Abstract
Imprinted methylation of the paternal Rasgrf1 allele in mice occurs at a differentially methylated domain (DMD) 30 kbp 5′ of the promoter. A repeated sequence 3′ of the DMD regulates imprinted methylation, which is required for imprinted expression. Here we identify the mechanism by which methylation controls imprinting. The DMD is an enhancer blocker that binds CTCF in a methylation-sensitive manner. CTCF bound to the unmethylated maternal allele silences expression. CTCF binding to the paternal allele is prevented by repeat-mediated methylation, allowing expression. Optimal in vitro enhancer-blocking activity requires CTCF binding sites. The enhancer blocker can be bypassed in vivo and imprinting abolished by placing an extra enhancer proximal to the promoter. Together, the repeats and the DMD constitute a binary switch that regulates Rasgrf1 imprinting.
Approximately 70 transcripts undergo genomic imprinting, in which expression is primarily or exclusively from one parental allele while the other allele remains silent. Accompanying allele-specific expression is allele-specific DNA methylation, which is important for imprinted expression (17). In mice, the paternal Rasgrf1 allele is exclusively expressed in neonatal brain, while the maternal allele is silent. The paternal allele is also methylated within a differentially methylated domain (DMD) located 30 kbp 5′ of the promoter. Immediately 3′ of the DMD is a repeated sequence element containing 40 copies of a 41-nucleotide (nt) element. DMD methylation requires the repeats. Mice lacking the repeats on the paternal allele fail to establish proper paternal specific DNA methylation during gametogenesis (11, 29). Removal of the paternal repeats after fertilization but before implantation causes a loss of previously established methylation (R. Holmes, Y. Chang, and P. D. Soloway, submitted for publication). Collectively, the results show the Rasgrf1 repeats provide a positive signal for establishing and maintaining DNA methylation in mice. In other studies, sequences have been identified that regulate methylation at transgene insertion sites (4, 5, 15) and at the endogenous position of the imprinted H19/Igf2 locus (8, 25, 27).
Paternal allele-specific expression of Rasgrf1 in neonatal brain requires DMD methylation. Mutations that cause inappropriate loss of paternal allele methylation silence paternal allele expression, while mutations that induce maternal allele methylation activate the normally silent maternal allele (11, 29; Holmes et al., submitted). The correlation between DMD methylation and expression at Rasgrf1 is reminiscent of the relationship between methylation of the H19 DMD and expression of the tightly linked Igf2 locus. The H19 DMD is a methylation-sensitive enhancer-blocking element that binds CTCF when unmethylated, as on the maternal allele. This enables the enhancer-blocking activity of the DMD to prevent interaction between a downstream enhancer with the upstream Igf2 promoter, thus silencing the maternal Igf2 allele. However, when the DMD is methylated, as on the paternal allele, two things occur: methylation spreads to the H19 promoter, preventing H19 transcription, and CTCF can no longer bind the DMD. Because CTCF binding is required for enhancer-blocking activity, its absence from the methylated paternal allele permits interaction between the downstream enhancer and the upstream Igf2 promoter, allowing expression of the paternal allele (2, 10, 13). We show that a similar mechanism applies to regulation of Rasgrf1 imprinting. These data, combined with our identification of cis-acting sequences controlling DNA methylation, provide a detailed model describing regulation of imprinting at Rasgrf1.
MATERIALS AND METHODS
Enhancer blocking assays.
We performed enhancer blocking assays as described previously, inserting all test fragments into the AscI or SalI sites of pNI or slightly altered derivatives (3). The DMD repeat sequence was on a 2,103-bp EcoRI-to-EcoRV fragment. The DMD was amplified using primers, DMDFORWD (5′-TTGGCGCGCCGGACTCTTCAGAGAGTATGTAAAGCC-3′) and 92BRASC (5′-TTGGCGCGCCGAAGTGCGGCAGCAGCAGCGATAGC-3′), to generate a 349-bp product. This PCR product was cloned, sequence was verified, and mutations were prepared at positions 160, 230, and 320, where a 5′-GCnGCCnC-3′ consensus sequence shared with CTCF sites from H19 was found using a QuickChange kit (Stratagene). The position 160 consensus was changed from 5′-GCGGCCGC-3′ to 5′-ATGATTGT-3′ using oligonucleotides 5′-TCATGATTGTGCTGCTGCTCCCACATCC-3′ and 5′-GCACAATCA TGAAACGGTAGCGAAGTGC-3′; the position 230 consensus was changed from 5′-GCTGCCGC-3′ to 5′-ATTATTGT-3′ using oligonucleotides 5′-CCATTATTGTTAAGCTATGGCTGCCGCA-3′ and 5′-TAACAATAATGGTGCAGCAACAGCAATA-3′; the position 320 consensus was changed from 5′-GCTGCCGC-3′ to 5′-ATTATTGT-3′ using oligonucleotides 5′ CGATTATTGTGCTATCGCTGCTGCTGCC-3′ and 5′-GCACAATAATCGTAGCGCAACGGTAGTG-3′.
Gel shift analysis.
