-Pex5 import assays were done using PNS fraction from ZP105 with or without supplementation of 1 mM ATP plus ARS or after pretreatment with apyrase. As the ATP level was differentially lowered, 35S-Pex5 was more discernible in the organelle fraction (Fig. 2B, upper panel, lanes 2, 6, and 10) and concomitantly more resistant to protease digestion (lower panel, lanes 4, 8, and 12), suggesting that 35S-Pex5 accumulated in peroxisomes under the ATP-lowering conditions. The results obtained with ZP105 were apparently contrary to those using CHO-K1. We interpreted these findings to mean that 35S-Pex5 was imported into peroxisomes in CHO-K1 and peroxisome remnants in ZP105 in the same rate in an ATP-independent manner, where import of 35S-Pex5 was partially inhibited by preexisting endogenous Pex5 in CHO-K1 on peroxisomal membranes owing to the ATP depletion. Therefore, it is most likely that ATP is required for the Pex5 export step from peroxisomes.
FIG. 2.
ATP is not required for Pex5 import. (A) An in vitro Pex5 import assay was performed in the presence or the absence of ATP. Upper panel, 35S-Pex5 was incubated with PNS from CHO-K1 in import buffer containing 1 mM ATP plus ARS at 26°C for 60 min (lanes 1 to 4). Imported 35S-Pex5 was assessed as described in the legend to Fig. 1A. The 35S-Pex5 import assay was likewise done except for using PNS that had been pretreated with 5 U/ml of apyrase at 26°C for 10 min for ATP depletion (lanes 5 to 8). Lower panel, 35S-Pex5 in all fractions was quantitated, and the imported 35S-Pex5 (lanes 4 and 8) was represented as percentages of the total (S + P, 100%). (B) Upper panel, in vitro 35S-Pex5 import was verified at 26°C for 60 min using PNS from PEX5-deficient ZP105 under three different ATP conditions. PNS was treated with 5 U/ml of apyrase at 26°C for 30 min (lanes 1 to 4) or with no addition (lanes 5 to 8) and supplemented with 1 mM ATP plus ARS (lanes 9 to 12). Lower panel, the imported 35S-Pex5 was quantitated as for panel A. (C) Temperature dependence of Pex5 import. 35S-Pex5 was incubated with PNS from ZP105 at 0°C (lanes 1 to 4) or 26°C (lanes 5 to 8) for 60 min in import buffer with 1 mM ATP plus ARS. Pex5 import was assessed as for Fig. 1A.
Next, we examined temperature dependence of 35S-Pex5 import using PNS from ZP105. Upon incubation at 0°C, 35S-Pex5 was detected in the organelle fraction in amounts less than that at 26°C (Fig. 2C, lanes 1, 2, 5, and 6). However, none of the organelle-associated 35S-Pex5 was protected from protease digestion, in contrast to the protease-resistant 35S-Pex5 after the import reaction at 26°C (lanes 4 and 8), strongly suggesting that Pex5 import was dependent on temperature, interaction with its partners, and translocation using thermodynamic energy.
35S-Pex5 is imported into peroxisome ghosts in PEX1-, PEX6-, and PEX26-defective mutants.
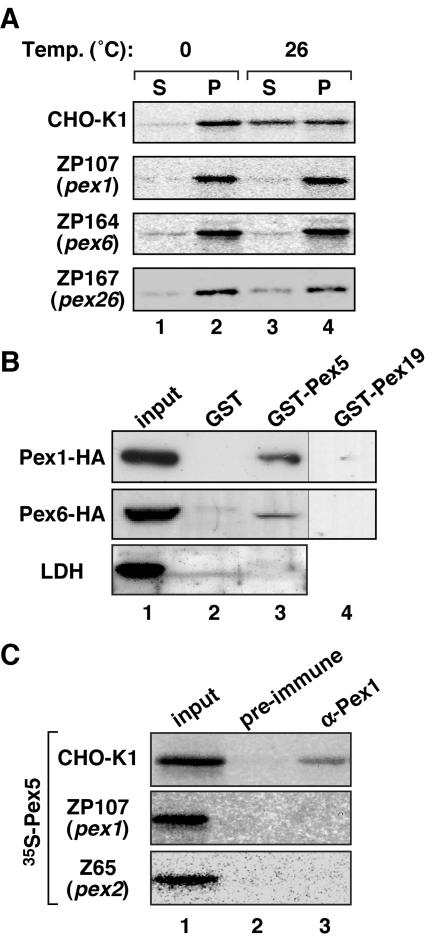
Impairment of Pex1 and Pex6 of the AAA ATPase family (26, 40, 42, 47, 52, 57) and the recruiter Pex26 of the Pex1-Pex6 complexes (24, 25) causes defects in matrix protein import. We investigated whether these peroxins are involved in Pex5 import. A 35S-Pex5 import reaction was carried out using PNS fractions from CHO mutants, peroxisome ghost-positive pex1 ZP107 (13, 24, 47), pex6 ZP164 (21), and pex26 ZP167 (13, 24, 47). About half of the input 35S-Pex5 was detected in the organelle fraction, showing proteinase K resistance (Fig. 3A, lanes 1 to 4) but less than that with CHO-K1 and yet indicative of 35S-Pex5 import into such peroxisome ghosts. Clear separation of Pex14 and LDH and their sensitivity to the protease treatment indicated that the assays were well controlled as for Fig. 1A. Hence, it is conceivable that endogenous Pex5 in these mutant cells partly blocked the 35S-Pex5 import. In human fibroblasts from PBD patients with PEX1-, PEX6-, and PEX26-defective CG1 (CG-E), CG4 (CG-C), and CG8 (CG-A), the Pex5 level was significantly reduced (data not shown) (8), implying that the effect of endogenous Pex5 on 35S-Pex5 import might be lower than that with CHO pex5 mutants. Accordingly, we likewise performed the 35S-Pex5 import assays using fibroblasts of CG1, CG4, and CG8. Upon incubation with PNS from these three types of fibroblasts, 35S-Pex5 in membrane fractions was partly protease resistant, nearly to the same or a higher extent as with fibroblasts from a normal control (Fig. 3B). To verify both types of findings, we carried out a competition assay using bacterially expressed Pex5, termed rPex5. A 35S-Pex5 import reaction was carried out using the CHO-K1-derived PNS fraction in the presence of rPex5. As the amount of rPex5 increased, the amount of protease-resistant 35S-Pex5 in the organelle fraction concomitantly decreased (Fig. 3C), strongly suggesting that rPex5 competitively inhibited the import of 35S-Pex5. Taken together, Pex1, Pex6, and Pex26 are dispensable for translocation of Pex5 into peroxisomes.
