Abstract
Rho family guanine nucleotide exchange factors (GEFs) regulate diverse cellular processes including cytoskeletal reorganization, cell adhesion, and differentiation via activation of the Rho GTPases. However, no studies have yet implicated Rho-GEFs as molecular regulators of the mesenchymal cell fate decisions which occur during development and repair of tissue damage. In this study, we demonstrate that the steady-state protein level of the Rho-specific GEF GEFT is modulated during skeletal muscle regeneration and that gene transfer of GEFT into cardiotoxin-injured mouse tibialis anterior muscle exerts a powerful promotion of skeletal muscle regeneration in vivo. In order to molecularly characterize this regenerative effect, we extrapolate the mechanism of action by examining the consequence of GEFT expression in multipotent cell lines capable of differentiating into a number of cell types, including muscle and adipocyte lineages. Our data demonstrate that endogenous GEFT is transcriptionally upregulated during myogenic differentiation and downregulated during adipogenic differentiation. Exogenous expression of GEFT promotes myogenesis of C2C12 cells via activation of RhoA, Rac1, and Cdc42 and their downstream effector proteins, while a dominant-negative mutant of GEFT inhibits this process. Moreover, we show that GEFT inhibits insulin-induced adipogenesis in 3T3L1 preadipocytes. In summary, we provide the first evidence that the Rho family signaling pathways act as potential regulators of skeletal muscle regeneration and provide the first reported molecular mechanism illustrating how a mammalian Rho family GEF controls this process by modulating mesenchymal cell fate decisions.
The Rho family of small GTPases has been shown to regulate a variety of cytoskeletal-dependent cell functions such as cell morphology changes, formation of focal adhesions and stress fibers, platelet aggregation, cytokinesis, cell cycle progression, and neurite outgrowth and guidance (27). In addition, the Rho family proteins are also involved in the differentiation of many cell types, including neurons, T lymphocytes, myocytes, adipocytes, and keratinocytes (11, 16, 19, 33). One intriguing aspect of the Rho family is their role in regeneration following injury. Rho family proteins have been shown to promote mouse and chick embryonic epidermal healing following injury via formation of a rapidly assembled actin purse string (7, 9, 69). Indeed, even in the adult, the Rho proteins play a role in the formation of “re-epithelium” whereby the initial inflammatory response leads to the influx of macrophages and neutrophils, which subsequently release cytokines, growth factors, and nitric oxide to induce nearby keratinocytes to migrate in a Rho protein-dependent manner across the wounded epithelium (35, 81). In addition, the small GTPases are important intracellular targets for promoting axon regrowth following injury, whereby studies have shown that inactivation of RhoA induces spontaneous plasticity of axonal and dendritic remodeling after spinal cord injury (25, 26, 30, 47). While several reports have demonstrated the function of these proteins in wound healing and neuronal regeneration, to date no studies have examined the effects of the Rho family or its regulators in skeletal muscle regeneration. This is surprising considering the intimate function of this very important family of proteins in skeletal muscle differentiation (11).
Damage to skeletal muscle occurs throughout life via sports and other related activities, leading to a continuous recurrence of regeneration in order to maintain proper muscle form. In degenerating diseases such as diabetes and Duchenne muscular dystrophy, muscle regeneration is impaired and often leads to the well-documented symptoms of these diseases. The process of muscle regeneration, whether induced by everyday or debilitating disease-induced damage, remains an active process for long periods of time after the initial muscle injury. The capacity of tissue for repair occurs via satellite stem cells located between the basal lamina and the sarcolemma in mature skeletal myofibers (39). Upon muscle injury, satellite cells exit G0 and enter the cell cycle to proliferate. At this time, they either renew the quiescent stem cell pool by reentering G0 or differentiate into terminal myofibers.
The Rho family guanine nucleotide exchange factor GEFT is capable of activating RhoA, Rac1, and Cdc42 via the catalysis of the exchange of bound GDP for GTP on the Rho proteins, thereby inducing their activation and the activity of their downstream targets (10). Because GEFT has been shown to be highly expressed in the adult skeletal muscle (10, 34), we sought to determine if this protein plays a role in the regulation of skeletal muscle regeneration. Our data demonstrate that endogenous GEFT protein levels are highly modulated during the process of cardiotoxin-induced muscle injury and subsequent regeneration of the mouse tibialis anterior muscle. Moreover, infection of the tibialis anterior muscle with a GEFT-expressing adeno-associated virus following cardiotoxin-induced skeletal muscle injury resulted in significantly improved regeneration by day 15 compared to vector control infection. Because muscle regeneration involves the Rho family-dependent process of myogenesis, we examined the molecular mechanism by which GEFT promotes skeletal muscle regeneration utilizing the multipotent C2C12 mesenchymal progenitor cells which are capable of differentiating into a number of cell types, including skeletal muscle cells. Our studies demonstrate that GEFT is strongly expressed in differentiated C2C12 myocytes, and, via activation of the Rho-GTPases and their downstream signaling pathways, GEFT enhances the myogenic cascade, as evidenced by myotube formation and an increased expression of differentiated muscle markers following GEFT expression. In addition, our data demonstrate that GEFT expression is downregulated during adipogenesis and that exogenous expression of this protein strongly inhibits adipogenesis of 3T3L1 cells. Importantly, our novel findings are the first reported which implicate the Rho-signaling pathways in the regulation of skeletal muscle regeneration and which demonstrate the involvement of a mammalian Rho family GEF in the regulation of myogenic and adipogenic cell fate determination.
MATERIALS AND METHODS
Animal model of hind-limb muscle injury.
Injury in the tibialis anterior muscle was induced by injecting 25 μl of 1 mM cardiotoxin (Sigma) as previously described (4). To quantify cardiotoxin-induced injury and subsequent regeneration, microphotographs of histological samples of transversal sections of the tibialis anterior muscle were examined in a blinded fashion by two different examiners. Quantification was performed on histological sections from the upper, middle, and lower regions of the tibialis anterior muscle (three sections per region; n = 6 animals per group). The areas of injury, as defined by absence or subconfluence of myofiber formation, as well as the cross-sectional fiber area, were quantified and expressed as the percentage ± standard deviation.
Adeno-associated virus (AAV) production and infection.
Human GEFT cDNA was subcloned from a previously constructed GEFT-pCMVTag2B expression vector (10) into the EcoRI and XhoI sites of the pAAV-internal ribosome entry site (IRES)-human recombinant green fluorescence protein (GFP) AAV2 vector (Stratagene). To produce the AAV2/8 viruses expressing human GEFT, the AAV8 (p5E18-VD2/8) vector, the adeno-helper plasmid pAdΔF6, and pAAV2-GEFT-IRES-hrGFP were cotransfected into 293 cells using the calcium phosphate-mediated transfection method (31a). After 72 h of incubation, the cells were harvested, pelleted down, and resuspended in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8.5). Purification of the virus was performed using an Iodixanol density gradient centrifugation procedure as described previously (94, 95). The purified viral stocks were titrated using SYBR-Green real-time PCR to determine the genome copies. As a control, vector AAVs were prepared as described above.
In the hind-limb regeneration mouse model, the 3 × 1011 recombinant AAV2/8 virus particles were injected in the tibialis anterior as previously described (4). All experiments were performed in groups including 6 mice from 4 to 6 weeks of age.
In situ hybridization and immunohistochemistry.
Digoxigenin (DIG)-labeled GEFT-specific antisense and sense probes were produced using the DIG Nucleic Acid Detection kit (Roche) according to the manufacturer's directions and used in in situ staining to detect GEFT mRNA expression in paraffin sections of adult skeletal muscle using a DIG Wash and Block Buffer Set (Roche) according to the manufacturer's instructions.
For whole-mount in situ hybridization, embryos were rehydrated and subsequent Proteinase K digestion was performed according to stages between 7 min and 1.5 h. Prehybridization was done in hybridization buffer (50% formamide, 50 μg/ml heparin, 0,1% Tween 20, 5 mg/ml torula RNA, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) at 65°C for 2 h. Denatured DIG-labeled antisense (specific for GEFT) and sense (control) probes were hybridized for 12 h at 65°C. All washing steps were performed at 65°C. The first three washes were done with 50% formamide-2× sodium citrate-sodium chloride-Tween 20 (SSCT) (two washes for 15 min, one wash for 30 min). These were followed by two washes with 2× SSCT (15 min each) and two washes with 0.2× SSCT (15 min each). Blocking of the embryos was performed for 2 h with 5% sheep serum-PTW (phosphate-buffered saline [PBS], pH 7.5, 0.1% Tween 20). The embryos were incubated for 2 h in preabsorbed anti-DIG-probes at a 1:2,000 dilution in PTW. After eight washes for 10 min with PTW, two incubations of 7 min each with staining buffer (100 mM Tris-Cl, pH 9.5, 100 mM NaCl, 50 mM MgCl2, 0.1% Tween 20) concluded the hybridization procedure. Staining was done for a maximum of 6 h, whereby individual reactions were stopped according to the signal intensity. The staining reaction was stopped by several washes with PTW and postfixed overnight in 1% paraformaldehyde-PTW at 4°C.
Immunohistochemistry (IHC) of paraffin-embedded tissues was performed using the ABC-Staining System according to manufacturer's instructions (Santa Cruz Biotechnology). All sections were counterstained with Harris Modified Hematoxylin with acetic acid (Fisher Scientific). Anti-GEFT peptide antibody raised in rabbits against the last 13 amino acids of the C terminus of GEFT (Proteintech Group, Inc.) was used at a 1:100 dilution for IHC. Control IHC experiments (data not shown) were performed using the rabbit preimmune serum and anti-GEFT blocked with saturating levels of its specific peptide antigen.
Northern blot analysis.
Total RNA from cultured cells was isolated with Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA gel electrophoresis and transfer onto nitrocellulose were performed by standard techniques. Expression of GEFT or hypoxanthine-guanine phosphoribosyltransferase (HPRT) in C2C12 cell lysates was assayed via hybridization of a [32P]dCTP-labeled gene-specific probe against the membrane. Radiolabeled probes were purified using prepacked Sephadex G50 columns (Amersham). Northern hybridizations were carried using standard methods, exposed to autoradiography film overnight, and then developed using a Kodak Processor.
Western blot analysis.