We performed the gel shift analysis shown in Fig. 2A and B as described previously (3). Binding reactions included 5 μg of partially purified chicken CTCF or chicken erythrocyte extracts, 20 mM HEPES, pH 7.9, 150 mM KCl, 5 mM MgCl2, 5% glycerol, 1 mM dithiothreitol, 0.5% triton X-100, 50 ng/μl poly(dA-dT), and 20 fmol labeled oligonucleotide probe. Oligonucleotide probes include some described earlier (2, 3) or double-stranded forms of the following primers labeled with polynucleotide kinase prior to annealing to the complement: DMD160 (5′-AGGCGCGCCCTGCTGCCGCGCTTCGCGCCTGCACTTCGCTACCGTTTCGCGGCCGCGCTGCTGCTC CCACATCCATCCGGGCGCGCCT-3′), DMD230 (5′-AGGCGCGCCTCC ATCCGTGGCTACCGCTATTGCTGTTGCTGCACCGCTGCCGCTAAG CTATGGCTGCCGCACTTCACTGGGCGCGCCT-3′), and DMD320 (5′-AGG CGCGCCCCACGACTGCTACTGCTGCTGCTGCACTACCGTTGCGCT ACGGCTGCCGCGCTATCGCTGCTGCTGCCGCGGCGCGCCT-3′). 320m was DMD320 synthesized with methylcytosines at the eight CG dinucleotides. Incubation was at room temperature for 45 min, and products were run on a 5% (29:1, acrylamide-bisacrylamide) gel in 1× Tris-borate-EDTA at 150 V for 1.5 to 2 h.
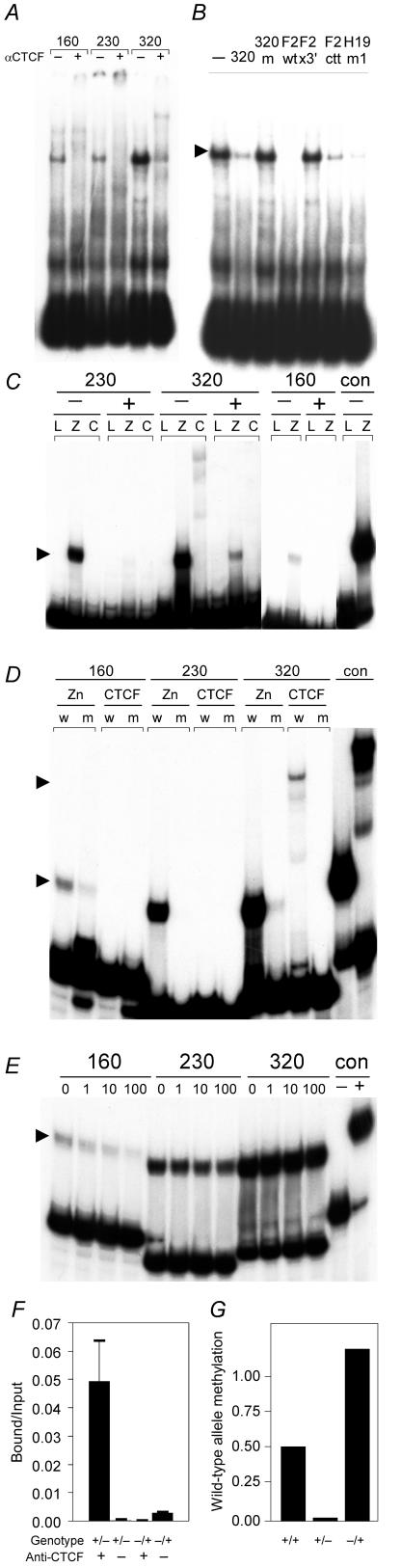
FIG. 2.
CTCF binds the unmethylated Rasgrf1 DMD. A. Oligonucleotide probes corresponding to three sequences in the DMD (230, 320, and 160) were tested for CTCF binding by gel shift analysis. Reactions included CTCF-containing extracts from chicken erythrocytes with (+) or without (−) anti-CTCF antibody. B. One of the probes (320) was used in further gel shift experiments using CTCF purified from chicken erythrocytes and several competitor probes. Binding reactions were done with no competing oligonucleotide (−) or 200-fold molar excesses of competitors that included self (320), methylated self (320m), the wild-type CTCF binding site at the chicken β-globin locus (F2wt), mutated forms of the chicken β-globin CTCF site that abolished (F2x3′) or attenuated (F2ctt) CTCF binding, and a CTCF site from H19. C. Methylation-sensitive binding was confirmed using larger probes containing the three Rasgrf1 CTCF sites prepared by PCR and methylated in vitro (+) or left unmethylated (−) prior to incubation with in vitro-transcribed and -translated luciferase (L), Zn-finger domain of CTCF (Z), or full-length CTCF (C). An unmethylated human XIST probe (con) was used as a positive control for binding. D. Probes generated by PCR corresponding to wild-type (w) or mutated (m) forms of Rasgrf1 CTCF sites 160, 230, and 320 were tested for binding to the in vitro-translated and -transcribed Zn-finger domain of CTCF. The mutated forms of these sites were those used in enhancer-blocking tests in Fig. 1. E. The wild-type probes from panel D were used in competition assays that included the Zn-finger domain of CTCF and increasing molar excesses (0 or 1-, 10-, or 100-fold) of unlabeled, mutated site probes. An unmethylated human XIST probe (con) was used with (+) or without (−) added protein as a control. Arrowheads in panels B, C, D, and E indicate positions of complexes with the full-length CTCF or Zn-finger domain complexes. F. Chromatin immunoprecipitation was performed using embryonic fibroblasts from mice with a paternally (+/−) or maternally (−/+) inherited repeat deletion (29) with (+) or without (−) antibody against CTCF. Precipitates were analyzed using real-time PCR with primers specific for the wild-type allele only. The experiment was done in triplicate, and the fraction of input that was precipitated is reported. Error bars show the standard deviation. G. The methylation state of HhaI sites in the wild-type allele was measured by real time PCR. DNAs from wild-type (+/+), +/−, and −/+ cells used in panel F were amplified with wild-type-specific primers that spanned five HhaI sites in the DMD. Amplification was done either before or after digestion with HhaI. Wild-type allele methylation is the ratio of product amplified from the digested templates to that from undigested templates. Of the two wild-type alleles in +/+ cells, only the paternal is methylated (ratio = 0.50), the single wild-type maternal allele in +/− cells is not appreciably methylated (ratio = 0.014), and the single wild-type paternal allele in −/+ cells is fully methylated (ratio = 1.2).