FIG. 3.
Import of Pex5 into peroxisomal ghosts in pex mutant cells. (A) Pex5 import assay was performed using several pex mutants defective in matrix protein import. 35S-Pex5 was incubated with PNS from the CHO pex1 mutant ZP107, pex6 ZP164, or pex26 ZP167 in the presence of 1 mM ATP plus ARS. Imported 35S-Pex5 was verified as described in the legend to Fig. 1A. (B) The 35S-Pex5 import assay was likewise carried out using PNS of human fibroblasts from a normal control and patients with PBD of PEX1-defective CG1 (CG-E), PEX6-deficient CG4 (CG-C), or PEX26-impaired CG8 (CG-A). (C) The 35S-Pex5 import assay was performed as for panel A, using CHO-K1-derived PNS in the presence of purified recombinant Pex5 (rPex5) as indicated. Only the proteinase K-treated organelle fractions are shown.
Pex5 is exported from peroxisomes in a cell-free system.
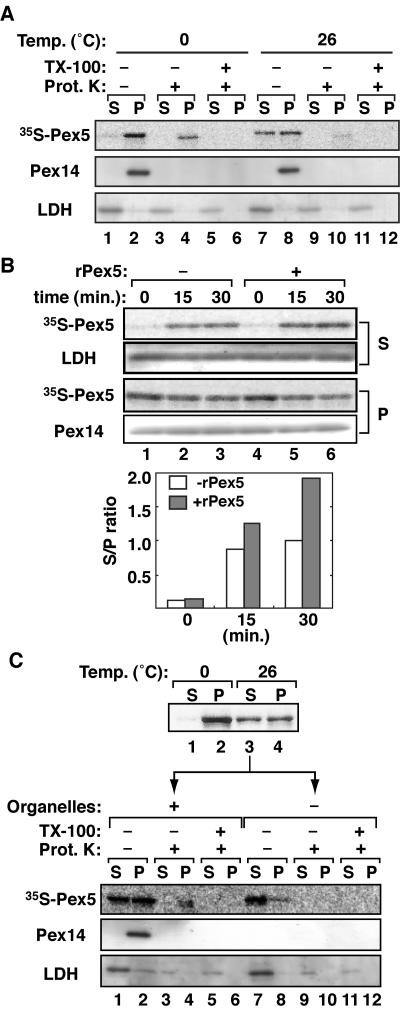
Next, we investigated whether Pex5 exits from peroxisomes. We first constructed an in vitro 35S-Pex5 export assay system. The organelle fraction of PNS of CHO-K1 cells that had been incubated with 35S-Pex5 as for Fig. 1A was suspended in the export buffer (see Materials and Methods) containing CHO-K1-derived cytosol and 1 mM ATP plus ARS. The reaction mixture was incubated at 26°C or 0°C for 30 min and was then divided into three aliquots, two of which were treated with proteinase K in the absence and presence of 1% Triton X-100. 35S-Pex5 was barely detected in cytosolic fraction at 0°C (Fig. 4A, upper panel, lane 1) and before the incubation (data not shown). After the reaction at 26°C, 35S-Pex5 was increased in the cytosolic fraction (lane 7), while the amount of 35S-Pex5 in the organelle fraction was decreased with a concomitantly reduced level of proteinase K-resistant 35S-Pex5, which became readily sensitive to the protease digestion in the presence of Triton X-100 (lanes 2, 4, 8, 10, and 12), strongly suggesting that the imported 35S-Pex5 was exported from peroxisomes. Organelles and the cytosol fraction were well separated, as assessed by immunoblotting of Pex14 and LDH (middle and lower panels). 35S-Pex5 in the cytosolic fraction and Pex14 of organelle fraction were sensitive to proteinase K (lanes 7 and 8), hence confirming the Pex5 export. Moreover, 35S-Pex5 was exported from peroxisomes in a time-dependent manner, as demonstrated in the time course experiment where 35S-Pex5 was exported at a nearly maximal level at 15 min (Fig. 4B, upper panel, lanes 1 to 3). This finding implied that the export and import of 35S-Pex5 reached equilibrium within 15 min. To verify this, export assays were performed in the presence of 50 μg/ml of bacterially expressed Pex5 to inhibit reimport of the exported 35S-Pex5. The amount of 35S-Pex5 exported to the cytosol was elevated about twofold at 30 min compared to that in the absence of recombinant Pex5 (Fig. 4B, upper panel, lanes 4 to 6, and lower panel). The level of marker proteins, Pex14 and LDH, in P and S fractions was unchanged during the assays. These results suggested that 35S-Pex5 was exported from peroxisomes in a time-dependent manner and then reimported to peroxisomes.
FIG. 4.
In vitro export of Pex5. (A) 35S-Pex5 import was done with PNS from CHO-K1 in 1 mM ATP plus ARS, as for Fig. 1A. The organelle fraction was centrifuged and resuspended with the cytosolic fraction in export buffer containing 1 mM ATP and ARS. An export reaction was carried out for 30 min on ice (lanes 1 to 6) or at 26°C (lanes 7 to 12). The reaction mixtures were mock-treated (−) or treated with 90 μg/ml of proteinase K (+) and were centrifuged to organelle (P) and cytosol (S) fractions. 35S-Pex5 in S and P fractions was detected by SDS-PAGE and a Fujix FLA5000 autoimaging analyzer (upper panel). Endogenous Pex14 and LDH were detected with respective antibodies (middle and lower panels). (B) Kinetics of Pex5 export. Upper panels, 35S-Pex5 export assays were carried out as for panel A for the indicated time in the absence (−) (lanes 1 to 3) or presence (+) (lanes 4 to 6) of an excess amount (50 μg/ml) of recombinant Pex5 (rPex5). Lower panel, 35S-Pex5 bands were quantitated, where the recovery of 35S-Pex5 in S plus P fractions at each time point was within 90 to 110% of the input. The ratio of 35S-Pex5 in S to P was plotted in the absence (open bars) or presence (solid bars) of rPex5. (C) Reimport of Pex5. A 35S-Pex5 export reaction was conducted as for panel A (upper panel). 35S-Pex5 exported from peroxisomes in the cytosolic fraction (upper panel, lane 3) was verified for import using PNS (lanes 1 to 6) or cytosolic fraction (lanes 7 to 12) from CHO-K1, as for Fig. 1A.