To prepare whole-cell lysates, cells were lysed in cell lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM sodium orthovanadate). The insoluble material was excluded by centrifugation. The resulting supernatant was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and was subjected to SDS-PAGE. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane, and the blot was incubated with the appropriate primary antibody. Following incubation with the primary antibody, the membrane was exposed to a horseradish peroxidase-conjugated secondary anti-mouse, anti-rabbit, or anti-goat antibody, subjected to SuperSignal West Pico Chemiluminescent reagent (Pierce Biotechnology, Inc.), and exposed to film. Antibodies were used in the following dilutions: 1:1,000 anti-GEFT peptide antibody (Proteintech Group, Inc.); 1:1,000 anti-actin (Santa Cruz Biotechnology); 1:25 anti-F5D myogenin antibody (Developmental Studies Hybridoma Bank; 1:25 anti-MF20 myosin heavy chain (MHC; Developmental Studies Hybridoma Bank); 1:1,000 anti-RhoA (Santa Cruz Biotechnology); 1:1000 anti-Rac1 (Santa Cruz Biotechnology); 1:300 anti-Cdc42 (Santa Cruz Biotechnology); 1:250 anti-phospho-Rho kinase (Thr396) (AnaSpec); 1:500 anti-Rho kinase (paired nonphospho) (AnaSpec); 1:1,000 anti-Pak1 (Santa Cruz Biotechnology); 1:500 anti-phospho-Pak1 (Thr423) (Chemicon); 1:2,000 anti-phospho-p38 (Promega); 1:1,000 anti-p38 (Santa Cruz Biotechnology); 1:2,000 anti-phospho-JNK (Promega); 1:1,000 anti-JNK (Santa Cruz Biotechnology); 1:2,500 anti-phospho-p42/44 (Promega); 1:1,000 anti-p42/44 (Santa Cruz Biotechnology); 1:100 anti-p21 (Santa Cruz Biotechnology); 1:25 anti-CT3 Troponin T (Developmental Studies Hybridoma Bank); 1:250 anti-MyoD (Santa Cruz Biotechnology).
Reverse transcription-PCR (RT-PCR) analysis.
Total RNA was isolated from 3T3L1 preadipocyte cells using Trizol reagent (Invitrogen). Total RNA of 1.0 μg was used as a template for first-strand cDNA synthesis using oligo(dT) as primer in the presence of Superscript II reverse transcriptase (Invitrogen). One milliliter of cDNA was subsequently used as a template for PCR amplification using Taq polymerase (New England Biolabs).
Cell culture methodology.
C2C12 cells were grown at subconfluent levels in growth medium (GM) consisting of Dulbecco's modified Eagle medium (DMEM) (HyClone) supplemented with 10% fetal bovine serum (FBS) (HyClone). To induce differentiation, cells were grown to 100% confluence and GM was replaced with differentiation medium (DM) consisting of DMEM (HyClone) supplemented with 2% horse serum (Gibco). Stable transfected cells were selected in medium supplemented with 400 μg/ml G418. DM was replaced every 24 h.
3T3L1 preadipocyte cells were grown at subconfluent levels in growth medium (GM) consisting of DMEM (HyClone) supplemented with 10% bovine calf serum (BCS) (Gibco). To induce differentiation, cells were grown to 100% confluence and GM was replaced with differentiation medium (DM) consisting of DMEM (HyClone) supplemented with 10% fetal calf serum (Gibco), 0.5 mM isobutyl methyl xanthine (IBMX) (Sigma), 1 μM dexamethasone (Sigma), and 500 ng/ml insulin (Sigma).
Plasmids and transfections.
The pCMV-Tag2B mammalian expression vector containing the human GEFT cDNA sequence was generated previously (11). The dominant-negative GEFT mutant was generated using PCR with primers specific for the gene resulting in the following amino acids of GEFT remaining: 165 to 467. RhoA, Rac1, and Cdc42 constructs were obtained from the Guthrie cDNA Resource Center. Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's directions. For all experiments other than reporter assays, pools of cells were stably selected with G418 prior to experimental analysis. Via Western blot analysis, these pools were found to exhibit a 5- to 10-fold increase in GEFT protein levels compared to vector-transfected C2C12 and 3T3L1 cells (data not shown).
Immunofluorescence.
Immunofluorescence for GFP was performed on frozen sections of mouse muscle that were fixed in ice-cold methanol for 10 min and washed in PBS. Cells growing on glass coverslips were fixed in ice-cold methanol for 10 min, followed by 10 min of permeabilization in 0.1% Triton X-100 in PBS. Reactions were blocked for 30 min with 0.2% bovine serum albumin, followed by 60 min of incubation with a rabbit polyclonal anti-GEFT antibody (1:100 dilution; Proteintech Group, Inc.), a mouse monoclonal MF-20 antibody (1:5 dilution; Iowa Hybridoma Bank), mouse monoclonal anti-FLAG epitope antibody (1:500; Strategene), or rabbit polyclonal anti-hemagglutinin (HA) epitope antibody (1:500; Santa Cruz Biotechnology). Fluorescein-conjugated goat anti-rabbit or goat anti-mouse antibody or Texas Red-conjugated anti-rabbit were added for 30 min (1:1,000; Molecular Probes). Polymerized actin was visualized by incubation with Texas Red-conjugated phalloidin (1:1,000 dilution; Sigma). Nuclear staining was observed after 10 min of 4′,6-diamidino-2-phenylindole (DAPI) treatment (1:500 dilution; Molecular Probes). Fluorescence images were captured at 400× on a CCD camera mounted on an inverted research microscope using Ultraview imaging software (Olympus, Inc.).
GTPase activation assays.
Lysates were incubated with glutathione S-transferase (GST)-rhotekin or GST-Pak1 previously bound to glutathione-Sepharose beads and washed four times with lysis buffer, and associated GTP-bound forms of RhoA, Rac1, or Cdc42 were released with protein loading buffer and revealed by Western blot analysis.
Subcellular fractionation.
Collected cells were washed with PBS and resuspended in 400 ml ice-cold cytoplasmic lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, pH 8.0, 0.1 mM EGTA, 1 mM dithiothreitol, 1.0 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 0.5 mg/ml benzamidine) and incubated for 15 min on ice. A volume of 12.5 μl of 10% NP-40 was added, the reaction was centrifuged at full speed for 1 min, and the supernatant was collected corresponding to the cytoplasmic extract. The pelleted nuclear extract was resuspended in 25 μl nuclear extraction buffer (20 mM HEPES, pH 7.9, 400 mM NaCl, 1.0 mM EDTA, pH 8.0, 1.0 mM EGTA, pH 7.0) and incubated on ice for 30 min with intermittent vortexing. The nuclear extract was collected following centrifugation.
Luciferase reporter assays.
Cells were grown in 24-well plates and 1 μg total DNA per well was transfected using Lipofectamine (Invitrogen) according to the manufacturers suggestions. Cells were harvested 24 h posttransfection for C2C12 cells or 48 h posttransfection for 3T3L1 cells in reporter lysis buffer (Promega). Luciferase expression was detected with a luciferase assay system (Promega). As a transfection control, β-galactosidase (β-gal) activities were determined with the β-gal reporter gene assay (Roche).
ORO staining.
Oil Red O (ORO) staining for lipids was performed on 3T3L1 cells that were fixed with 4% paraformaldehyde, 0.2% Triton X-100 in PBS for 10 min and washed in PBS. The cells were then incubated with 0.6% (wt/vol) Oil Red O solution (Avocado Research Chemicals Ltd.) dissolved in 50% dimethyl sulfoxide for 1 h at room temperature.
RESULTS
Gene transfer of GEFT promotes skeletal muscle regeneration following severe injury.
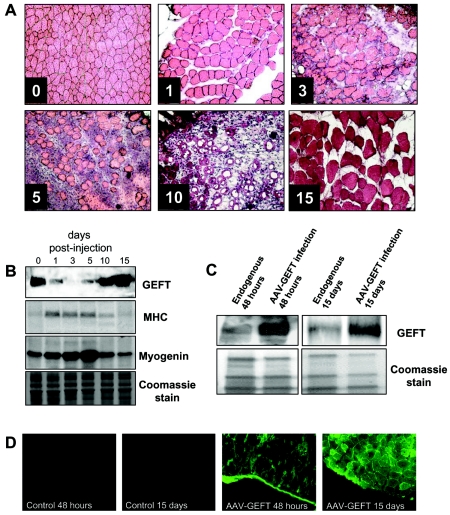
Day-to-day wear and tear of adult skeletal muscle accounts for approximately 1 to 2% replacement of muscle fibers per week (75); however, mammalian skeletal muscle has the ability to induce an extensive regeneration in response to severe damage. The use of myotoxins such as bupivacaine, cardiotoxin, and notexin is perhaps the easiest and most reproducible way to induce muscle regeneration in animal models (23, 36-39). Muscle regeneration, both physically and chemically induced, is characterized by two phases: a degenerative phase and a regenerative phase. For example, the initial event of muscle degeneration following cardiotoxin injection into the mouse tibialis anterior muscle is characterized by significant necrosis of muscle fibers (Fig. 1A) and is accompanied by increased levels of muscle proteins, including myosin heavy chain and myogenin (92) (Fig. 1B). Muscle degeneration is followed by the onset of active muscle repair in which satellite stem cells from the basal lamina proliferate and differentiate to fully repair the damaged skeletal muscle (17, 20, 42, 84).
FIG. 1.
Infection of AAV2/8-GEFT in the mouse tibialis anterior muscle. (A) Representative immunohistochemical sections of cardiotoxin-injured tibialis anterior muscle sections stained with H&E and taken from untreated and 1, 3, 5, 10, and 15 days postcardiotoxin-treated muscles. (B) Western blot analysis was performed to examine the steady-state protein levels of GEFT and the myogenic proteins, myosin heavy chain and myogenin, during cardiotoxin-induced regeneration. (C) Western blot analysis demonstrating sustained exogenous expression of GEFT in normal tibialis anterior muscles at 48 h and 15 days postinfection. (D) Immunofluorescence of GFP signal from infected and uninfected mouse tibialis anterior muscles taken 48 h and 15 days postinjection, further demonstrating the sustained expression of the AAV2/8-GEFT virus.
Because the Rho family of small GTPases has been implicated in the regulation of the myogenic cascade (11), we examined if this signaling pathway plays a role in skeletal muscle regeneration following cardiotoxin-induced muscle regeneration. The Rho family specific guanine nucleotide exchange factor, GEFT, has been shown to be highly expressed in adult skeletal muscle and can activate the small GTPases RhoA, Rac1, and Cdc42 using both in vivo and in vitro assays (10, 34). Western blot analysis of endogenous GEFT steady-state protein levels in untreated and cardiotoxin-injected tibialis anterior muscles revealed high levels of GEFT in healthy muscle tissue, accompanied by a significant reduction in expression during the initial phases of muscle degeneration at 1, 3, and 5 days postcardiotoxin injection (Fig. 1B). At 10 and 15 days following cardiotoxin treatment, endogenous GEFT levels returned to approximately the level of untreated muscles. These data indicate that regulation of GEFT is important for skeletal muscle regeneration and suggests that GEFT may play an active role in this process. Additionally, these findings are significant in that most GEFs are regulated posttranslationally, while our data suggest that GEFT is regulated at the level of expression.