The gel shift analysis shown in Fig. 2C, D, and E, using PCR-generated probes, was done as described previously (9). Briefly, binding reactions were at room temperature in a 20-μl volume and included 5 μg in vitro-transcribed and -translated full-length CTCF, the CTCF Zn finger fragment, or luciferase as a negative control and labeled probe in 5 mM MgCl2, 0.65 mM ZnSO4, 0.35 mM β-mercaptoethanol, 7.5% glycerol, 0.065% NP-40, 2.5 μg/ml salmon sperm DNA, and 50 μg/ml poly(dI-dC). We used the following primers to PCR amplify probes used in Fig. 2C, D, and E using cloned fragments as templates: 160 was amplified with P2F (5′-GGAATTCTGGGGACTCTTCAGAGAGTTT-3′) and P2R (5′-CGGTAGCCACGGATGGATGTGGG-3′); 230 was amplified with P3F (5′-CTTCGCTACCGTTTCGCGGCC-3′) and P3R (5′-GGTAGTTGTAGCGCAGCGGTAGCG-3′); 320 was amplified with P4F (5′-GCTGCACCGCTGCCGCTAAG-3′) and P4R (5′-CAGCACGGCAGCGAAGTGCGG-3′). We methylated PCR products with SssI methyltransferase and verified the extent of methylation by digestion overnight with BstUI restriction endonuclease. The XIST probe was described previously (24). Reactions were electrophoresed on 5% polyacrylamide gels run in 0.5× Tris-borate-EDTA buffer.
ChIP.
For chromatin immunoprecipitation (ChIP) analysis, we used established protocols (http://www.upstate.com/misc/protocols.q.prot.e.chips/Chromatin+Immunoprecipitation++ChIPs++Assay+Kit) with modifications. Briefly, we fixed approximately 1 × 107 cells by adding formaldehyde (1% volume basis) to the medium for 10 min at room temperature with agitation followed by a 10-min quench with 0.5 M glycine. After washing cells twice with 50 ml phosphate-buffered saline (PBS) containing Roche Complete proteinase inhibitor (catalog no. 11697498001), reconstituted as recommended by the manufacturer, we collected cells in 2 ml ice-cold PBS with proteinase inhibitor, centrifuged cells, and resuspended them in the same solution at a concentration of 1 × 107 cells per ml. We sonicated cells to shear the DNA into 700-bp fragments, diluted with 9 volumes of PBS with proteinase inhibitor, and then performed the immunoprecipitation on one-quarter of the lysate using anti-CTCF antibody (Abcam catalog no. 06-917). As a negative control, antibody was omitted. After the final washes of the precipitate, elution of the CTCF-DNA complex, and reversal of the cross-links, we performed real-time PCR to detect the amount of bound wild-type DNA using primers P2F and P6R described above.
Methylation analysis of cells used in ChIP studies.
DNA templates were amplified using primers PDS12 (5′-CACATCCATCCGTGGCTACCGCTATTGCTGT-3′) and PDS13 (5′-GCGAAGTGCGGCAGCAGCAGCGA-3′), which span five HhaI sites in the DMD. Real-time PCRs including SYBR green were done with an ABI 7500 instrument.
Development and characterization of mutant mice.
We prepared the Rasgrf1tm2Pds targeting vector, pBJR2, as follows. We amplified, by PCR, a 2-kbp 5′ homologous arm using a 7-kbp BamHI genomic clone with sequences 5′ of the repeats as a template. The forward primer (5′-CATGCTCCTTGGGATGTTGA-3′) was from the plasmid pSPL3, in which the BamHI fragment was cloned; the reverse primer (5′-CGAAGTGCGGCTGCAGAAGCTTTAGCGCGGCAGCCGTAGCG-3′) was located at the 5′ junction of the repeat. The reverse primer differed from the wild type at three nucleotide positions to generate PstI and HindIII sites specific for the mutated allele (mutated sequences are in bold). We placed 3′ of this a 1.5-kbp neo cassette and a 500-nt Pgk enhancer-and-promoter fragment in reverse orientation that replaced the Rasgrf1 repeats. The 3′ homologous arm was a 3-kbp EcoRV-to-BamHI fragment located 3′ of the repeats. The BRP1.0 probe used for Southern blots was located 5′ of the 5′ arm. We used standard methods for embryonic stem cell culture and blastocyst injections. Southern blots for methylation analysis and reverse transcriptase PCR for expression analysis were described previously (29). All animal research complied with all relevant federal guidelines and institutional policies.
RESULTS
Enhancer blocking elements at Rasgrf1.