Next, to verify the reimport to peroxisomes of the exported Pex5, we performed the reimport assay using exported 35S-Pex5. The cytosol fraction containing exported 35S-Pex5 after the 30-min export reaction (Fig. 4C, upper panel, lane 3) was incubated with PNS or the cytosol from CHO-K1, and import of 35S-Pex5 was assessed by proteinase K treatment (lower panel). Using the PNS fraction for the second import reaction, about two-thirds of the exported 35S-Pex5 in the first export assay was recovered in the organelle fraction and partly protected from protease digestion (Fig. 4C, lower panel, lanes 2 and 4), indicating that exported 35S-Pex5 was reimported into peroxisomes. In contrast, with the CHO-K1-derived cytosol, the exported 35S-Pex5 was barely sedimented and was completely digested with proteinase K (lanes 7 to 12). These data demonstrated that Pex5 was imported into the peroxisome at least twice, evidently being translocated through the peroxisomal membranes at least three times.
Pex5 is exported from peroxisomes in an ATP- and cytosol-dependent manner.
As found in Fig. 2, ATP is apparently essential for the export, but not for import, of Pex5. To verify the ATP requirement of Pex5 export, we carried out Pex5 export assays with the export buffer containing the cytosolic fraction and 1 mM ATP plus ARS or apyrase-pretreated cytosol. In the presence of ATP plus ARS, 35S-Pex5 was partly detected in the cytosol fraction with a concomitant decrease of 35S-Pex5 in the organelle fraction (Fig. 5A, lanes 1 to 4), while no 35S-Pex5 was detected in the cytosol fraction pretreated with apyrase (lanes 5 to 8). The similar export defect was also observed with 10 mM AMP-PNP, a nonhydrolyzable ATP analogue (data not shown). Together, these results strongly suggested that Pex5 was exported from peroxisomes using ATP hydrolysis energy. Next, we investigated whether the cytosol fraction was required for Pex5 export. A 35S-Pex5 export reaction was carried out using the cytosolic fraction with or without prior boiling at 95°C for 2 min. No 35S-Pex5 was exported with the heat-treated cytosol (Fig. 5B, lanes 5 to 8), while 35S-Pex5 was efficiently exported with the intact cytosol (lanes 1 to 4), hence implying that protein factors in the cytosol are involved in the Pex5 export.
FIG. 5.
Pex5 export requires ATP and cytosol. (A) The 35S-Pex5 export assay was performed for 30 min on ice or at 26°C in the presence of 1 mM ATP and ARS (lanes 1 to 4) as for Fig. 4A or after pretreatment of the cytosolic fraction with 5 U/ml of apyrase at 26°C for 10 min (lanes 5 to 8). (B) The 35S-Pex5 export assay was carried out with the reaction mixture containing the cytosol fraction as for panel A (lanes 1 to 4) or with boiled cytosol.
Pex1 and Pex6 are involved in Pex5 export.
Based on the findings shown in Fig. 3 and 5, including Pex5 export in an ATP- and cytosolic protein- dependent manner, it is conceivable that the AAA-ATPase peroxins, Pex1 and Pex6, are involved in Pex5 export. Inactivating mutation of such peroxins may result in failure of Pex5 export, hence inducing the defect in matrix protein import, i.e., the phenotype of pex1 and pex6 cell mutants including CHO ZP107 and ZP164 as well as fibroblasts from PBD patients.
To determine whether Pex5 is exported from peroxisome ghosts in ZP107 and ZP164, a Pex5 export assay was performed using PNS from these cell mutants. 35S-Pex5 was first incubated with PNS from ZP107, ZP164, and CHO-K1 in the import buffer with 1 mM ATP plus ARS. Organelle fractions were isolated by centrifugation, resuspended in export buffer containing 1 mM ATP, ARS, and the cytosol of respective cell types, and incubated as for Fig. 5A. After a 30-min reaction, 35S-Pex5 was barely detectable in cytosolic fractions of ZP107 and ZP164, indicative of impaired export of Pex5, in contrast to efficient export in the case of CHO-K1 (Fig. 6A). Likewise, 35S-Pex5 export was significantly affected when the assay was carried out using Pex26-defective ZP167 (Fig. 6A). Together, these data strongly suggested that Pex1 and Pex6 as well as their complex receptor Pex26 are involved in the Pex5 export. Furthermore, we investigated whether Pex5 interacts with Pex1 and Pex6. Cell lysates of CHO-K1 expressing Pex1-HA or Pex6-HA were incubated with bacterially expressed GST, GST-Pex5, and a control, GST-Pex19. After thorough washing, proteins bound to GST and its fusion proteins were analyzed by SDS-PAGE and immunoblotting. Pex1-HA and Pex6-HA were specifically detected in Pex5-bound fractions, not in the fractions of GST and GST-Pex19 (Fig. 6B, upper and middle panels), thereby indicating that Pex5 interacted with Pex1 and Pex6. Only small amounts of Pex1 and Pex6 were recovered in Pex5-bound fractions, implying that the interaction was weak or transient. To assess the specificity of interaction of Pex1 and Pex6 with Pex5, we examined an abundant cytosolic protein, LDH, in the fractions bound to GST and GST-Pex5. LDH was barely detectable (Fig. 6B, lower panel), thereby confirming the specific binding of Pex1 and Pex6 to Pex5. We also verified if Pex1 and Pex6 interact with Pex5 on peroxisomes. After the 35S-Pex5 import reaction using CHO-K1-, ZP107-, and pex2 Z65-derived PNS, organelle fractions were isolated and solubilized with 1% digitonin. 35S-Pex5 was specifically coimmunoprecipitated with Pex1 from CHO-K1 (Fig. 6C), while 35S-Pex5 was not detectable from Pex1-defective ZP107, thereby suggesting that Pex1 interacted with Pex5 in the export step from peroxisomes. Furthermore, no 35S-Pex5 was coimmunoprecipitated from Pex2-deficient Z65, suggesting that Pex1 failed to interact with Pex5 on peroxisomal ghosts lacking Pex2. The finding implies that Pex2, a RING peroxin, functions upstream of Pex1. However, our antibody raised against the N-terminal peptide of Pex6 (48) was not potent enough to immunoprecipitate endogenous Pex6 (data not shown).
FIG. 6.