The potential of adeno-associated virus (AAV)-mediated gene transfer into cells has been exploited in multiple studies, offering a unique opportunity to study the effects of gene expression for prolonged periods of time in vivo in the absence of inflammatory or immune response (8, 21). To investigate directly whether GEFT might affect the regenerative capacity of skeletal muscles during cardiotoxin-induced regeneration, we injected purified 3 × 1011 AAV2/8-GEFT virus particles into the cardiotoxin-damaged right tibialis anterior muscles of 4- to 6-week-old adult mice. Western blot analysis indicated that exogenous GEFT is detectable within 48 h of infection and remained high at least until 15 days postinfection (Fig. 1C). Moreover, IRES-GFP signal was detected in frozen sections collected from the infected muscle at 48 h after AAV2/8-GEFT injection and remained strong at 15 days postinfection, while untreated muscle exhibited no significant background fluorescence, further confirming the fidelity and stability of the AAV-GEFT virus for use in gene transfer in skeletal muscle (Fig. 1D).
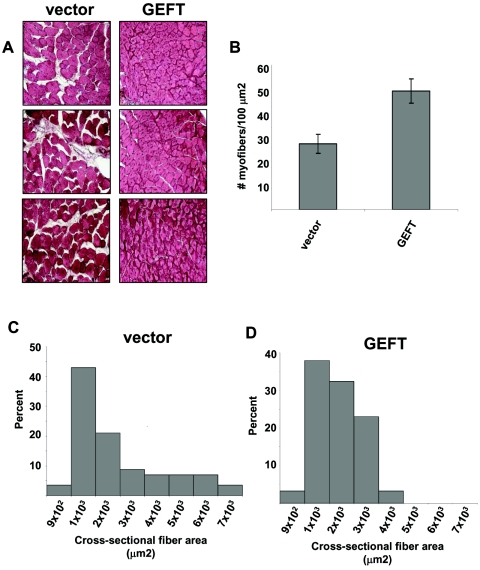
To explore the therapeutic potential of AAV2/8-GEFT administration after skeletal muscle damage, we administered an injection of 3 × 1011 AAV2/8-GEFT or control virus particles directly into cardiotoxin-damaged mouse tibialis anterior muscles and harvested the mice 15 days following initial muscle damage. Compared to the control treatment, AAV2/8-GEFT resulted in a remarkable improvement in the regeneration process. While at day 15 after injury hematoxylin- and eosin-stained (H&E) sections showed centronucleated myofibers in both vector- and GEFT-treated muscles (a characteristic hallmark of regenerating muscle fibers), the damaged area in AAV2/8-GEFT-treated muscles encompassed less than 10% of the transversal muscle section area, compared with greater than 40% of the control (Fig. 2A). To quantify the effect of the AAV2/8-GEFT treatment more precisely, we measured the myofiber density and muscle fiber cross-sectional area. Compared to control treatment, GEFT-injected muscles exhibited significantly more densely packed myofibers at 15 days postcardiotoxin injection (Fig. 2B). Moreover, the fiber area distribution of the control-injected muscles skewed to the right and exhibited a much broader distribution of myofiber area, indicative of less uniformly regenerated skeletal muscle fibers (Fig. 2C). However, the distribution of the cross-sectional fiber area was relatively narrow and symmetric in the AAV2/8-GEFT-injected muscles, suggesting a more uniform myofiber size indicative of regenerated muscle (Fig. 2D).
FIG. 2.
Sustained expression of GEFT in cardiotoxin-damaged tibialis anterior muscles induces muscle fiber regeneration. (A) H&E-stained sections of tibialis anterior muscle from control and GEFT AAV2/8-infected mice at 15 days after cardiotoxin-induced injury. (B) Number of myofibers per 100-μm2 area at 15 days postcardiotoxin injection. (C and D) The fiber cross-sectional area distribution of control and GEFT AAV2/8-infected tibialis anterior muscles measured at 15 days postcardiotoxin injection.
GEFT steady-state mRNA and protein levels are upregulated during myogenic differentiation.
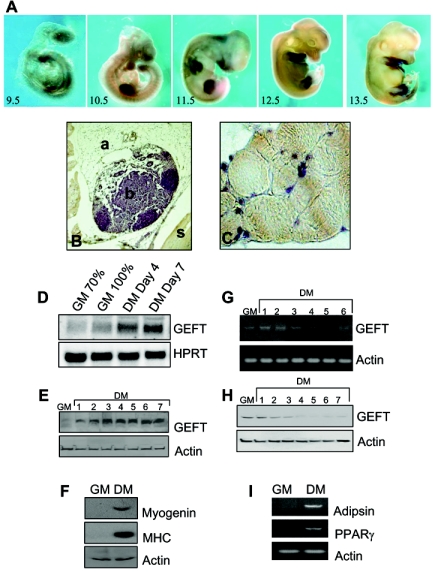
Many of the signaling pathways underlying skeletal muscle regeneration have been identified and are highly studied. Intriguingly, many of these pathways resemble those that are activated or repressed during skeletal muscle formation from mesenchymal progenitor cells during development. Indeed, whole-mount in situ hybridization on mouse embryos at the embryonic days 9.5 to 13.5 exhibited a strong positive staining (dark staining) for GEFT mRNA throughout the limb bud, suggesting that GEFT is highly expressed and potentially important in mesodermal cells which will specify the developing limb (Fig. 3A). Moreover, terminally differentiated skeletal muscle of the adult hind limb revealed intense staining of GEFT in skeletal muscle (s) but not in bone (b) or adipocyte tissue (a) (Fig. 3B and C), suggesting that GEFT is important not only for early skeletal muscle differentiation but also in the muscle in adulthood.
FIG. 3.
Expression of GEFT at mRNA and protein levels is highly regulated during myogenesis and adipogenesis. (A) Whole-mount in situ hybridizations of GEFT mRNA expression at mouse embryonic days 9.5 to 13.5. (B and C) Immunohistochemical detection of GEFT protein in the adult hind limb. Positive immunostaining appears brown (a, adipocytes; b, bone; s, skeletal muscle). Magnification for panel B, 40×; for panel C, 100×. (D) Northern blot analysis of GEFT steady-state mRNA expression in C2C12 cells subjected to proliferative growth medium (GM) at 70% and 100% confluence as well as at days 4 and 7 of differentiation conditions (DM). As a loading control, hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA expression was detected. (E) Western blot analysis of GEFT steady-state protein levels in C2C12 cells grown in growth medium (GM) or days 1 to 7 of differentiation (DM). (F) Analysis of myogenic markers by Western blot. To ensure that myogenic differentiation is properly occurring, Western blotting was performed in growth medium (GM) and day 5 differentiation (DM) for the myogenic markers myogenin and MHC. (G) RT-PCR detecting GEFT steady-state mRNA expression in 3T3L1 cells subjected to nondifferentiation (GM) and days 1 to 6 of differentiation (DM) conditions. (H) Western blot analysis of GEFT steady-state protein levels in 3T3L1 cells subjected to nondifferentiation (GM) and days 1 to 7 of differentiation (DM) conditions. (I) RT-PCR analysis of adipogenic differentiation in growth medium (GM) and day 4 differentiation (DM) for the adipogenic markers adipsin and PPARγ.
In order to fully understand the molecular signaling pathways mediated by GEFT during skeletal muscle regeneration and differentiation, we utilized the highly studied multipotent C2C12 mesenchymal progenitor cells. Epithelial derived mesenchymal cells, muscle satellite cells, and cell lines such as C2C12 cells are unique in that they are capable of differentiating into a number of cell types, including chondrocytes, osteoblasts, myocytes, and adipocytes (1, 6, 59, 63, 65). Recently, much interest has cumulated over the cell fate decision between myogenesis and adipogenesis, two lineages in which regenerating satellite cells are capable of becoming myocytes and adipocytes, suggesting that RhoA plays an important role in the insulin-directed control of these two processes (73, 79). In order to examine if GEFT may perform a function in this process, we first examined the expression profiles of GEFT during the C2C12 myogenic cascade and the 3T3L1 adipogenic cascade. Specifically, upon serum starvation under high confluence conditions, C2C12 cells will enter the myogenic cascade, thus forming multinucleated myotubes and expressing markers of terminal skeletal muscle differentiation. As evidenced by both Northern and Western blot analyses, GEFT mRNA and protein levels are highly upregulated upon subjecting the cells to differentiation conditions (Fig. 3D and E). As a control to demonstrate that the myogenic cascade is occurring in our experiments, we examined the expression of myogenin and MHC, both important muscle-specific proteins that are strongly upregulated upon terminal myogenic differentiation. Indeed, no myogenin or MHC is present in proliferative conditions (GM); however, upon differentiation under differentiation medium (DM), a strong band is detected for myogenin and MHC, respectively, demonstrating the fidelity of our experiments (Fig. 3F).
3T3L1 preadipocyte cells are capable, upon treatment with isobutyl methyl xanthine (IMBX), dexamethasone, and insulin, to differentiate from fibroblast cells to adipocyte cells (45). These differentiated cells possess biochemical and morphological features of adipocytes whereby differentiation is accompanied by massive increases in the specific activities of enzymes and receptors involved in fatty acid and triglyceride synthesis. As opposed to the trends observed during C2C12 myogenesis, we demonstrate that GEFT is significantly downregulated at the steady-state mRNA and protein levels during 3T3L1 differentiation with RT-PCR and Western blot analysis (Fig. 3G and H). In order to demonstrate that we are truly differentiating the 3T3L1 cells, we utilized RT-PCR to detect the expression of adipogenic markers, adipsin and peroxisome proliferator-activated receptor γ (PPARγ), both of which were detectable in lanes correlating to differentiated 3T3L1 cells but not in lanes correlating to undifferentiated cells (Fig. 3I).
Subcellular localization of GEFT in proliferating and differentiated C2C12 cells.