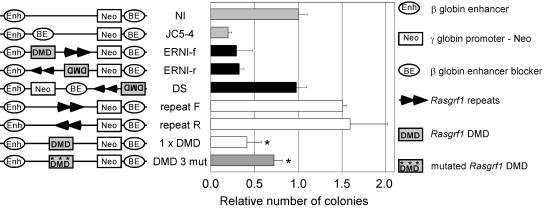
We tested the Rasgrf1 DMD and repeat-containing sequences for enhancer-blocking activity using a previously described assay (3, 6). The system utilized the chicken β-globin enhancer augmenting transcription of a neo reporter by a weak human Aγ-globin promoter. We placed sequences to be tested in either orientation between the enhancer and promoter or outside the enhancer-to-promoter interval, electroporated these into K562 human erythroleukemia cells, plated them in soft agar containing G418, selected for 2 to 3 weeks, and counted colonies (Fig. 1). When placed between the enhancer and promoter, the Rasgrf1 DMD and repeats together reduced the colony number regardless of their orientation. The magnitude of this reduction was comparable to that provided by the enhancer blocker found at the chicken β-globin locus (3). In contrast, the Rasgrf1 sequences did not reduce the colony number when placed outside the enhancer-to-promoter interval, indicating that the reduction in colony number was due to enhancer-blocking activity and not nonspecific silencing. The DMD alone exhibited enhancer-blocking activity at a level indistinguishable from that of the DMD repeat combination. The repeats alone had no blocking activity and in fact provided significant but modest stimulation of the colony number in the forward orientation. The numbers of G418-resistant colonies produced by pNI electroporation into K562 cells had been shown to be unaffected by changes in enhancer-to-promoter spacing caused by insertion of sequences from λ phage (3). Collectively, the data indicate the Rasgrf1 enhancer blocking activity resides in the DMD.
FIG. 1.
The Rasgrf1 DMD has enhancer-blocking activity that depends upon CTCF binding sites. The enhancer-blocking test constructs shown at the left were prepared and transfected into K562 cells as described previously (3, 6). Each contained the chicken β-globin enhancer (Enh), the human Aγ-globin promoter directing transcription of a neo reporter (Neo), the chicken β-globin enhancer blocker (BE), and various test fragments. Each test was done 2 to 23 times. The colony number for each test is expressed relative to the number observed with the negative control plasmid (NI) (3), which lacked test sequences and produced an average of 226 colonies. JC5-4 containing the chick β-globin enhancer blocker served as a positive control (7). Rasgrf1 sequences tested included the DMD and repeats (two black triangles) in both orientations or downstream of the neo reporter, one copy of the DMD alone (1 × DMD), and a mutated DMD carrying mutations in the three known CTCF sites (designated 160, 230, and 320 in Fig. 2). Colony counts from 1 × DMD and the DMD with repeats are not significantly different. The difference between results for 1 × DMD and the mutant DMD 3 mut is significant (*, P = 0.008 by t test).
CTCF binding at Rasgrf1.
Vertebrate enhancer blockers have been shown to require CTCF binding for their activity (3). Furthermore, an enhancer blocker in the H19 DMD has been shown to bind CTCF in a methylation-sensitive manner, and abrogation of binding by mutation of the binding sites or depletion of CTCF has been shown to eliminate Igf2 imprinting (2, 10, 13, 21, 25). This led to the model that lack of maternal methylation at the H19 DMD silences the maternal Igf2 allele by supporting CTCF binding and enhancer-blocking activity, which prevents the 3′ enhancers from interacting with the 5′ Igf2 promoters (2). We asked if the Rasgrf1 DMD also binds CTCF in a methylation-sensitive manner using two independent assays.
First, we performed gel shift experiments using CTCF-containing extracts from chicken erythrocytes, oligonucleotide probes from the DMD, and anti-CTCF antibody to supershift CTCF complexes (Fig. 2A). Three CTCF binding sites were detected within the DMD. One of these (320) bound CTCF, more effectively than the other two. We used the 320 oligonucleotide in additional gel shift experiments using purified chicken CTCF and competitor probes (Fig. 2B). Competitors included self, a methylated form of self, CTCF sites from the mouse H19 and chicken β-globin loci, as well as mutated forms of the chicken β-globin probes. CTCF binding to the Rasgrf1 DMD 320 site was completely blocked by the H19 and wild-type chicken β-globin competitors and partially blocked by self and a mutated form of the chicken β-globin probe that has attenuated CTCF binding. However, binding was not competed at all by a methylated form of the 320 probe, suggesting CTCF binding to 320 was methylation sensitive. CTCF binding at H19 and chicken β-globin may be stronger than its binding to Rasgrf1.
To confirm that CTCF binds the DMD in a methylation-sensitive manner, gel shift studies were repeated using PCR-generated probes containing the three individual CTCF sites in the DMD that were either methylated or unmethylated (Fig. 2C). Interacting proteins were prepared by in vitro translation of templates encoding CTCF, the Zn-finger domain of CTCF, or luciferase as a negative control. The in vitro-translated CTCF did not bind as well as the native CTCF used in Fig. 2A and B; however, the unmethylated form of each probe bound the CTCF Zn-finger domain. When the probes were methylated, their binding to the CTCF Zn-finger domain was greatly reduced or eliminated altogether, confirming that CTCF binding to the DMD is methylation sensitive (Fig. 2C).