Pex1, Pex6, and Pex26 are involved in Pex5 export from peroxisomes. (A) The Pex5 export assay was performed as for Fig. 5, using PNS and cytosolic fractions from CHO-K1, pex1 ZP107, pex6 ZP164, or pex26 ZP167 cells. (B) Pex5 interacts with AAA ATPase family peroxins. GST-fused Pex5, GST-Pex19, and GST were incubated with lysates of CHO-K1 cells expressing Pex1-HA or Pex6-HA. Proteins bound to GST, GST-Pex19, or GST-Pex5 on glutathione-Sepharose were separated by SDS-PAGE and probed with anti-HA antibody. GST- and GST-Pex5-bound fractions from CHO-K1 were also probed with anti-LDH antibody. Input, one-twentieth of the lysate used for GST-pull-down assay. (C) Pex1 interacts with Pex5 on peroxisomes. The 35S-Pex5 import reaction was carried out with PNS from CHO-K1, ZP107, or Z65 in the presence of 1 mM ATP plus ARS, as for Fig. 1A. The organelle fraction was sedimented, solubilized with 1% digitonin, and subjected to immunoprecipitation with preimmune serum (lane 2) or anti-Pex1 antibody (lane 3). The immunoprecipitates were analyzed by SDS-PAGE and autoradiography. Input was loaded as for panel B.
Pex5 is translocated into two distinct import complexes on peroxisomes.
Pex5 has been thought to translocate through the potential matrix protein import machinery on peroxisomes, comprising Pex2, Pex10, Pex12, Pex13, and Pex14, via initial docking to Pex14 and recycling back to the cytosol at the final step (36, 38). We attempted to detect such import machinery complexes using the cell-free system for Pex5 import and export established in the present work. 35S-Pex5 import was done using PNS from CHO-K1, pex5 ZP105, and pex14 ZP161 in 1 mM ATP plus ARS. Organelle fractions were isolated, solubilized, and analyzed by BN-PAGE and autoradiography. In PNS from CHO-K1 and ZP105, 35S-Pex5 was detected with two distinct migrations: one with a molecular mass of ∼800 kDa and the other with ∼500 kDa (Fig. 7A, lanes 1 and 2). In contrast, in PNS from Pex14-deficient ZP161, none of these complexes containing 35S-Pex5 was discernible (lane 3), thereby suggesting that Pex14 is essential for Pex5 translocation to the high-molecular-mass complexes. 35S-Pex5-containing complexes were also examined using pex2 and pex12 cell mutants, Z65 and ZP109, respectively (Fig. 7A, lanes 4 and 5). Interestingly, 35S-Pex5 failed to translocate to the 500-kDa complexes, suggesting that the 800-kDa complexes were involved in the 35S-Pex5 transport upstream of the 500-kDa complexes, and Pex2 and Pex12 were required for 35S-Pex5 translocation from the 800-kDa complex to the 500-kDa one. Next, to verify whether AAA ATPase peroxins, such as Pex1, are involved in assembly of these two complexes, 35S-Pex5 translocation was likewise examined using PNS fractions of fibroblasts from a normal control and a PEX1-defective PBD patient. Two types of 35S-Pex5-containing complexes with molecular masses of ∼800 kDa and ∼500 kDa were similarly identified (Fig. 7B), hence indicating that Pex1 deficiency did not affect Pex5 translocation to or assembly of these two complexes. Furthermore, we investigated the ATP requirement for assembly of these 35S-Pex5-containing complexes, using Pex5-deficient ZP105 cells. 35S-Pex5-loaded, ∼800-kDa and ∼500-kDa complexes were detected at a significantly higher level under the condition of ATP-depletion with apyrase than with ATP supplementing with 1 mM ATP plus ARS (Fig. 7C). We interpreted this finding to mean that 35S-Pex5-containing complexes are more stable in the absence of ATP. 35S-Pex5 is readily released from the import/translocation complexes in an ATP-dependent manner.
FIG. 7.
Pex5 translocation machinery on peroxisomal membranes. (A) Pex5-containing complexes on peroxisomal membrane were analyzed by BN-PAGE. The 35S-Pex5 import reaction was carried out with PNS factions each from CHO-K1, pex5 ZP105, pex14 ZP161, pex2 Z65, and pex12 ZP109, as for Fig. 1A. Organelle membrane fractions were isolated, solubilized with 1% digitonin, and analyzed by BN-PAGE. 35S-Pex5 was detected by a Fujix autoimaging analyzer. The figure represents a composite of two separate experiments. Arrowheads indicate two distinct complexes containing 35S-Pex5. Molecular mass markers are on the left. (B) AAA ATPase peroxin, Pex1, is not involved in the two types of import complexes. 35S-Pex5 import was done using PNS from fibroblasts from a normal control (lane 1) and a PEX1-defective PBD patient of CG1 (CG-E) (lane 2), as for Fig. 3B. Organelle fractions were analyzed as for panel A. (C) Assembly of the two import complexes does not require ATP. 35S-Pex5 import was done with PNS from ZP105 in the presence of 1 mM ATP plus ARS (lane 1) or after preincubation with apyrase (lane 2), as for Fig. 2A. Organelle fractions were analyzed as for panel A. (D) Antibody shift assays using antibodies against Pex14 and Pex2 were performed. 35S-Pex5 import was done as for panel A. Solubilized organelle fractions of CHO-K1, Z65, and ZP109 were incubated with rabbit preimmune serum (lanes 1, 5, and 8) or antibodies to Pex14 (lanes 2, 6, and 9) or Pex2 (lanes 3, 7, and 10) or the mixture of antibodies to Pex14 and Pex2 (lane 4) and were analyzed as for panel A. Arrowheads indicate positions of 35S-Pex5-containing complexes.
Next, to identify the constituents of these high-mass complexes, we performed antibody shift assays using antibodies against several peroxins. The 35S-Pex5 import reaction was carried out as for Fig. 7A, using PNS from CHO-K1, pex2 Z65, and pex12 ZP109, and analyzed by BN-PAGE. Decoration with anti-Pex14 antibody shifted only the 800-kDa complexes to a higher-molecular-mass region (Fig. 7D, lanes 2, 6, and 9). With anti-Pex2 antibody, the 500-kDa complexes were apparently shifted to one with a mass larger than the 800-kDa complex in the case of CHO-K1 (lane 3). Furthermore, upon decorating with the mixture of anti-Pex14 and anti-Pex2 antibodies, both of the 800-kDa and 500-kDa complexes were shifted to the higher-molecular-mass region (lane 4). Together, these results suggested that the 800-kDa complexes contained Pex14 and the 500-kDa complexes contained Pex2 as a component, in addition to 35S-Pex5. Furthermore, the mobilities of the 800-kDa complexes of Z65 and ZP109 were likewise shifted upon treatment with anti-Pex14 antibody but not with anti-Pex2 antibody (lanes 6, 7, 9, and 10). The 500-kDa complexes were not detectable in the case of Z65 and ZP109 cells. Pex12 may be required for assembly of 500-kDa complexes, as evidently assessed for Pex2 using Pex2-deficient Z65 cells. It is also equally plausible that 35S-Pex5 was not translocated to such 500-kDa complexes, if any, in ZP109 lacking Pex12.