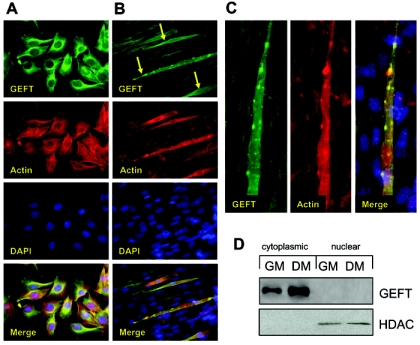
Immunofluorescent staining of endogenous GEFT was performed in proliferative growth conditions (GM) and differentiating conditions (DM). In GM conditions, endogenous GEFT appears to be exclusively cytoplasmic and partially colocalizes with actin in a perinuclear fashion (Fig. 4A). Fluorescent GEFT staining in C2C12 cells differentiated for 5 days demonstrates a significantly stronger staining in multinucleated myotubes (arrows) than in nonfused surrounding cells (Fig. 4B), suggesting that in addition to the initial upregulation of GEFT mRNA and protein levels upon shift to DM treatment, a further increase in GEFT protein expression occurs specifically in cells that have undergone myotube fusion. A higher magnification examination of the GEFT-rich myotubes illustrates that, while GEFT protein is present throughout the cell, it is highly concentrated in actin-rich regions lining the myotube (Fig. 4C). This unique subcellular patterning occurs in approximately 40% of myotubes. Moreover, no studies have examined the distinct subcellular localization of GEFT. Therefore, we performed cytoplasmic-nuclear fractionation of C2C12 cells under GM and DM conditions, respectively. Given that no putative nuclear localization sequence exists in the amino acid sequence, GEFT is expressed exclusively in the cytoplasmic compartment in either condition with no protein present in the nuclear fraction (Fig. 4D). Our previous studies have shown that GEFT is capable of activating RhoA, Rac1, and Cdc42 (10, 34), and considering that GEF activation often targets the Rho family to specific subcellular regions, our data suggest that the activation of these proteins occurs in the cytoplasm.
FIG. 4.
GEFT subcellular localization in C2C12 cells. Endogenous GEFT subcellular localization was detected by immunofluorescence with an anti-GEFT specific antibody in proliferative (A) and day 5 differentiation (B) conditions (400× magnification). (C) High-magnification image of GEFT subcellular localization in a multinucleated myotube. (D) Analysis of GEFT in cytoplasmic and nuclear fractions of C2C12 cells in growth medium (GM) and 4 days differentiation medium (DM). Western blot analysis was performed with an anti-GEFT specific antibody to confirm the subcellular localization of GEFT in cytoplasm. HDAC, histone deacetylase.
Activation of RhoA, Rac1, Cdc42, and their downstream signaling cascades by GEFT.
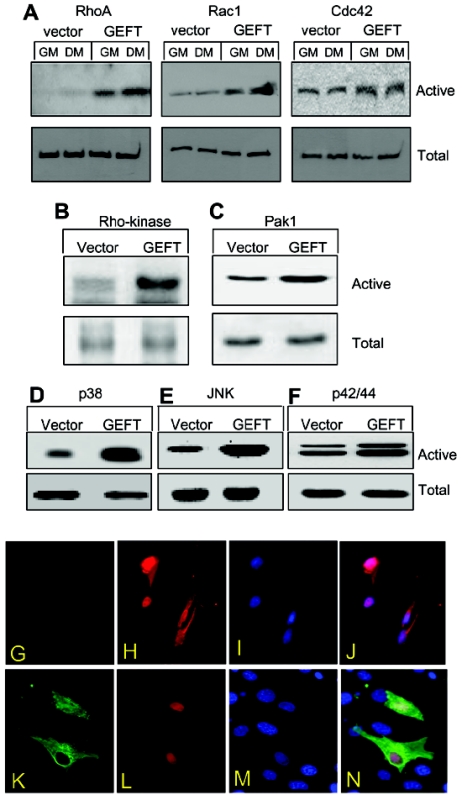
Our previous report (10) demonstrated that GEFT functions as a promiscuous GEF, regulating the activation of RhoA, Rac1, and Cdc42 in Neuro2A cells. In order to examine if this trend holds true in C2C12 cells, we performed GTPase activation assays examining the levels of the active GTP-bound forms of each GTPase. Active Rac1 and Cdc42 were pulled down with GST bead-bound Pak1, which is only capable of binding these two GTPases in their active GTP-bound form, while active RhoA was pulled down with GST bead-bound Rhotekin, which is only capable of binding RhoA in its active GTP-bound form. Western blots were performed with anti-RhoA, Rac1, and Cdc42-specific antibodies detecting the active forms from the GST fusion protein pull-down assays as well as the total GTPase levels from the cell lysate. As illustrated in Fig. 5A, exogenous expression of GEFT promotes the active forms of all three GTPases in either GM or DM conditions. Moreover, Pak1 and Rho kinase are well studied downstream effectors of Rac1/Cdc42 and RhoA, respectively, (18, 43, 54, 70). Using phosphospecific Pak1 and Rho kinase antibodies, which only detects the activated forms of these proteins, we observe that GEFT strongly upregulates the activation of these two proteins (Fig. 5B and C), suggesting that GEFT mediates Rac1, Cdc42, and RhoA pathways in this cell type. Additionally, Rac1 and Cdc42 are known to regulate p38, JNK, and p42/44 mitogen-activated protein kinase pathways (58, 83). Using phosphospecific antibodies that recognize only the activated forms of these proteins in Western blot analysis of vector- and GEFT-transfected cells, we determined that GEFT is capable of strongly upregulating the activation of these proteins (Fig. 5D to F), further providing evidence that GEFT signals, in addition to RhoA, through the Rac1 and Cdc42 cascades. All three GTPase members have been shown to regulate the subcellular localization of the smooth, cardiac, and skeletal muscle-specific transcription factor serum response factor (SRF) (41, 50, 60, 61, 86), which has been shown to upregulate essential myogenic proteins, including α-actin and MyoD (18, 32, 48, 74). In order to test whether exogenous expression of GEFT is capable of regulating the nuclear shuttling of SRF, we coexpressed either an untagged empty vector or a FLAG-tagged GEFT expression vector with an HA-tagged SRF expression vector in C2C12 cells. Immunofluorescent staining demonstrates that in control cells, SRF is localized both in the cytoplasm and nucleus (Fig. 5G to J), while in GEFT-transfected cells, SRF is exclusively nuclear (Fig. 5K to N), confirming that overexpression of GEFT is also capable of regulating the nuclear translocation of this important transcription factor involved in skeletal myogenesis.
FIG. 5.
Regulation of RhoA, Rac1, Cdc42, and their downstream signaling pathways in C2C12 cells by exogenous expression of GEFT. (A) C2C12 cells were stably transfected with either a vector or GEFT plasmids. Cell lysates were collected and RhoA, Rac1, and Cdc42 were affinity purified by binding to GST-Rhotekin (for RhoA) or GST-Pak1 (for Rac1/Cdc42) beads. The protein bound to GST beads (active forms) as well as the total protein were analyzed by Western blotting with antibodies against RhoA, Rac1, or Cdc42. (B to F) Activation of downstream protein kinases by GEFT. Vector or GEFT stably transfected C2C12 cells were collected and the lysates were subjected to Western blot analysis with phosphospecific antibodies specific for the active, phosphorylated forms of (B) Rho kinase, (C) Pak1, (D) p38, (E) JNK, and (F) p42/44. As a loading control, Western blotting was performed with complementary antibodies (not phosphospecific) to determine the total levels of each protein. (G) Nuclear translocation of SRF promoted by GEFT. C2C12 cells were plated on glass coverslips and transiently cotransfected HA-tagged SRF with either an empty vector or FLAG-tagged GEFT. Immunofluorescent staining with anti-FLAG and anti-HA antibodies reveal the subcellular localization of SRF response to the presence or absence of exogenous GEFT.
GEFT promotes C2C12 skeletal myogenesis.
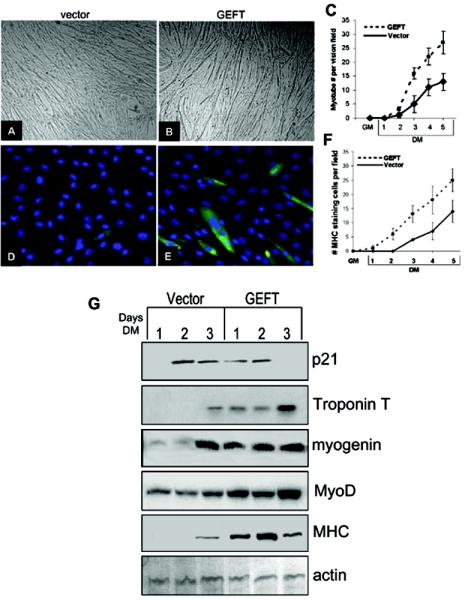
C2C12 cells are capable of proliferative growth when subjected to serum-rich, subconfluent growth conditions (GM). However, upon 100% confluence and serum deprivation, these cells will withdraw from the cell cycle, polarize with respect to each other, and fuse to form multinucleated myotubes. In order to test the overall effect of GEFT on myogenesis, we analyzed the GEFT-mediated effects on the above-mentioned processes. GEFT overexpression in C2C12 cells subjected to 3 days of differentiation resulted in significantly more myotube formation than vector cells (Fig. 6A to C). In addition, we observed that the percentage of immunofluorescently labeled myosin heavy chain-positive C2C12 cells was significantly higher in GEFT-transfected cells than in vector-transfected cells (Fig. 6D to F).
FIG. 6.
GEFT strongly upregulates biomarkers of myogenic differentiation. (A and B) C2C12 cells, stably transfected with either vector (A) or GEFT (B) plasmids, were subjected to differentiation conditions. Photos were taken at day 3 of differentiation, showing a significant increase of myotubes in GEFT-expressing cells. (C) Histogram illustrating the mean number of myotubes per vision field in vector- and GEFT-transfected cells from growth media up to 5 days of differentiation. The data are the means ± standard errors of the means of at least three independent experiments. (D and E) Immunofluorescent staining with the myosin heavy chain-specific MF-20 antibody on vector (D) and GEFT (E) transfected C2C12 cells at day 2 of differentiation. Myosin heavy chain-positive cells appear green. (F) Graph illustrating the mean number of myosin heavy chain positive cells per vision field in vector- and GEFT-transfected cells from growth media up to 5 days differentiation. The data are means ± standard errors of the means of at least three independent experiments. (G) C2C12 cells stably transfected with either vector control or GEFT were differentiated, and cell lysates were collected at days 1, 2, and 3 of differentiation. Subsequent Western blot analysis was performed to detect the levels of different myogenic markers.
Several molecular markers of myogenesis are commonly used to judge the degree of differentiation in C2C12 cells. Myosin heavy chain (MHC) (a structural component of muscle fibers), MyoD (a transcription factor upregulated in myogenesis), myogenin (a transcription factor upregulated in myogenesis), troponin T (a structural component of the contractile fibers), and p21 (a cell cycle inhibitor) have been shown to be highly upregulated during differentiation (2, 3, 64, 93). Western blot analysis of these markers demonstrates that p21, troponin T, myogenin, MyoD, and MHC are all upregulated at earlier time points in GEFT-transfected than vector-transfected cells (Fig. 6G), suggesting that GEFT enhances the progression of C2C12 cell myogenesis. Expression of these protein markers, with the exception of MyoD (at equal levels between vector and GEFT), is not detected at significant levels in either vector- or GEFT-transfected cells under proliferating conditions (data not shown).
GEFT promotes the regulation of myogenic markers via the activation of RhoA, Rac1, and Cdc42.