To determine if the CTCF binding sites in the Rasgrf1 DMD were important for the enhancer-blocking activity, we mutated the three CTCF sites within the DMD we identified, changing each of six conserved nucleotides in those sites with a transition mutation. When we performed gel shift and competition experiments similar to those described above, results showed the mutations diminished, but did not completely abolish, CTCF Zn-finger domain binding (Fig. 2D and E). In enhancer-blocking assays, the DMD that was mutated at all three CTCF sites was significantly less effective at blocking the enhancer-to-promoter interactions than the wild-type DMD (Fig. 1), though enhancer blocking activity was not eliminated. This may be due to residual CTCF binding activity in the mutated sites or additional CTCF sites within the DMD that we did not find.
We extended these results to confirm that the Rasgrf1 DMD binds CTCF in a methylation-sensitive manner and that binding occurs at the Rasgrf1 locus in cells and not only to synthetic probes in biochemical tests. For these assays, we performed ChIP experiments (Fig. 2F) using embryonic fibroblasts from heterozygous mice with a maternally (−/+) or paternally (+/−) derived repeat deletion (29). All precipitated material was analyzed by a PCR assay specific for the wild-type allele. This enabled us to separately monitor CTCF binding to the wild-type paternal allele, which is methylated in −/+ cells, and the wild-type maternal allele, which is unmethylated in +/− cells (Fig. 2G). This assay does not detect the mutated (−) allele. Consistent with our gel shift data, we could not detect CTCF binding to the methylated, wild-type, paternal DMD in −/+ cells. However, when we looked for binding to the unmethylated, wild-type maternal DMD in +/− cells, CTCF binding was readily detected. This too was consistent with our gel shift data showing that CTCF binds to its sites in the Rasgrf1 DMD when they are unmethylated.
Collectively, these studies indicate that the Rasgrf1 DMD is a methylation-sensitive enhancer blocker that silences the unmethylated maternal allele by binding CTCF. The methylated paternal allele is expressed because repeat-induced methylation prevents CTCF binding at Rasgrf1.
Enhancer location controls Rasgrf1 imprinting.
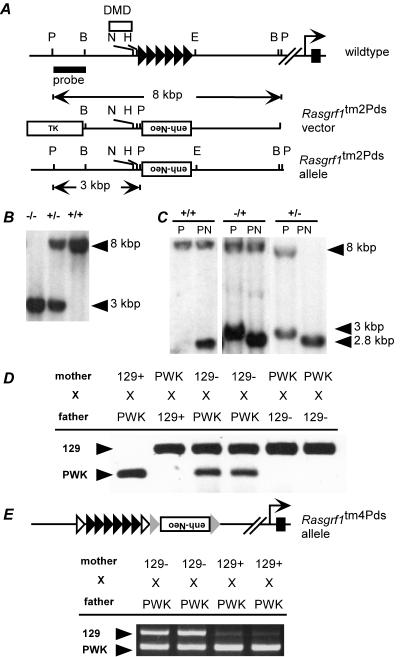
We wondered if imprinted expression of Rasgrf1 in vivo in fact relies upon regulation of enhancer-to-promoter interactions by the enhancer blocker within the DMD. If this is the case, we reasoned that we should be able to bypass the imprinting switch altogether by placing an extra enhancer downstream of the DMD repeat interval. At this location, the enhancer could interact with the promoter, unencumbered by an intervening enhancer blocker, enabling the modified allele to escape imprinting control. We tested this hypothesis using two independently derived mouse mutants. In one mutant, we deleted the Rasgrf1 repeats and replaced them with a neo cassette containing a 500-bp transcriptional control sequence from the mouse Pgk gene (Fig. 3A and B). The 500-bp sequence contains the Pgk promoter as well as the enhancer (19). We designated this extra enhancer allele Rasgrf1tm2Pds. Consistent with our earlier studies (29), removal of the paternal repeats caused a loss of paternal allele methylation. This was shown by Southern blot analysis of the NotI site in the DMD (Fig. 3C) and by bisulfite analysis of 19 CpGs in the paternal DMD (data not shown). These results using an allele independent of the one described in our earlier studies (29) verified that the repeats positively regulate paternal allele DNA methylation at Rasgrf1.
FIG. 3.
An extra enhancer at Rasgrf1 bypasses imprinted regulation caused by the DMD enhancer-blocking element. A. The wild-type Rasgrf1 locus was mutated to create a new allele (Rasgrf1tm2Pds) that placed an enhancer (enh-neo) in an inverted orientation at the locus in place of the repeats (filled triangles). B. Southern blot analysis using the probe shown in panel A and PstI-digested DNA revealed the expected 8.0-kbp and 3.0-kbp bands from the wild-type (+) and mutated (−) alleles, respectively, confirming homologous recombination. C. Methylation analysis by Southern blotting using PstI (P) and NotI (N) produced a 2.8-kbp band when methylation was absent from either the wild-type or mutated allele. Maternal transmission of the Rasgrf1tm2Pds allele (−/+) had no effect on methylation, while in mice with paternal transmission (+/−), the sole band at 2.8 kbp indicated that methylation of the paternal allele was lost. D. Allele-specific reverse transcriptase PCR using cDNA prepared from neonatal brains of progeny from reciprocal crosses between wild-type PWK mice and animals with the Rasgrf1tm2Pds mutation on the 129S4/SvJae (129) background. The extra enhancer-containing neo cassette facilitated expression of the otherwise silent maternal allele when it was maternally transmitted and enabled paternal allele expression even though paternal methylation was absent. E. Expression analysis of an independently derived allele with loxP sites (open triangles) flanking the repeats and frt sites (shaded triangles) flanking the enh-neo cassette (Rasgrf1tm4Pds) (R. Holmes et al., submitted) revealed the same escape from imprinted expression as for the Rasgrf1tm2Pds allele.