DISCUSSION
According to the PTS1 receptor recycling model (38, 43, 45), cargo-loaded Pex5 targets peroxisomes, translocates across the peroxisomal membrane, unloads the cargoes, and finally exits back to the cytosol. This protein import process has been thought to require several peroxisomal peroxins, such as Pex14, Pex13, Pex2, Pex10, and Pex12 (2, 3, 4, 10, 15, 32, 36, 38, 44, 50). Defining the components involved at each step of the whole process remains elusive.
In the present work, we established a cell-free system for Pex5 translocation. By making use of the wild type and several pex mutants of CHO cells, we investigated the dynamism of Pex5, including its import to and export from peroxisomes, as well as Pex5 translocation steps through potential protein import machinery complexes on peroxisomal membranes. 35S-labeled Pex5 was specifically imported into peroxisomes in PNS from CHO-K1 cells as well as those isolated from rat liver. 35S-Pex5 was not imported to peroxisome ghosts of Pex14-deficient ZP161, demonstrating that Pex14 is the initial Pex5-docking peroxin on peroxisomes, consistent with our earlier morphological and biochemical findings (36). We also showed here that ATP was not required for the import of Pex5. Nevertheless, Pex5 import was temperature sensitive, implying that Pex5 translocates across the peroxisomal membrane driven by thermodynamic energy. Such a property in protein import is distinct from that in mitochondria and the ER, where proteins are imported in an ATP-dependent manner (31, 41). We also developed an in vitro Pex5 export system (Fig. 4). Using this system, we evidently demonstrated that Pex5 imported to peroxisomes was exported back to the cytosol in an ATP-dependent manner, in sharp contrast to the ATP independence of its import, in good agreement with the findings by Oliveira et al. (34). Moreover, we showed that Pex5 recycled multiple times between peroxisomes and the cytosol. It is noteworthy that 35S-Pex5 was efficiently exported from peroxisomes in the presence of the excess amount of Pex5 in the cytosol, implying that Pex5 is exported independently on its concentration gradient, consistent with the requirement of ATP. The Pex5 export required heat-labile cytosol fraction, implying that protein factors are involved in this step. The peroxins mostly localized in the cytosol, including Pex1, Pex6, and Pex19, could be the candidates for such factors. However, 35S-Pex5 was efficiently exported from peroxisomes of CHO-K1 when cytosol fractions from the mutant cells, pex1 ZP107, pex6 ZP164, and pex19 ZP119, were used (data not shown), suggesting that these peroxins are not responsible for the cytosol dependence of Pex5 export. Alternatively, the organelle-associated forms of these peroxins may function in Pex5 export. Thus, unknown cytosolic factors essential for Pex5 export remain to be identified.
We also showed that Pex1 and Pex6 were essential for Pex5 export, not its import, consistent with the ATP dependence of the export step. This is in good agreement with the findings by epistatic analysis (5). Furthermore, in our preliminary data, only a small amount of 35S-Pex5 could be exported from peroxisome ghosts of pex1 ZP107 and pex6 ZP164, only by addition of the cytosol fraction from CHO-K1 (data not shown). The export of 35S-Pex5 from peroxisomes of CHO-K1 was not affected even when the cytosol fraction from ZP107 and ZP164 was used in the export reaction (data not shown), strongly suggesting that organelle-associated Pex1 and Pex6 play a major role in Pex5 export. Furthermore, we found that Pex1 and Pex6 bind to Pex5 (Fig. 6). Therefore, it is conceivable that Pex1 and Pex6 pull out Pex5 from peroxisome membranes in an ATP-dependent manner. Such pulling-out activity is similar to that of other members of the AAA ATPase family, such as P97, mitochondrial AAA ATPase protease, and bacterial FtsH, all tightly linked to substrate degradation (20, 23, 58). Distinct from these AAA proteins, Pex5 is destined for recycling instead of degradation. It is noteworthy that the involvement of Pex1 and Pex6 in Pex5 dislocation to the cytosol in Saccharomyces cerevisiae was very recently reported (39), while this paper was in review.
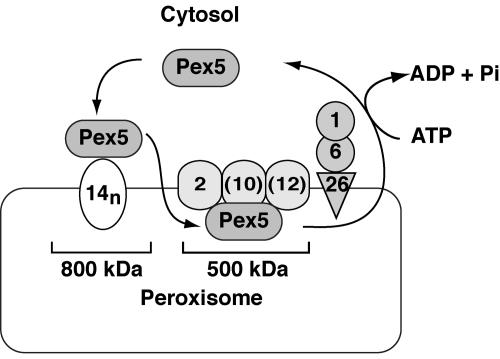
We identified by BN-PAGE two novel and distinct Pex5-containing import complexes with molecular masses of about 800 kDa and 500 kDa in peroxisomal membranes, as shown in Fig. 7 and 8. The 800-kDa complexes contained Pex14, and the 500-kDa one contained Pex2. Pex14 has been shown to function as the initial docking site of Pex5 (2, 10, 36, 38). Thus, the 800-kDa complexes are possibly the initial docking machinery for peroxisomal matrix proteins. After targeting to peroxisomes via 800-kDa complexes, Pex5 appears to translocate to the 500-kDa, Pex2-containing complexes. Three RING peroxins, Pex2, Pex10, and Pex12, interact with each other and form RING core complexes (1) (K. Okumoto and Y. Fujiki, unpublished data). Hence, it is likely that 500-kDa complexes also contain Pex12 and Pex10. Consistent with this, Pex5 indeed translocated only to the 800-kDa complexes, but not to the 500-kDa complexes, in pex2 Z65 and pex12 ZP109. On the other hand, these two complexes are assembled on peroxisomal ghosts in pex1 fibroblasts from CG1 PBD patients, suggesting that these complexes do not contain the AAA ATPase peroxins. Therefore, it is less likely that the AAA ATPase peroxins affect the assembly of such complexes. Nevertheless, Pex1 was evidently demonstrated to interact with Pex5 downstream of Pex2 on peroxisomes (Fig. 6). Therefore, we interpret these findings to mean that the interaction between Pex5 and the AAA ATPase peroxins may be transient, so that such complexes could not be detected by BN-PAGE. Taken together, Pex5 targets Pex14 in the 800-kDa import machinery and then translocates to the 500-kDa complexes, finally exiting from peroxisomes, possibly mediated by AAA ATPase peroxins. Such mobility shifts were not discernible with our antibody raised against Pex13 (48) (data not shown), implying that Pex13, if present in these complexes, was not readily recognized by the antibody. Therefore, Pex13 was not included in the 800-kDa complexes (Fig. 8). However, Pex14 and Pex13 have recently been reported to be involved in the initial docking complexes of Pex5 (2, 10, 36, 38). Thus, it is likely that Pex13 is present in the 800-kDa complexes. Meanwhile, Pex14 was detectable in oligomeric forms in vitro (35) (R. Itoh and Y. Fujiki, unpublished data) as well as in vivo (R. Itoh and Y. Fujiki, unpublished data). Thus, it is possible that Pex14 is in an oligomeric form in the 800-kDa complexes (Fig. 8).