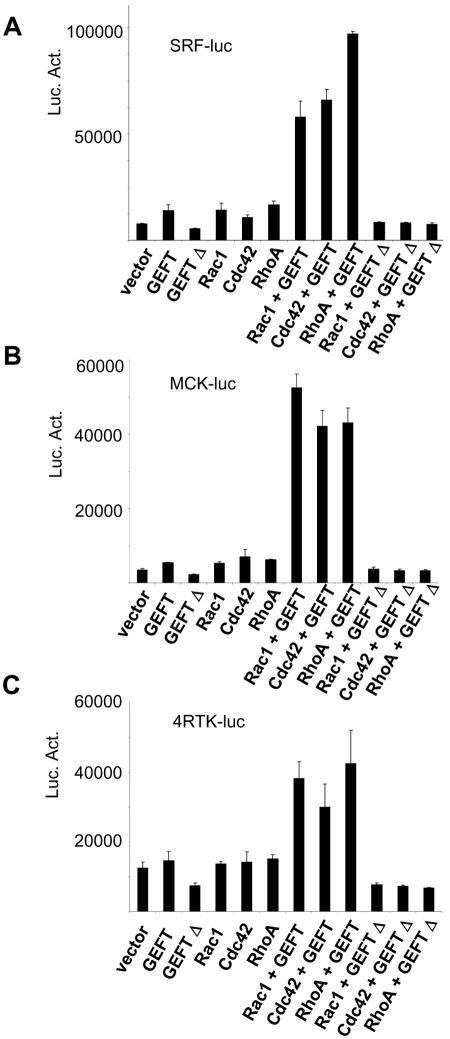
Many reports have attempted to characterize the role of the Rho family GTPases during skeletal myogenesis. While it appears to be universally agreed that RhoA is essential in promoting this process, there are conflicting reports as to the function of Rac1 and Cdc42 in this process which are likely attributed to the reported limitations of using dominant-negative and constitutively active mutants of the Rho proteins (11). Because GEFT appears to promiscuously regulate each of these GTPases in C2C12 cells, we sought to determine which GEFT-mediated GTPase signaling cascade is important for our observed promotion of myogenesis. Using luciferase constructs driven by 4,800 bases of the muscle creatine kinase (MCK) promoter (termed MCK4800), SRF promoter, and 4RTK promoter (an artifical promoter composed of four tandem E-box transcription factor binding sites from the MCK promoter), we analyzed the ability of GEFT together with RhoA, Rac1, or Cdc42 to regulate these promoters. Overexpression of GEFT or any of the GTPases resulted in a modest upregulation of SRF-driven luciferase activity, while coexpression of GEFT with any single GTPase exhibited strong synergistic activation of this reporter construct (Fig. 7A), suggesting that GEFT is capable of regulating SRF-target gene expression via any one of the GTPases. In MCK4800 or 4RTK promoter-driven luciferase assays, expression of GEFT or any single GTPase again resulted in a small increase in luciferase activity (Fig. 7B and C). However, coexpression of GEFT with wild-type RhoA, Rac1, or Cdc42 exhibited a strong synergistic activation of MCK4800 and 4RTK luciferase expression. Lutz et al. (52) reported that a C-terminal truncation of p63RhoGEF, a known splice variant arising from the same gene as GEFT, resulted in a modest dominant-negative effect on its downstream signaling pathways. Using a double N- and C-terminal truncation of GEFT which leaves amino acids 165 to 467 (termed GEFTΔ), we demonstrate a dominant-negative effect that, in our hands, is significantly greater than the single C-terminal truncation previously reported. In SRF, MCK, and 4RTK luciferase assays, expression of this dominant-negative GEFT construct exhibited a nearly 50% reduction compared to the vector level (Fig. 7A to C). Cotransfection of any single Rho-GTPase with the dominant-negative GEFT mutant led to a significant reduction in the luciferase activity to a level well below the expression of any single Rho-GTPase alone. These data suggest that GEFT is capable of regulating myogenic gene expression via activation of RhoA, Rac1, and Cdc42. Moreover, ablation of wild-type GEFT activity with a dominant-negative GEFT mutant led to a significant reduction in the transcriptional activation of the reporter genes, suggesting that at some level, GEFT is essential for myogenic gene expression. Additionally, these data confirm several reports suggesting that RhoA as well as Rac1 and Cdc42 are important positive regulators of myogenesis.
FIG. 7.
Regulation of myogenic transcription factors by the GEFT-mediated activation of the Rho proteins. C2C12 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the (A) muscle creatine kinase (MCK4800), (B) serum response factor (SRF), and (C) 4RTK promoter. Data are the means ± standard errors of the means of at least three independent experiments. Luc. Act., luciferase activity.
GEFT promotes MCK4800-luciferase activity through a synergistic regulation of MyoD, E12, and E47.
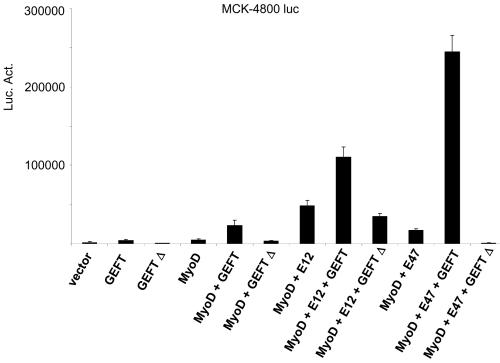
Myogenic regulatory factors bind as heterodimers with E proteins to E-box consensus sites in the promoters of genes regulated at the transcriptional level during myogenesis (12, 13, 14, 64, 67). MyoD, one such myogenic regulatory factor, is capable of binding with E12 or E47 in order to regulate the expression of myogenic proteins. Using MCK4800-luciferase assays, we demonstrate that singular transfection of GEFT or MyoD is capable of a moderate upregulation of MCK4800-luciferase activity, while a strong synergistic activation is observed upon cotransfection of GEFT with MyoD (Fig. 8). Additionally, cotransfection of GEFT and MyoD with either E12 or E47 resulted in a manyfold increase of luciferase activation compared to transfection with any of these constructs alone, suggesting that GEFT is capable of synergistically enhancing the activity of MyoD and its binding partners, E12 and E47, to activate genes and proteins involved in myogenesis. Again, use of the dominant-negative GEFT mutant resulted in a significant reduction in the activation of the MCK4800 luciferase assays when expressed alone or when coexpressed with MyoD, E12, or E47. These data suggest that activation of the Rho-signaling pathways by GEFT, whether direct or indirect, can synergistically promote E-box protein binding and activation of myogenic regulatory factors, such as MyoD, to enhance the expression of myogenic markers.
FIG. 8.
GEFT promotes the activation of MyoD and its heteromeric binding partners, E12 and E47. C2C12 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the muscle creatine kinase (MCK4800) promoter. Data are the means ± standard errors of the means of at least three independent experiments. Luc. Act., luciferase activity.
Inhibition of adipogenesis of 3T3L1 cells by GEFT.
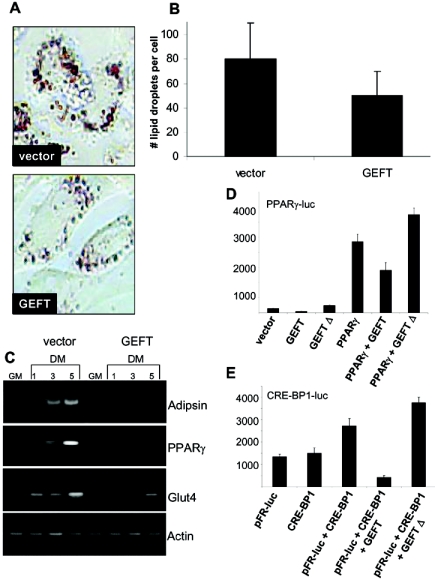
Muscle satellite cells are believed to represent a committed stem cell population that is responsible for the postnatal growth and regeneration of skeletal muscle. However, many findings demonstrate that cultured myoblasts are capable of differentiation into multiple cell lineages, including osteocytes or adipocytes (1, 6, 59, 65, 63). These observations suggest some degree of plasticity within the mesenchymal lineage. To further investigate this phenomenon, we explored the adipogenic potential of mesenchymal lineages with respect to GEFT. Our data demonstrate a strong down-regulation of GEFT expression, both at the steady-state mRNA and protein levels, during 3T3L1 adipogenesis (Fig. 3G and H), suggesting this protein may be essential for maintaining the undifferentiated state of these cells. In order to determine if GEFT can modulate the process of adipogenesis, we utilized fibroblast-derived 3T3L1 preadipocytes, which can be induced to differentiate into mature adipocytic cells upon treatment with supersaturating levels of insulin, dexamethasone, and IBMX. Fully differentiated 3T3L1 cells produce lipid granules that can be stained with Oil Red O (ORO), which strongly binds to neutral lipids and fatty acids. As a visual measure of the effect of GEFT on adipogenesis, we quantitated ORO staining in 3T3L1 cells and found that vector control cells exhibited significantly more stained lipid droplets than GEFT-transfected cells (Fig. 9A and B). Additionally, using RT-PCR analysis measuring the adipogenic markers adipsin, PPARγ, and Glut4, we observed a strong inhibition of marker gene expression in GEFT-transfected cells compared to vector transfected cells (Fig. 9C), demonstrating that GEFT is capable of inhibiting the adipogenic program.
FIG. 9.
GEFT inhibits adipogenesis in 3T3L1 cells. (A) Oil Red O staining of vector- and GEFT-transfected 3T3L1 cells subjected to 5 days of differentiation. (B) Histogram illustrating the mean number of Oil Red O lipid droplets per cell in vector- and GEFT-transfected 3T3L1 cells at day 5 of differentiation. The data are means ± standard errors of the means of at least three independent experiments. P < 0.05 by Student’s t test. (C) 3T3L1 preadipocyte cells were stably transfected with vector control or GEFT. Cell lysates were collected in growth media (GM) and days 1, 3, and 5 of differentiation (DM). Subsequent RT-PCR analysis was performed for the adipogenic markers adipsin, PPARγ, and Glut4. (D and E) 3T3L1 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the (D) PPARγ and (E) the CRE-BP1 promoter. Data are the means ± standard errors of the means of at least three independent experiments.
In order to further confirm that GEFT is capable of regulating signaling cascades that are central in adipogenesis, the effect of GEFT on PPARγ and CRE-BP1 luciferase assays was measured. PPARγ is a nuclear hormone receptor induced very early in the differentiation of several cultured adipocyte cell lines and has been suggested to be a dominant regulator of the AP2 gene which encodes an intracellular lipid binding protein and is expressed only in adipose cells (28, 29, 68). Cotransfection of GEFT with the PPARγ luciferase construct resulted in a reduction from baseline luciferase activity, while cotransfection with the dominant-negative GEFT truncation mutant resulted in a modest increase of luciferase activity (Fig. 9D). Moreover, cotransfection with a PPARγ expression vector led to a strong increase in PPARγ-mediated luciferase activity, while expression of GEFT strongly down-regulated the PPARγ-mediated luciferase activity. Coexpression of PPARγ with the dominant-negative GEFT truncation mutant resulted in a modest increase in activation of the PPARγ reporter.