When we monitored Rasgrf1 expression in mice carrying the extra enhancer by using an allele-specific assay, we observed that the enhancer insertion enabled expression of Rasgrf1 regardless of whether it was transmitted maternally or paternally (Fig. 3D). Expression was due entirely to the presence of the Pgk-enh-pro-neo insertion and not due to repeat deletion, because mice with only a repeat deletion and no enhancer-containing Pgk-neo cassette failed to express Rasgrf1 from the mutated allele (29). Importantly, while DMD methylation is normally required for expression of Rasgrf1 in neonatal brain, the extra enhancer allowed expression even in the absence of methylation.
The results from the Rasgrf1tm2Pds mutation were confirmed by analysis of another independent mutation we prepared in which the enhancer-containing cassette was inserted 3′ of the Rasgrf1 repeats (Fig. 3E) (Holmes et al., submitted). In these mice, the maternal allele was also activated by the cassette insertion, demonstrating that the Pgk-enh-pro-neo insertion bypassed imprinted expression of Rasgrf1 whether the repeats were present or not. These results, in combination with the in vitro results described above, indicated the enhancer-blocking activity of the Rasgrf1 DMD is central to regulation of Rasgrf1 imprinting in vivo. Enhancer relocation studies at H19 similarly supported the model that Igf2 is regulated by the methylation-sensitive enhancer blocker in the H19 DMD (28).
Model for Rasgrf1 imprinting.
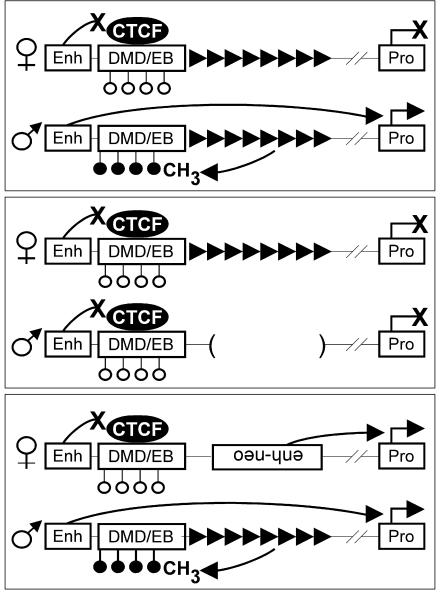
In the female germ line of wild-type mice (Fig. 4, top), the Rasgrf1 repeat element does not induce DMD methylation and the unmethylated allele transmitted from mother to progeny binds CTCF. CTCF binding facilitates the enhancer-blocking activity of the DMD, which prevents a yet-to-be-identified upstream enhancer from stimulating Rasgrf1 transcription in neonatal brain, and the maternal allele is silent. In the male germ line, the Rasgrf1 repeat element establishes methylation at the DMD. The methylated allele transmitted from father to progeny maintains its methylation and cannot bind CTCF. Because CTCF is not bound, the enhancer blocker does not function on the paternal allele and enhancer-to-promoter interactions are permitted, causing expression of the paternal allele in neonatal brain. In mice carrying the repeat-deficient Rasgrf1tm1Pds allele on the paternal chromosome (Fig. 4, middle), normal DMD methylation never becomes established. In this case, neither allele is methylated at the DMD, and CTCF binds both alleles and enables the maternal and paternal enhancer blockers to silence both alleles.
FIG. 4.
Model depicting the Rasgrf1 DMD and repeat as a binary switch that regulates Rasgrf1 imprinting. Shown are the putative neonatal enhancer (Enh), promoter (Pro), repeats (rightward-pointing filled triangles) and the DMD methylated (filled circles) and unmethylated (open circles) on the paternal and maternal alleles, respectively. Curved lines ending in an X indicate blocked interactions or activities, and lines ending in an arrowhead indicate those that are permitted. The repeat-deficient Rasgrf1 allele (Rasgrf1tm1Pds) was described earlier (29). See the text for details.
In mice carrying the extra enhancer allele (Rasgrf1tm2Pds) on the maternal chromosome (Fig. 4, bottom), the enhancer-blocking function is still active because the DMD is unmethylated and CTCF can bind. But the location of the extra enhancer allows it to bypass regulation by the DMD enhancer blocker and activate the maternal promoter, resulting in biallelic expression. When the mutation is inherited paternally, the DMD fails to become methylated because the repeats are missing (Fig. 3C). The absence of paternal methylation can support CTCF binding and enhancer-blocking activity, which would silence the paternal allele. However, the placement of the extra enhancer bypasses the DMD enhancer blocker, allowing apparently normal expression of the paternal allele (Fig. 3D). This model of imprinted expression is similar to the model described for H19/Igf2 in that allele-specific expression is mediated by a methylation-sensitive enhancer blocker.