FIG. 8.
A schematic model for the matrix protein import reaction mediated by the shuttling receptor Pex5 and protein import machinery on the peroxisomal membrane. Pex5 initially targets to an 800-kDa complex containing Pex14 and then translocates to a 500-kDa complex comprising RING peroxins. At the terminal step of the protein import reaction, Pex1 and Pex6 catalyze the export of Pex5. The numbers indicate peroxins, where those in parenthesis are introduced from the published findings. In the 800-kDa complexes, 14n designates the Pex14 oligomer.
As demonstrated in this report for the first time, BN-PAGE is a potential method for investigating the protein import machinery on peroxisomes, as used for mitochondrial translocase of the outer membrane (TOM) complexes (7, 56). Investigations using combinations of several detergents and cross-linkers with BN-PAGE would elucidate further detailed mechanisms underlying peroxisomal protein import, including the issue with regard to a step of cargo unloading from Pex5.
Acknowledgments
We thank K. Okumoto for construction of the His6-Flag-PEX5L plasmid, U. K. Rhee and C. M. Koeller for technical advice on BN-PAGE, M. Nishi for preparing the figures, and many members of the Fujiki laboratory for discussion.
This work was supported in part by a SORST grant (to Y.F.) from the Science and Technology Corporation of Japan; Grants-in-Aid for Scientific Research (12308033, 12557017, 12206069, 13206060, 14037253, 15032242, and 15207014 to Y.F.), a grant from the National Project on Protein Structural and Functional Analyses (to Y.F.) and The 21st Century COE Program from The Ministry of Education, Culture, Sports, Science, and Technology of Japan; and a grant from the Japan Foundation for Applied Enzymology. N.M. is a Research Fellow of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Agne, B., N. M. Meindl, K. Niederhoff, H. Einwaechter, P. Rehling, A. Sickmann, H. E. Meyer, W. Girzalsky, and W.-H. Kunau. 2003. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol. Cell 11:635-646. [DOI] [PubMed] [Google Scholar]
- 2.Albertini, M., P. Rehling, R. Erdmann, W. Girzalsky, J. A. K. W. Kiel, M. Veenhuis, and W.-H. Kunau. 1997. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell 89:83-92. [DOI] [PubMed] [Google Scholar]
- 3.Bottger, G., P. Barnett, A. T. J. Klein, A. Kragt, H. F. Tabak, and B. Distel. 2000. Saccharomyces cerevisiae PTS1 receptor Pex5p interacts with the SH3 domain of the peroxisomal membrane protein Pex13p in an unconventional, non-PXXP-related manner. Mol. Biol. Cell 11:3963-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, C.-C., D. S. Warren, K. A. Sacksteder, and S. J. Gould. 1999. PEX12 interacts with PEX5 and PEX10 and acts downstream of receptor docking in peroxisomal matrix protein import. J. Cell Biol. 147:761-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, C. S., J. E. Kalish, J. C. Morrell, J. M. McCaffery, and S. J. Gould. 2000. The peroxisome biogenesis factors Pex4p, Pex22p, Pex1p, and Pex6p act in the terminal steps of peroxisomal matrix protein import. Mol. Cell. Biol. 20:7516-7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dammai, V., and S. Subramani. 2001. The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell 105:187-196. [DOI] [PubMed] [Google Scholar]
- 7.Dekker, P. J., F. Martin, A. C. Maarse, U. Bomer, H. Mueller, B. Guiard, M. Meijer, J. Rassow, and N. Pfanner. 1997. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16:5408-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodt, G., and S. J. Gould. 1996. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J. Cell Biol. 135:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransen, M., C. Brees, E. Baumgart, J. C. Vanhooren, M. Baes, G. P. Mannaerts, and P. P. V. Veldhoven. 1995. Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J. Biol. Chem. 270:7731-7736. [DOI] [PubMed] [Google Scholar]
- 10.Fransen, M., S. R. Terlecky, and S. Subramani. 1998. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc. Natl. Acad. Sci. USA 95:8087-8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiki, Y. 2000. Peroxisome biogenesis and peroxisome biogenesis disorders. FEBS Lett. 476:42-46. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda, S., N. Shimozawa, Y. Suzuki, Z. Zhang, S. Tomatsu, T. Tsukamoto, N. Hashiguchi, T. Osumi, M. Masuno, K. Imaizumi, Y. Kuroki, Y. Fujiki, T. Orii, and N. Kondo. 1996. Human peroxisome assembly factor-2 (PAF-2): a gene responsible for group C peroxisome biogenesis disorder in humans. Am. J. Hum. Genet. 59:1210-1220. [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaedi, K., A. Itagaki, R. Toyama, S. Tamura, T. Matsumura, A. Kawai, N. Shimozawa, Y. Suzuki, N. Kondo, and Y. Fujiki. 1999. Newly identified Chinese hamster ovary cell mutants defective in peroxisome assembly represent complementation group A of human peroxisome biogenesis disorders and one novel group in mammals. Exp. Cell Res. 248:482-488. [DOI] [PubMed] [Google Scholar]
- 14.Ghaedi, K., A. Kawai, K. Okumoto, S. Tamura, N. Shimozawa, Y. Suzuki, N. Kondo, and Y. Fujiki. 1999. Isolation and characterization of novel peroxisome biogenesis-defective Chinese hamster ovary cell mutants using green fluorescent protein. Exp. Cell Res. 248:489-497. [DOI] [PubMed] [Google Scholar]