The activating transcription factor CRE-BP1 (also called ATF2) binds to both AP-1 and CRE DNA response elements and is a member of the ATF/CREB family of leucine zipper proteins (46). CRE-BP1 has been implicated in the transcriptional regulation of cytokines, cell cycle control, and apoptosis. This transcription factor is an important substrate of signals upstream of the activation of genes associated with promoting adipogenesis. As indicated in Fig. 9E, cotransfection of GEFT with the CRE-BP1 responsive trans-reporter construct resulted in a strong decrease in activity compared to the vector control, again demonstrating that GEFT is capable of inhibiting adipogenesis-responsive signaling pathways. Moreover, a modest increase in luciferase activity compared to the vector control was observed with transfection of the dominant-negative GEFT truncation mutant. Together, these data suggest that GEFT down-regulates the adipogenic cascade by inhibiting the transcriptional activation of PPARγ and CRE-BP1.
DISCUSSION
Data from our laboratory demonstrate that the Rho family GEF GEFT regulates important signaling pathways involved in the processes of skeletal muscle regeneration and myogenic differentiation. Our findings represent the first reported study to show that Rho-signaling pathways are capable of regulating skeletal muscle regeneration and are also the first to implicate, at the molecular level, a Rho-GEF in the process of myogenic-adipogenic cell fate determination. Our data show that GEFT steady-state protein levels are modulated during cardiotoxin-induced skeletal muscle regeneration, and infection of mouse skeletal muscle with AAV-GEFT led to a substantial enhancement on muscle regeneration compared to the control. We sought to characterize the molecular mechanisms controlling this process by utilizing well-established differentiation models. GEFT steady-state mRNA and protein levels were found to be strongly increased during myogenic differentiation of multipotent mesenchymal C2C12 cells and downregulated during adipogenic differentiation of fibroblastic 3T3L1 preadipocytes. To gain insight into the role of GEFT in mesenchymal cell fate determination, we expressed this protein in C2C12 and 3T3L1 cells and examined its effect on myogenesis and adipogenesis, respectively. We found that GEFT strongly promotes myogenesis in C2C12 cells through activation of RhoA, Rac1, and Cdc42 and, conversely, inhibits the adipogenic program in 3T3L1 cells. Our data suggest that during skeletal muscle regeneration, GEFT promotes the myogenic lineages of infiltrating progenitor cells, thus promoting the healing process after injury.
Expression of GEFT in vivo and during in vitro cell differentiation.
At present, there are over 70 known Rho family GEFs in mammals (71). This striking number of proteins, each with similar functions and targets, is due in part to the remarkable temporal and spatial complexity of the mammalian development and function. Aside from tissue-specific expression, most GEFs are regulated at the level of posttranslational modifications, including phosphorylation, subcellular localization, and protein-protein interactions. GEFT appears to be unique in that it is regulated highly at the transcriptional level. This is not surprising, given that GEFT is one of the smallest known GEFs with limited N and C termini and putative regulatory domains. Consistent with our observations that GEFT expression is modulated during the skeletal muscle regeneration process, we find that upon differentiation of C2C12 cells, GEFT steady-state mRNA and protein levels are strongly upregulated. Interestingly, skeletal muscle differentiation undergoes dual regulation by both growth factor abundance and cell adhesion. Endogenous C2C12 GEFT levels remained low in growth medium where cells were either 70% confluent or 100% confluent; however, the upregulation occurred upon serum deprivation, suggesting that GEFT expression is not regulated in a cell contact-dependent manner but perhaps in response to growth factor concentrations. Furthermore, immunofluorescence analysis of endogenous GEFT localization reveals that during differentiation, GEFT expression is higher in multinucleated myotubes than in nonmyotube cells, suggesting that GEFT is markedly upregulated in a certain percentage of the cells in the later stages of myogenic differentiation. This finding is consistent with our observation that GEFT steady-state protein levels are upregulated late in the regenerative process at 10 and 15 days postcardiotoxin injection. The upregulation of GEFT during myogenesis is not surprising given that the GEFT promoter contains many high-stringency putative myogenic regulatory factor and SRE elements, both of which play essential roles as regulatory elements in the genes of important function during myogenic differentiation.
Muscle satellite cells possess multipotential mesenchymal stem cell activity and are capable of forming osteocytes and adipocytes as well as myocytes. Intracellular and extracellular signals are responsible for the cell fate decision during muscle regeneration toward the formation of myoblasts but not adipocytes or other incorrect cell types. As an excellent model system to examine adipogenesis, 3T3L1 preadipocyte cells are capable of differentiation from fibroblast cells to adipocyte cells upon treatment with IMBX, dexamethasone, and insulin. In correlation to our IHC experiments, differentiation of 3T3L1 preadipocytes results in a substantial decrease in GEFT steady-state mRNA and protein levels by day 3 of treatment. Interestingly, day 3 to 4 of treatment marks a well studied biochemical switch in 3T3L1 differentiation where adipogenic markers, including adipsin, PPARγ, and Glut4, have been shown to be strongly upregulated, triglycerides are produced, and intracellular lipid granules begin to accumulate. An interesting observation is that though we observe a reduction in GEFT activity by day 4 of differentiation, its levels are still present, albeit at a lower level, up to day 7 of differentiation, suggesting that much like members of the Rho-GTPases, there are likely more complex functions than simply a “black and white” role for this protein during adipogenesis.
GEFT regulates the Rho family of small GTPases and their downstream signaling pathways.
GEFT has been shown to regulate RhoA, Rac1, and Cdc42 in a cell-type-specific manner (10, 34). In order to examine the signaling pathways modulated by GEFT during the mesenchymal cell fate decision, a process that is essential for skeletal muscle repair, we exogenously expressed GEFT in undifferentiated multipotent C2C12 cells and assayed the activation of the Rho family and their downstream signaling pathways. Similar to our previous report (10), GEFT is capable of promiscuously activating RhoA, Rac1, and Cdc42 in C2C12 cells. In this study, we observed a GEFT-mediated activation of Pak1, Rho-kinase, p38, JNK, and p42/p44, all important downstream effectors of the Rho family. It is interesting to note that, while p38 is essential for skeletal myogenesis to occur (5, 15, 22, 49), this process is strongly inhibited by JNK (56, 57, 72). Indeed, for myogenesis to occur correctly, JNK must be down-regulated either at the level of expression or activity; however, the mechanism by which this occurs remains unknown. The overactivation of JNK by use of constitutively active mutants of Rac1 and Cdc42 in previous reports likely contributes to the controversial ideology that Rac1 and Cdc42 ultimately lead to the inhibition of myogenesis.
C2C12 skeletal myogenesis is enhanced upon exogenous expression of GEFT.
Given the recent high-profile studies illustrating that RhoA and its regulator, p190-B RhoGAP, are essential mediators in the IGF-1-mediated mesenchymal cell fate decision between myogenesis and adipogenesis (73, 78, 79), we were prompted to examine if GEFT, given its unique expression patterns in vivo and during myogenesis and adipogenesis in cell culture, is capable of regulating this process in a similar manner. To date, no mammalian GEFs have been molecularly implicated in directly controlling either myogenesis or adipogenesis. One study has suggested that the well studied Trio-GEF could play a role in myogenic differentiation (62). While the proliferation of secondary myoblasts appeared normal in Trio-deficient mice, terminally differentiated secondary myofibers were absent, possibly due to defects in myoblast alignment or fusion. However, no molecular characterization of the role of Trio in myogenesis has been reported. Interestingly, Trio is highly homologous to and expressed in the same adult tissues as GEFT, with the highest adult expression in the brain, heart, and skeletal muscle (24). This suggests that these two proteins may perform similar functions in the same systems; however, it poses the question as to why there is redundancy of these two proteins in the adult brain, heart, and skeletal muscle.
In this report, we present several lines of evidence suggesting that GEFT promotes myogenesis via its downstream targets: RhoA, Rac1, and Cdc42. GEFT is capable of enhancing myotube formation and key proteins involved in myogenesis, including myosin heavy chain, myogenin, and muscle creatine kinase, among many others. Observations that GEFT mediates myogenesis via RhoA, Rac1, and Cdc42 are interesting given that the roles of the Rho proteins in myogenesis are highly controversial (11). It is generally agreed that RhoA promotes myogenesis in a number of cell lines via its activation of SRF and MyoD (18, 60, 66, 76, 80), while the roles of Rac1 and Cdc42 remain a point of argument. Previous reports have demonstrated negative roles for these two GTPases (31, 40, 57) as well as positive roles (51, 82, 85). A recent study suggests that each GTPase is regulated in a temporal manner in order for proper myogenic differentiation to occur (85). This implies that previous reports revealing an inhibitory role of Rac1 and Cdc42 are likely artifacts of the limitation of using constitutively active and dominant-negative mutants to examine a process that is composed of multiple stages leading to terminal differentiation. We circumvented the use of dominant-negative and constitutively active mutants in our experiments by simply activating exogenous wild-type RhoA, Rac1, and Cdc42 by cotransfection with GEFT, thus demonstrating that all three GTPases can promote skeletal myogenesis.
To date, no reports have attempted to knock down the effect of endogenous GEFT with small interfering RNA. Unfortunately, after multiple unsuccessful attempts by our laboratory to generate effective GEFT-specific retroviral RNA interference constructs or small interfering oligonucleotides, we opted to utilize a dominant-negative mutant of GEFT based on a previously reported mutant of p63RhoGEF, a splice variant of GEFT arising from the same gene (52). Use of the double N- and C-terminal truncation dominant-negative GEFT mutant in several assays measuring the progression of myogenesis demonstrate that when GEFT activity is inhibited, myogenesis is strongly hampered, suggesting GEFT expression is essential for proper myogenesis to occur.
3T3L1 adipogenesis is inhibited upon exogenous expression of GEFT.