DISCUSSION
It is not clear how the Rasgrf1 repeats regulate paternal DMD methylation. In plants, transcripts that include inverted repeats can be processed into 21- to 25-nt small interfering RNAs (siRNAs) that regulate local DNA methylation (1, 20). Such mechanisms have been reported in mammalian cells, too (16). Furthermore, if models depicting direct repeats as a source for siRNAs are valid (18), then the Rasgrf1 repeats may regulate local DNA methylation by RNA-mediated mechanisms. However, we have been unable to detect RNAs emanating from the Rasgrf1 repeats in the siRNA size range (R. Holmes and P. D. Soloway, unpublished data). Second, it is not clear how paternal allele methylation is erased in the female germ line. This may be a passive process in which methylation is not maintained during female germ line development, but it may be an active process, involving local cis-acting methyl erasure signals. Transgenic studies suggest that the Rasgrf1 repeats are sufficient for paternal allele methylation of the DMD, but additional sequences are necessary for demethylation of the maternal Rasgrf1 allele (H. Herman, B. Hu, and P. D. Soloway, unpublished data). There is precedent for the existence of such demethylating signals at Snrpn (26).
It is clear that mechanisms governing imprinted methylation are varied and that no one unifying mechanism applies to all loci. This is true even if one considers only imprinted methylation at paternal alleles. For example, key differences exist between paternal methylation of Rasgrf1 and H19. Notably, regulation of methylation at Rasgrf1 is by a positive mechanism requiring the repeats. Without the repeats, no methylation is established. At H19, no positive regulators for establishing DMD methylation have been identified. In fact, it is possible that methylation is the default state at the paternal H19 DMD and that methylation is inhibited at the maternal allele by a CTCF-dependent mechanism (8, 21, 22, 25). Studies of Dnmt3a mutant mice also reveal multiple mechanisms for establishing paternal allele methylation. In primordial germ cells taken from male mice lacking DNMT3a, it was shown that H19 and the intergenic DMD at Dlk1-Gtl2 failed to acquire methylation normally seen there, yet Rasgrf1 became methylated (14). Although this suggests a common DNMT requirement for H19 and Dlk1-Gtl2 and a different one for Rasgrf1, H19 methylation also required Dnmt3L, but Dlk1-Gtl2 did not. These results illustrate that paternal methylation at each of these imprinted loci involves distinct mechanisms.
Three details regarding imprinted expression of Rasgrf1 are missing. First, it is not clear if the DMD contains additional CTCF binding sites other than the three we identified. The DMD with mutations in the three CTCF sites still had blocking activity, although it was significantly less than the blocking activity of the wild-type DMD. The residual blocking activity could be due to additional CTCF binding sites we did not find or because the mutations at positions 160 and 230 did not completely abolish CTCF binding (Fig. 2D and E). Second, the evidence for enhancers controlling Rasgrf1 expression remains circumstantial: we have yet to identify the endogenous enhancer(s) for Rasgrf1 expression that are regulated by the enhancer-blocking activity of the DMD. Because locating an extra enhancer 3′ of the DMD ablates imprinted expression, we looked 5′ of the DMD for enhancers. Sequence comparisons between mouse and human revealed several conserved sequences in this region. Similar comparisons at H19 were used to identify enhancers there (12); however, we were unable to demonstrate enhancer activity for the conserved Rasgrf1 sequences (R. Holmes, unpublished). Finally, paternal allele-specific expression of Rasgrf1 is restricted to the neonatal mouse brain. In other tissues and in adult brain, Rasgrf1 expression is biallelic. Based on our model, we predict that this can happen when the Rasgrf1 promoter escapes its requirement for an enhancer or when alternate enhancers 3′ of the repeats become active that can bypass the DMD enhancer blocker mechanism because of their location.
The model we present accounts for all imprinting phenotypes described for wild-type mice (23) and for mice with mutations in Rasgrf1 imprinting control sequences (11, 29) and with all biochemical and cell biological analyses of Rasgrf1 imprinting described here. This model may guide additional studies to elaborate mechanisms of genomic imprinting at Rasgrf1.
Acknowledgments
We are grateful to Gary Felsenfeld and Shirley Tilghman for helpful discussions.
Funding to P.D.S. was from the National Institutes of Health (CA98596 and EY11279), the U.S. Army (DAMD17-02-1-0652), and the Roswell Park Cancer Institute Alliance. Support was also provided by the National Cancer Institute Cancer Center Support Grant to Roswell Park Cancer Institute (P30CA016056).