- 15.Gould, S. J., J. E. Kalish, J. C. Morrell, J. Bjorkman, A. J. Urquhart, and D. I. Crane. 1996. Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTS1 receptor. J. Cell Biol. 135:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould, S. J., G.-A. Keller, N. Hosken, J. Wilkinson, and S. Subramani. 1989. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108:1657-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouveia, A. M., C. P. Guimaraes, M. E. Oliveira, C. Reguenga, C. Sa-Miranda, and J. E. Azevedo. 2003. Characterization of the peroxisomal cycling receptor, Pex5p, using a cell-free in vitro import system. J. Biol. Chem. 278:226-232. [DOI] [PubMed] [Google Scholar]
- 18.Gouveia, A. M., C. P. Guimaraes, M. E. Oliveira, C. Sa-Miranda, and J. E. Azevedo. 2003. Insertion of Pex5p into the peroxisomal membrane is cargo protein-dependent. J. Biol. Chem. 278:4389-4392. [DOI] [PubMed] [Google Scholar]
- 19.Kawajiri, K., T. Harano, and T. Omura. 1977. Biogenesis of the mitochondrial matrix enzyme, glutamate dehydrogenase, in rat liver cells. I. Subcellular localization, biosynthesis, and intracellular translocation of glutamate dehydrogenase. J. Biochem. 82:1403-1416. [DOI] [PubMed] [Google Scholar]
- 20.Kihara, A., Y. Akiyama, and K. Ito. 1999. Dislocation of membrane proteins in FtsH-mediated proteolysis. EMBO J. 18:2970-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita, N., K. Ghaedi, N. Shimozawa, R. J. A. Wanders, Y. Matsuzono, T. Imanaka, K. Okumoto, Y. Suzuki, N. Kondo, and Y. Fujiki. 1998. Newly identified Chinese hamster ovary cell mutants are defective in biogenesis of peroxisomal membrane vesicles (peroxisomal ghosts), representing a novel complementation group in mammals. J. Biol. Chem. 273:24122-24130. [DOI] [PubMed] [Google Scholar]
- 22.Lazarow, P. B., and Y. Fujiki. 1985. Biogenesis of peroxisomes. Annu. Rev. Cell Biol. 1:489-530. [DOI] [PubMed] [Google Scholar]
- 23.Leonhard, K., B. Guiard, G. Pellecchia, A. Tzagoloff, W. Neupert, and T. Langer. 2000. Membrane protein degradation by AAA proteases in mitochondria: extraction of substrates from either membrane surface. Mol. Cell 5:629-638. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, N., S. Tamura, and Y. Fujiki. 2003. The pathogenic peroxin Pex26p recruits the Pex1p-Pex6p AAA-ATPase complexes to peroxisomes. Nat. Cell Biol. 5:454-460. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto, N., S. Tamura, S. Furuki, N. Miyata, A. Moser, N. Shimozawa, H. W. Moser, Y. Suzuki, N. Kondo, and Y. Fujiki. 2003. Mutations in novel peroxin gene PEX26 that cause peroxisome biogenesis disorders of complementation group 8 provide a genotype-phenotype correlation. Am. J. Hum. Genet. 73:233-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto, N., S. Tamura, A. Moser, H. W. Moser, N. Braverman, Y. Suzuki, N. Shimozawa, N. Kondo, and Y. Fujiki. 2001. The peroxin Pex6p gene is impaired in peroxisome biogenesis disorders of complementation group 6. J. Hum. Genet. 46:273-277. [DOI] [PubMed] [Google Scholar]
- 27.Miura, S., I. Kasuya-Arai, H. Mori, S. Miyazawa, T. Osumi, T. Hashimoto, and Y. Fujiki. 1992. Carboxyl-terminal consensus Ser-Lys-Leu-related tripeptide of peroxisomal proteins functions in vitro as a minimal peroxisome-targeting signal. J. Biol. Chem. 267:14405-14411. [PubMed] [Google Scholar]
- 28.Miyazawa, S., T. Osumi, T. Hashimoto, K. Ohno, S. Miura, and Y. Fujiki. 1989. Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol. Cell. Biol. 9:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto, T., S. Matsuura, S. Sasaki, Y. Yashiro, and T. Omura. 1976. Immunochemical and immuno-electron microscopic studies on localization of NADPH-cytochrome c reductase on rat liver microsomes. J. Cell Biol. 68:189-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukai, S., K. Ghaedi, and Y. Fujiki. 2002. Intracellular localization, function, and dysfunction of the peroxisome-targeting signal type 2 receptor, Pex7p, in mammalian cells. J. Biol. Chem. 277:9548-9561. [DOI] [PubMed] [Google Scholar]
- 31.Neupert, W. 1997. Protein import into mitochondria. Annu. Rev. Biochem. 66:863-917. [DOI] [PubMed] [Google Scholar]
- 32.Okumoto, K., I. Abe, and Y. Fujiki. 2000. Molecular anatomy of the peroxin Pex12p: RING finger domain is essential for the Pex12p function and interacts with the peroxisome targeting signal type 1-receptor Pex5p and a RING peroxin, Pex10p. J. Biol. Chem. 275:25700-25710. [DOI] [PubMed] [Google Scholar]
- 33.Okumoto, K., A. Bogaki, K. Tateishi, T. Tsukamoto, T. Osumi, N. Shimozawa, Y. Suzuki, T. Orii, and Y. Fujiki. 1997. Isolation and characterization of peroxisome-deficient Chinese hamster ovary cell mutants representing human complementation group III. Exp. Cell Res. 233:11-20. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, M. E., A. M. Gouveia, R. A. Pinto, C. Sa-Miranda, and J. E. Azevedo. 2003. The energetics of Pex5p-mediated peroxisomal protein import. J. Biol. Chem. 278:39483-39488. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira, M. E. M., C. Reguenga, A. M. M. Gouveia, C. P. Guimaraes, W. Schliebs, W.-H. Kunau, M. T. Silvaa, C. Sa-Miranda, and J. E. Azevedo. 2002. Mammalian Pex14p: membrane topology and characterisation of the Pex14p-Pex14p interaction. Biochim. Biophys. Acta 1567:13-22. [DOI] [PubMed] [Google Scholar]
- 36.Otera, H., T. Harano, M. Honsho, K. Ghaedi, S. Mukai, A. Tanaka, A. Kawai, N. Shimizu, and Y. Fujiki. 2000. The mammalian peroxin Pex5pL, the longer isoform of mobile PTS1-transporter, translocates Pex7p-PTS2 protein complex into peroxisomes via its initial docking site Pex14p. J. Biol. Chem. 275:21703-21714. [DOI] [PubMed] [Google Scholar]