Progenitor cells which infiltrate damaged skeletal muscle have the capacity to differentiate into multiple cell lineages. However, a variety of signaling pathways must be precisely regulated in order to ensure that myogenic cell fate determination in maintained. Sordella et al. (79) demonstrated that differential activation of RhoA in response to IGF-1 provides an important switching mechanism in the cell fate decision between myogenesis and adipogenesis, and that the phosphorylation status of the Rho regulator, p190-B RhoGAP, controls this switch and consequently determines how the cell will respond to the IGF-1 stimulus. This report illustrates that the regulation of RhoA GTPase activity appears to be an important determinant of cell fate in the differentiation of adipocyte and myocyte precursors. Our data demonstrate that GEFT steady-state mRNA and protein levels are significantly downregulated upon adipogenic differentiation, suggesting that GEFT could function as an inhibitor of adipocyte differentiation. Using 3T3L1 preadipocyte cells, which undergo adipocyte differentiation in response to supersaturating levels of IBMX, dexamethasone, and insulin, we demonstrated that GEFT is capable of inhibiting adipogenesis, as evidenced by decreased lipid granule staining and inhibition of PPARγ, CRE-BP1, adipsin, and Glut4 expression, all markers of adipocyte differentiation. Moreover, a modest increase in the adipogenic cascade was observed in all adipogenesis assays performed with the dominant-negative GEFT mutant, suggesting that when the Rho-signaling pathways are inhibited, adipogenesis is enhanced.
Similar to the conflicting reports on the role of the Rho-GTPase in skeletal muscle differentiation, much confusion exists as to the precise mechanism by which these proteins regulate the adipogenic program. The Rho family has been shown to regulate adipogenesis, though the exact function of the GTPases in this process is largely unknown. In addition the RhoA and p190-B RhoGAP control of IGF-1-induced adipogenesis (78, 79), one recent study demonstrated that in human mesenchymal stem cells (hMSCs), expression of dominant-negative RhoA committed hMSCs to become adipocytes, while constitutively active RhoA promoted osteogenesis (55). Although several studies indicate that RhoA inhibits adipogenic differentiation, other reports demonstrate findings to the contrary. For example, insulin-stimulated prenylation of RhoA assures normal phosphorylation and activation of CREB that triggers the intrinsic cascade of adipogenesis (44). Moreover, these authors conclude that inhibition of RhoA prenylation arrests insulin-stimulated adipogenesis. In insulin-sensitive cells, such as adipocytes and skeletal muscle, the activation of phosphoinositide 3-kinase (PI3K) is thought to be critical in allowing insulin to stimulate both the uptake of glucose and the translocation of a specialized glucose transporter, Glut4, to the plasma membrane (77, 87-91). One study provides direct evidence that PI3K does not use Rac1 to couple the insulin receptor to glucose uptake in adipocytes (53), suggesting that some of the specificity in the biological responses elicited by PI3K may be mediated by the activation of different effector molecules. However, Cdc42 has been shown to mediate insulin signaling to Glut4 translocation and lies downstream of G alpha (q/11) and upstream of PI3K and protein kinase C lambda in this stimulatory pathway (86a).
In conclusion, our data indicate that Rho-signaling cascades activated by GEFT play a role in modulating skeletal muscle regeneration. Moreover, we have demonstrated GEFT, a GEF for the Rho family of GTPases, to be the first mammalian GEF reported to regulate mesenchymal cell fate decisions. Furthermore, we provide evidence suggesting that GEFT is capable of modulating the myogenic verses adipogenic cell fate decision of progenitor mesenchymal cells. These findings offer important contributions to our present understanding of the role of the Rho-GTPases and their regulator, GEFT, in skeletal muscle regeneration and mesenchymal differentiation, demonstrating that GEFT is capable of promoting skeletal muscle regeneration via a RhoA-, Rac1-, and Cdc42-mediated enhancement of myogenesis and inhibition of adipogenesis.
Acknowledgments
We thank Xin-Hua Feng at Baylor College of Medicine for the use of C2C12 cells and Xiang Tong in the Children's hospital at Baylor College of Medicine for the use of 3T3L1 cells. We also thank Robert Schwartz at Texas A&M University HSC-IBT for the SRF-HA mammalian expression vector and Stephen Safe at Texas A&M University HSC for the PPARγ reporter construct and the PPARγ mammalian expression vector.
This work is partially supported by the NIH grant 5 R01 HL064792 to M.L.
REFERENCES
- 1.Akimoto, T., T. Ushida, S. Miyaki, H. Akaogi, K. Tsuchiya, Z. Yan, R. S. Williams, and T. Tateishi. 2005. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem. Biophys. Res. Commun. 329:381-385. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, H. H., and T. Braun. 2000. Genetics of muscle determination and development. Curr. Top. Dev. Biol. 48:129-164. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, H. H., and B. Winter. 1998. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 8:539-544. [DOI] [PubMed] [Google Scholar]
- 4.Arsic, N., S. Zacchigna, L. Zentilin, G. Ramirez-Correa, L. Pattarini, A. Salvi, G. Sinagra, and M. Giacca. 2004. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 10:844-854. [DOI] [PubMed] [Google Scholar]
- 5.Baeza-Raja, B., and P. Munoz-Canoves. 2004. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol. Biol. Cell 15:2013-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bains, W., P. Ponte, H. Blau, and L. Kedes. 1984. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol. Cell. Biol. 4:1449-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bement, W. M., C. A. Mandato, and M. N. Kirsch. 1999. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 9:579-587. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard, S., T. C. MacKenzie, A. P. Radu, S. Hayashi, W. H. Peranteau, N. Chirmule, and A. W. Flake. 2003. Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice. J. Gene Med. 5:941-950. [DOI] [PubMed] [Google Scholar]
- 9.Brock, J., K. Midwinter, J. Lewis, and P. Martin. 1996. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J. Cell Biol. 135:1097-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan, B., V. Kumar, L. J. Stafford, Y. Cai, G. Wu, and M. Liu. 2004. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J. Biol. Chem. 279:45824-45832. [DOI] [PubMed] [Google Scholar]
- 11.Bryan, B. A., D. Li, X. Wu, and M. Liu. 2005. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell Mol. Life Sci. 62:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckingham, M. 2001. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 11:440-448. [DOI] [PubMed] [Google Scholar]
- 13.Buckingham, M. 2003. How the community effect orchestrates muscle differentiation. Bioessays 25:13-16. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham, M., L. Bajard, T. Chang, P. Daubas, J. Hadchouel, S. Meilhac, D. Montarras, D. Rocancourt, and F. Relaix. 2003. The formation of skeletal muscle: from somite to limb. J. Anat. 202:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabane, C., W. Englaro, K. Yeow, M. Ragno, and B. Derijard. 2003. Regulation of C2C12 myogenic terminal differentiation by MKK3/p38alpha pathway. Am. J. Physiol. Cell Physiol. 284:C658-666. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell, D. A. 2003. GTPases and T cell activation. Immunol. Rev. 192:122-130. [DOI] [PubMed] [Google Scholar]
- 17.Cao, B., and J. Huard. 2004. Muscle-derived stem cells. Cell Cycle 3:104-107. [PubMed] [Google Scholar]
- 18.Carnac, G., M. Primig, M. Kitzmann, P. Chafey, D. Tuil, N. Lamb, and A. Fernandez. 1998. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9:1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charrasse, S., M. Causeret, F. Comunale, A. Bonet-Kerrache, and C. Gauthier-Rouviere. 2003. Rho GTPases and cadherin-based cell adhesion in skeletal muscle development. J. Muscle Res. Cell Motil. 24:309-313. [PubMed] [Google Scholar]
- 20.Chen, J. C., and D. J. Goldhamer. 2003. Skeletal muscle stem cells. Reprod. Biol. Endocrinol. 1:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 22.Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341-4346. [DOI] [PubMed] [Google Scholar]
- 23.d'Albis, A., R. Couteaux, C. Janmot, A. Roulet, and J. C. Mira. 1988. Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur. J. Biochem. 174:103-110. [DOI] [PubMed] [Google Scholar]
- 24.Debant, A., C. Serra-Pages, K. Seipel, S. O'Brien, M. Tang, S. H. Park, and M. Streuli. 1996. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. USA 93:5466-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dergham, P., B. Ellezam, C. Essagian, H. Avedissian, W. D. Lubell, and L. McKerracher. 2002. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22:6570-6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellezam, B., C. Dubreuil, M. Winton, L. Loy, P. Dergham, I. Selles-Navarro, and L. McKerrac. 2002. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog. Brain Res. 137:371-380. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 28.Fajas, L., M. B. Debril, and J. Auwerx. 2001. Peroxisome proliferator-activated receptor-gamma: from adipogenesis to carcinogenesis. J Mol. Endocrinol. 27:1-9. [DOI] [PubMed] [Google Scholar]
- 29.Farmer, S. R. 2005. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. Relat. Metab. Disord. 29(Suppl. 1):S13-S16. [DOI] [PubMed] [Google Scholar]
- 30.Fournier, A. E., B. T. Takizawa, and S. M. Strittmatter. 2003. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23:1416-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallo, R., M. Serafini, L. Castellani, G. Falcone, and S. Alema. 1999. Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell 10:3137-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier-Rouviere, C., M. Vandromme, D. Tuil, N. Lautredou, M. Morris, M. Soulez, A. Kahn, A. Fernandez, and N. Lamb. 1996. Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol. Biol. Cell 7:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govek, E. E., S. E. Newey, and L. Van Aelst. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 19:1-49. [DOI] [PubMed] [Google Scholar]
- 34.Guo, X., L. J. Stafford, B. Bryan, C. Xia, W. Ma, X. Wu, D. Liu, Z. Songyang, and M. Liu. 2003. A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J. Biol. Chem. 278:13207-13215. [DOI] [PubMed] [Google Scholar]
- 35.Hackam, D. J., and H. R. Ford. 2002. Cellular, biochemical, and clinical aspects of wound healing. Surg. Infect. 3(Suppl. 1):S23-S35. [DOI] [PubMed] [Google Scholar]
- 36.Hall-Craggs, E. C. 1974. Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcain). Exp. Neurol. 43:349-358. [DOI] [PubMed] [Google Scholar]
- 37.Harris, J. B. 2003. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon 42:933-945. [DOI] [PubMed] [Google Scholar]