REFERENCES
- 1.Aufsatz, W., M. F. Mette, J. van der Winden, A. J. Matzke, and M. Matzke. 2002. RNA-directed DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 99:16499-16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, A. C., and G. Felsenfeld. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405:482-485. [DOI] [PubMed] [Google Scholar]
- 3.Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387-396. [DOI] [PubMed] [Google Scholar]
- 4.Birger, Y., R. Shemer, J. Perk, and A. Razin. 1999. The imprinting box of the mouse Igf2r gene. Nature 397:84-88. [DOI] [PubMed] [Google Scholar]
- 5.Chaillet, J. R. 1994. Genomic imprinting: lessons from mouse transgenes. Mutat. Res. 307:441-449. [DOI] [PubMed] [Google Scholar]
- 6.Chung, J. H., A. C. Bell, and G. Felsenfeld. 1997. Characterization of the chicken beta-globin insulator. Proc. Natl. Acad. Sci. USA 94:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, J. H., M. Whiteley, and G. Felsenfeld. 1993. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74:505-514. [DOI] [PubMed] [Google Scholar]
- 8.Fedoriw, A. M., P. Stein, P. Svoboda, R. M. Schultz, and M. S. Bartolomei. 2004. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science 303:238-240. [DOI] [PubMed] [Google Scholar]
- 9.Filippova, G. N., S. Fagerlie, E. M. Klenova, C. Myers, Y. Dehner, G. Goodwin, P. E. Neiman, S. J. Collins, and V. V. Lobanenkov. 1996. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol. Cell. Biol. 16:2802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hark, A. T., C. J. Schoenherr, D. J. Katz, R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405:486-489. [DOI] [PubMed] [Google Scholar]
- 11.Herman, H., M. Lu, M. Anggraini, A. Sikora, Y. Chang, B. J. Yoon, and P. D. Soloway. 2003. Trans allele methylation and paramutation-like effects in mice. Nat. Genet. 34:199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishihara, K., N. Hatano, H. Furuumi, R. Kato, T. Iwaki, K. Miura, Y. Jinno, and H. Sasaki. 2000. Comparative genomic sequencing identifies novel tissue-specific enhancers and sequence elements for methylation-sensitive factors implicated in Igf2/H19 imprinting. Genome Res. 10:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanduri, C., V. Pant, D. Loukinov, E. Pugacheva, C. F. Qi, A. Wolffe, R. Ohlsson, and V. V. Lobanenkov. 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr. Biol. 10:853-856. [DOI] [PubMed] [Google Scholar]
- 14.Kaneda, M., M. Okano, K. Hata, T. Sado, N. Tsujimoto, E. Li, and H. Sasaki. 2004. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429:900-903. [DOI] [PubMed] [Google Scholar]
- 15.Kantor, B., K. Makedonski, Y. Green-Finberg, R. Shemer, and A. Razin. 2004. Control elements within the PWS/AS imprinting box and their function in the imprinting process. Hum. Mol. Genet. 13:751-762. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki, H., and K. Taira. 2004. Induction of DNA methylation and gene silencing by short interfering RNAs in human cells. Nature 431:211-217. [DOI] [PubMed] [Google Scholar]
- 17.Li, E., C. Beard, and R. Jaenisch. 1993. Role for DNA methylation in genomic imprinting. Nature 366:362-365. [DOI] [PubMed] [Google Scholar]
- 18.Martienssen, R. A. 2003. Maintenance of heterochromatin by RNA interference of tandem repeats. Nat. Genet. 35:213-214. [DOI] [PubMed] [Google Scholar]
- 19.McBurney, M. W., L. C. Sutherland, C. N. Adra, B. Leclair, M. A. Rudnicki, and K. Jardine. 1991. The mouse Pgk-1 gene promoter contains an upstream activator sequence. Nucleic Acids Res. 19:5755-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mette, M. F., W. Aufsatz, J. van Der Winden, M. A. Matzke, and A. J. Matzke. 2000. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 19:5194-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pant, V., S. Kurukuti, E. Pugacheva, S. Shamsuddin, P. Mariano, R. Renkawitz, E. Klenova, V. Lobanenkov, and R. Ohlsson. 2004. Mutation of a single CTCF target site within the H19 imprinting control region leads to loss of Igf2 imprinting and complex patterns of de novo methylation upon maternal inheritance. Mol. Cell. Biol. 24:3497-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pant, V., P. Mariano, C. Kanduri, A. Mattsson, V. Lobanenkov, R. Heuchel, and R. Ohlsson. 2003. The nucleotides responsible for the direct physical contact between the chromatin insulator protein CTCF and the H19 imprinting control region manifest parent of origin-specific long-distance insulation and methylation-free domains. Genes Dev. 17:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plass, C., H. Shibata, I. Kalcheva, L. Mullins, N. Kotelevtseva, J. Mullins, R. Kato, H. Sasaki, S. Hirotsune, Y. Okazaki, W. A. Held, Y. Hayashizaki, and V. M. Chapman. 1996. Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS-M. Nat. Genet. 14:106-109. [DOI] [PubMed] [Google Scholar]
- 24.Pugacheva, E. M., V. K. Tiwari, Z. Abdullaev, A. A. Vostrov, P. T. Flanagan, W. W. Quitschke, D. I. Loukinov, R. Ohlsson, and V. V. Lobanenkov. 2005. Familial cases of point mutations in the XIST promoter reveal a correlation between CTCF binding and pre-emptive choices of X chromosome inactivation. Hum. Mol. Genet. 14:953-965. [DOI] [PubMed] [Google Scholar]
- 25.Schoenherr, C. J., J. M. Levorse, and S. M. Tilghman. 2003. CTCF maintains differential methylation at the Igf2/H19 locus. Nat. Genet. 33:66-69. [DOI] [PubMed] [Google Scholar]
- 26.Shemer, R., A. Y. Hershko, J. Perk, R. Mostoslavsky, B. Tsuberi, H. Cedar, K. Buiting, and A. Razin. 2000. The imprinting box of the Prader-Willi/Angelman syndrome domain. Nat. Genet. 26:440-443. [DOI] [PubMed] [Google Scholar]
- 27.Thorvaldsen, J. L., K. L. Duran, and M. S. Bartolomei. 1998. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12:3693-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webber, A. L., R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 1998. Location of enhancers is essential for the imprinting of H19 and Igf2 genes. Nature 391:711-715. [DOI] [PubMed] [Google Scholar]
- 29.Yoon, B. J., H. Herman, A. Sikora, L. T. Smith, C. Plass, and P. D. Soloway. 2002. Regulation of DNA methylation of Rasgrf1. Nat. Genet. 30:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]