- 37.Otera, H., K. Okumoto, K. Tateishi, Y. Ikoma, E. Matsuda, M. Nishimura, T. Tsukamoto, T. Osumi, K. Ohashi, O. Higuchi, and Y. Fujiki. 1998. Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: studies with PEX5-defective CHO cell mutants. Mol. Cell. Biol. 18:388-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otera, H., K. Setoguchi, M. Hamasaki, T. Kumashiro, N. Shimizu, and Y. Fujiki. 2002. Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: conserved Pex5p WXXXF/Y motifs are critical for matrix protein import. Mol. Cell. Biol. 22:1639-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platta, H. W., S. Grunau, K. Rosenkranz, W. Girzalsky, and R. Erdmann. 2005. Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat. Cell Biol. 7:817-822. [DOI] [PubMed] [Google Scholar]
- 40.Portsteffen, H., A. Beyer, E. Becker, C. Epplen, A. Pawlak, W.-H. Kunau, and G. Dodt. 1997. Human PEX1 is mutated in complementation group 1 of the peroxisome biogenesis disorders. Nat. Genet. 17:449-452. [DOI] [PubMed] [Google Scholar]
- 41.Rapoport, T. A., B. Jungnickel, and U. Kutay. 1996. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu. Rev. Biochem. 65:271-303. [DOI] [PubMed] [Google Scholar]
- 42.Reuber, B. E., E. Germain-Lee, C. S. Collins, J. C. Morrell, R. Ameritunga, H. W. Moser, D. Valle, and S. J. Gould. 1997. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat. Genet. 17:445-448. [DOI] [PubMed] [Google Scholar]
- 43.Sacksteder, K. A., and S. J. Gould. 2000. The genetics of peroxisome biogenesis. Annu. Rev. Genet. 34:623-652. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu, N., R. Itoh, Y. Hirono, H. Otera, K. Ghaedi, K. Tateishi, S. Tamura, K. Okumoto, T. Harano, S. Mukai, and Y. Fujiki. 1999. The peroxin Pex14p: cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization, and functional analysis. J. Biol. Chem. 274:12593-12604. [DOI] [PubMed] [Google Scholar]
- 45.Subramani, S., A. Koller, and W. B. Snyder. 2000. Import of peroxisomal matrix and membrane proteins. Annu. Rev. Biochem. 69:399-418. [DOI] [PubMed] [Google Scholar]
- 46.Tamura, S., N. Matsumoto, A. Imamura, N. Shimozawa, Y. Suzuki, N. Kondo, and Y. Fujiki. 2001. Phenotype-genotype relationships in peroxisome biogenesis disorders of PEX1-defective complementation group 1 are defined by Pex1p-Pex6p interaction. Biochem. J. 357:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura, S., K. Okumoto, R. Toyama, N. Shimozawa, T. Tsukamoto, Y. Suzuki, T. Osumi, N. Kondo, and Y. Fujiki. 1998. Human PEX1 cloned by functional complementation on a CHO cell mutant is responsible for peroxisome-deficient Zellweger syndrome of complementation group I. Proc. Natl. Acad. Sci. USA 95:4350-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura, S., N. Shimozawa, Y. Suzuki, T. Tsukamoto, T. Osumi, and Y. Fujiki. 1998. A cytoplasmic AAA family peroxin, Pex1p, interacts with Pex6p. Biochem. Biophys. Res. Commun. 245:883-886. [DOI] [PubMed] [Google Scholar]
- 49.Terlecky, S. R., W. M. Nuttley, D. McCollum, E. Sock, and S. Subramani. 1995. The Pichia pastoris peroxisomal protein PAS8p is the receptor for the C-terminal tripeptide peroxisome targeting signal. EMBO J. 14:3627-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toyama, R., S. Mukai, A. Itagaki, S. Tamura, N. Shimozawa, Y. Suzuki, N. Kondo, R. J. A. Wanders, and Y. Fujiki. 1999. Isolation, characterization, and mutation analysis of PEX13-defective Chinese hamster ovary cell mutants. Hum. Mol. Genet. 8:1673-1681. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto, T., S. Miura, and Y. Fujiki. 1991. Restoration by a 35K membrane protein of peroxisome assembly in a peroxisome-deficient mammalian cell mutant. Nature 350:77-81. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamoto, T., S. Miura, T. Nakai, S. Yokota, N. Shimozawa, Y. Suzuki, T. Orii, Y. Fujiki, F. Sakai, A. Bogaki, H. Yasumo, and T. Osumi. 1995. Peroxisome assembly factor-2, a putative ATPase cloned by functional complementation on a peroxisome-deficient mammalian cell mutant. Nat. Genet. 11:395-401. [DOI] [PubMed] [Google Scholar]
- 53.Tsukamoto, T., S. Yokota, and Y. Fujiki. 1990. Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J. Cell Biol. 110:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Klei, I. J., R. E. Hilbrands, G. J. Swaving, H. R. Waterham, E. G. Vrieling, V. I. Titorenko, J. M. Cregg, W. Harder, and M. Veenhuis. 1995. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J. Biol. Chem. 270:17229-17236. [DOI] [PubMed] [Google Scholar]
- 55.Van der Leij, I., M. M. Franse, Y. Elgersma, B. Distel, and H. F. Tabak. 1993. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 90:11782-11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiedemann, N., V. Kozjak, A. Chacinska, B. Schoenfisch, S. Rospert, M. T. Ryan, N. Pfanner, and C. Meisinger. 2003. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424:565-571. [DOI] [PubMed] [Google Scholar]
- 57.Yahraus, T., N. Braverman, G. Dodt, J. E. Kalish, J. C. Morrell, H. W. Moser, D. Valle, and S. J. Gould. 1996. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 15:2914-2923. [PMC free article] [PubMed] [Google Scholar]
- 58.Ye, Y., H. H. Meyer, and T. A. Rapoport. 2001. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414:652-656. [DOI] [PubMed] [Google Scholar]