- 38.Harris, J. B., and M. A. Johnson. 1978. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 5:587-600. [DOI] [PubMed] [Google Scholar]
- 39.Harris, J. B., and C. A. Maltin. 1982. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br. J. Pharmacol. 76:61-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heller, H., E. Gredinger, and E. Bengal. 2001. Rac1 inhibits myogenic differentiation by preventing the complete withdrawal of myoblasts from the cell cycle. J. Biol. Chem. 276:37307-37316. [DOI] [PubMed] [Google Scholar]
- 41.Hill, C. S., J. Wynne, and R. Treisman. 1995. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81:1159-1170. [DOI] [PubMed] [Google Scholar]
- 42.Huard, J., B. Cao, and Z. Qu-Petersen. 2003. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res. C Embryo Today 69:230-237. [DOI] [PubMed] [Google Scholar]
- 43.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell. Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 44.Klemm, D. J., J. W. Leitner, P. Watson, A. Nesterova, J. E. Reusch, M. L. Goalstone, and B. Draznin. 2001. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J. Biol. Chem. 276:28430-28435. [DOI] [PubMed] [Google Scholar]
- 45.Langer-Safer, P. R., S. R. Lehrman, and A. M. Skalka. 1985. v-src inhibits differentiation via an extracellular intermediate(s). Mol. Cell. Biol. 5:2847-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, M. Y., H. J. Kong, and J. Cheong. 2001. Regulation of activating transcription factor-2 in early stage of the adipocyte differentiation program. Biochem. Biophys. Res. Commun. 281:1241-1247. [DOI] [PubMed] [Google Scholar]
- 47.Lehmann, M., A. Fournier, I. Selles-Navarro, P. Dergham, A. Sebok, N. Leclerc, G. Tigyi, and L. McKerracher. 1999. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19:7537-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.L'Honore, A., N. J. Lamb, M. Vandromme, P. Turowski, G. Carnac, and A. Fernandez. 2003. MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol. Biol. Cell 14:2151-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, Y., B. Jiang, W. Y. Ensign, P. K. Vogt, and J. Han. 2000. Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal. 12:751-757. [DOI] [PubMed] [Google Scholar]
- 50.Liu, H. W., A. J. Halayko, D. J. Fernandes, G. S. Harmon, J. A. McCauley, P. Kocieniewski, J. McConville, Y. Fu, S. M. Forsythe, P. Kogut, S. Bellam, M. Dowell, J. Churchill, H. Lesso, K. Kassiri, R. W. Mitchell, M. B. Hershenson, B. Camoretti-Mercado, and J. Solway. 2003. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am. J. Respir. Cell Mol. Biol. 29:39-47. [DOI] [PubMed] [Google Scholar]
- 51.Luo, L., Y. J. Liao, L. Y. Jan, and Y. N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787-1802. [DOI] [PubMed] [Google Scholar]
- 52.Lutz, S., A. Freichel-Blomquist, Y. Yang, U. Rumenapp, K. H. Jakobs, M. Schmidt, and T. Wieland. 2005. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J. Biol. Chem. 280:11134-11139. [DOI] [PubMed] [Google Scholar]
- 53.Marcusohn, J., S. J. Isakoff, E. Rose, M. Symons, and E. Y. Skolnik. 1995. The GTP-binding protein Rac does not couple PI 3-kinase to insulin-stimulated glucose transport in adipocytes. Curr. Biol. 5:1296-1302. [DOI] [PubMed] [Google Scholar]
- 54.Maruta, H., T. V. Nheu, H. He, and Y. Hirokawa. 2003. Rho family-associated kinases PAK1 and rock. Prog. Cell Cycle Res. 5:203-210. [PubMed] [Google Scholar]
- 55.McBeath, R., D. M. Pirone, C. M. Nelson, K. Bhadriraju, and C. S. Chen. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6:483-495. [DOI] [PubMed] [Google Scholar]
- 56.Meriane, M., S. Charrasse, F. Comunale, and C. Gauthier-Rouviere. 2002. Transforming growth factor beta activates Rac1 and Cdc42Hs GTPases and the JNK pathway in skeletal muscle cells. Biol. Cell. 94:535-543. [DOI] [PubMed] [Google Scholar]
- 57.Meriane, M., Roux, P., M. Primig, P. Fort, and C. Gauthier-Rouviere. 2000. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 11:2513-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 59.Minguell, J. J., A. Erices, and P. Conget. 2001. Mesenchymal stem cells. Exp. Biol. Med. 226:507-520. [DOI] [PubMed] [Google Scholar]
- 60.Miralles, F., G. Posern, A. I. Zaromytidou, and R. Treisman. 2003. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113:329-342. [DOI] [PubMed] [Google Scholar]
- 61.Montaner, S., R. Perona, L. Saniger, and J. C. Lacal. 1999. Activation of serum response factor by RhoA is mediated by the nuclear factor-kappaB and C/EBP transcription factors. J. Biol. Chem. 274:8506-8515. [DOI] [PubMed] [Google Scholar]
- 62.O'Brien, S. P., K. Seipel, Q. G. Medley, R. Bronson, R. Segal, and M. Streuli. 2000. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. USA 97:12074-12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohyama, M., N. Suzuki, Y. Yamaguchi, M. Maeno, K. Otsuka, and K. Ito. 2002. Effect of enamel matrix derivative on the differentiation of C2C12 cells. J. Periodontol. 73:543-550. [DOI] [PubMed] [Google Scholar]
- 64.Perry, R. L., and M. A. Rudnick. 2000. Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5:D750-767. [DOI] [PubMed] [Google Scholar]
- 65.Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147. [DOI] [PubMed] [Google Scholar]
- 66.Posern, G., F. Miralles, S. Guettler, and R. Treis. 2004. Mutant actins that stabilise F-actin use distinct mechanisms to activate the SRF coactivator MAL. EMBO J. 23:3973-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pownall, M. E., M. K. Gustafsson, and C. P. Emerson, Jr. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18:747-783. [DOI] [PubMed] [Google Scholar]
- 68.Qi, C., Y. Zhu, and J. K. Reddy. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem. Biophys. 32:187-204. [DOI] [PubMed]
- 69.Redd, M. J., L. Cooper, W. Wood, B. Stramer, and P. Martin. 2004. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rikitake, Y., and J. K. Liao. 2005. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev. Cardiovasc. Ther. 3:441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossman, K. L., C. J. Der, and J. Sondek. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell. Biol. 6:167-180. [DOI] [PubMed] [Google Scholar]
- 72.Rousse, S., F. Lallemand, D. Montarras, C. Pinset, A. Mazars, C. Prunier, A. Atfi, and C. Dubois. 2001. Transforming growth factor-beta inhibition of insulin-like growth factor-binding protein-5 synthesis in skeletal muscle cells involves a c-Jun N-terminal kinase-dependent pathway. J. Biol. Chem. 276:46961-46967. [DOI] [PubMed] [Google Scholar]
- 73.Saltiel, A. R. 2003. Muscle or fat? Rho bridges the GAP. Cell 113:144-145. [DOI] [PubMed] [Google Scholar]
- 74.Sartorelli, V., K. A. Webster, and L. Kedes. 1990. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 4:1811-1822. [DOI] [PubMed] [Google Scholar]
- 75.Schmalbruch, H., and D. M. Lewis. 2000. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23:617-626. [DOI] [PubMed] [Google Scholar]
- 76.Settleman, J. 2003. A nuclear MAL-function links Rho to SRF. Mol. Cell 11:1121-1123. [DOI] [PubMed] [Google Scholar]
- 77.Shepherd, P. R. 2005. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol. Scand. 183:3-12. [DOI] [PubMed] [Google Scholar]
- 78.Sordella, R., M. Classon, K. Q. Hu, S. F. Matheson, M. R. Brouns, B. Fine, L. Zhang, H. Takami, Y. Yamada, and J. Settleman. 2002. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell. 2:553-565. [DOI] [PubMed] [Google Scholar]
- 79.Sordella, R., W. Jiang, G. C. Chen, M. Curto, and J. Settleman. 2003. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113:147-158. [DOI] [PubMed] [Google Scholar]
- 80.Sotiropoulos, A., D. Gineitis, J. Copeland, and R. Treisman. 1999. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98:159-169. [DOI] [PubMed] [Google Scholar]
- 81.Stramer, B., W. Wood, M. J. Galko, M. J. Redd, A. Jacinto, S. M. Parkhurst, and P. Martin. 2005. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takano, H., I. Komuro, T. Oka, I. Shiojima, Y. Hiroi, T. Mizuno, and Y. Yazaki. 1998. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18:1580-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tibbles, L. A., Y. L. Ing, F. Kiefer, J. Chan, N. Iscove, J. R. Woodgett, and N. J. Lassam. 1996. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 15:7026-7035. [PMC free article] [PubMed] [Google Scholar]
- 84.Tidball, J. G. 2005. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R345-353. [DOI] [PubMed] [Google Scholar]
- 85.Travaglione, S., G. Messina, A. Fabbri, L. Falzano, A. M. Giammarioli, M. Grossi, S. Rufini, and C. Fiorentini. 2005. Cytotoxic necrotizing factor 1 hinders skeletal muscle differentiation in vitro by perturbing the activation/deactivation balance of Rho GTPases. Cell Death Differ. 12:78-86. [DOI] [PubMed] [Google Scholar]
- 86.Treisman, R., A. S. Alberts, and E. Sahai. 1998. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb. Symp. Quant. Biol. 63:643-651. [DOI] [PubMed] [Google Scholar]
- 86a.Usui, I., T. Imamura, J. Huang, H. Satoh, and J. M. Olefsky. 2003. Cdc42 is a Rho GTPase family member that can mediate insulin signaling to glucose transport in 3T3-L1 adipocytes. J. Biol. Chem. 278:13765-13774. [DOI] [PubMed] [Google Scholar]
- 87.Watson, R. T., M. Furukawa, S. H. Chiang, D. Boeglin, M. Kanzaki, A. R. Saltiel, and J. E. Pessin. 2003. The exocytotic trafficking of TC10 occurs through both classical and nonclassical secretory transport pathways in 3T3L1 adipocytes. Mol. Cell. Biol. 23:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watson, R. T., A. H. Khan, M. Furukawa, J. C. Hou, L. Li, M. Kanzaki, S. Okada, K. V. Kandror, and J. E. Pessin. 2004. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 23:2059-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watson, R. T., and J. E. Pessin. 2001. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 56:175-193. [DOI] [PubMed] [Google Scholar]
- 90.Watson, R. T., and J. E. Pessin. 2001. Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4. Exp. Cell Res. 271:75-83. [DOI] [PubMed] [Google Scholar]
- 91.Watson, R. T., S. Shigematsu, S. H. Chiang, S. Mora, M. Kanzaki, I. G. Macara, A. R. Saltiel, and J. E. Pessin. 2001. Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan, Z., S. Choi, X. Liu, M. Zhang, J. J. Schageman, S. Y. Lee, R. Hart, L. Lin, F. A. Thurmond, and R. S. Williams. 2003. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 278:8826-8836. [DOI] [PubMed] [Google Scholar]
- 93.Yun, K., and B. Wold. 1996. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr. Opin. Cell Biol. 8:877-889. [DOI] [PubMed] [Google Scholar]
- 94.Zhong, S., S. Sun, and B. B. Teng. 2004. The recombinant adeno-associated virus vector (rAAV2)-mediated apolipoprotein B mRNA-specific hammerhead ribozyme: a self-complementary AAV2 vector improves the gene expression. Genet. Vaccines Ther. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zolotukhin, S., B. J. Byrne, E. Mason, I. Zolotukhin, M. Potter, K. Chesnut, C. Summerford, R. J. Samulski, and N. Muzyczka. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973-985. [DOI] [PubMed] [Google Scholar]