Abstract
Spo0F is a secondary messenger in the sporulation phosphorelay, and its structure has been characterized crystallographically in the apo-state, in the metal-bound state, and in an interacting state with a phosphotransferase. Additionally, the solution structure of the molecule has been characterized by nuclear magnetic resonance techniques in the unliganded state and in complex with beryllofluoride. Spo0F is a single-domain protein with a well-defined three-dimensional structure, but it is capable of adapting to specific conformations for catching and releasing the phosphoryl moiety. This commentary deals with the conformational fluctuations of the molecule as it moves from an apo-state to a metal-coordinated state, to a phosphorylated state, and then to a phosphoryl-transferring state.
Spo0F is a component of the sporulation phosphorelay of Bacillus subtilis (10) which acts as a gateway for all sporulation signals processed by various histidine kinases (Fig. 1). The initiation of sporulation is controlled by a single parameter, the degree of phosphorylation of the transcription factor Spo0A. It is Spo0F that takes the phosphoryl moiety from the sensor kinases and passes it on to Spo0A through the phosphotransferase Spo0B. Hence, the activity of Spo0F involves interactions with the sensor kinases and Spo0B in receiving and donating the phosphoryl group. Therefore, to describe the conformational pathway of Spo0F, it is necessary to consider four distinct conformational states for Spo0F: (i) isolated unphosphorylated state, (ii) unphosphorylated Spo0F interacting with KinA, (iii) phosphorylated Spo0F, and (iv) phosphorylated Spo0F interacting with Spo0B. Although the documented structural data are inadequate to fully describe all these distinct states, the available data are reviewed here to gain the best understanding of the conformational states of Spo0F.
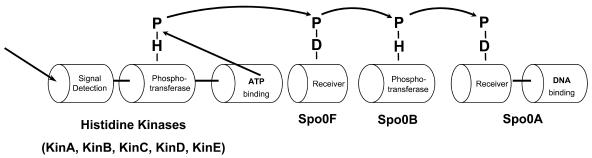
FIG. 1.
The phosphorelay that controls the sporulation in Bacillus subtilis. Five different histidine kinases are known to be involved in processing the signals. Histidine kinases have a signal detection domain and an autokinase domain which can be subdivided into a phosphotransferase domain and an ATP binding domain. The histidine kinases pass on the phosphoryl group to Spo0F. From Spo0F, it is transferred to the transcription factor Spo0A through Spo0B. The flow of the phosphoryl group is as follows: His→Asp→His→Asp.
STRUCTURAL STUDIES OF Spo0F
Unphosphorylated state.
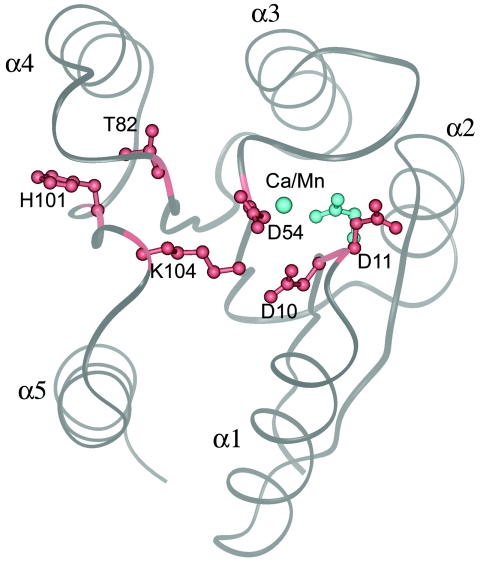
The structure of Spo0F has been studied by both crystallographic and nuclear magnetic resonance (NMR) techniques. This section focuses on the crystallographic results, and NMR studies are discussed in a different section. Structural analysis has been carried out on the metal-free form (17) and on the metal-bound forms (18, 19). Spo0F, like all other response regulators, has an α/β fold with a central β-sheet and five helices surrounding it (Fig. 2). The active site is located on the top of the β-strands and comprises an acid pocket with three conserved aspartates: Asp10, Asp11, and Asp54. The site of phosphorylation, Asp54, is located at the bottom of a shallow pocket. All phosphoryl transfer reactions make use of cations, and in the two component/phosphorelay systems, it is the response regulators that bind the cations and present them for catalysis. The crystal structures of the metal-free and metal-bound forms are very similar, and the changes are confined mainly to the metal binding site. The most significant difference between the two states of Spo0F is that, in the metal-free structure, the metal cavity is not fully formed and the metal induces the formation of the cavity. Figure 2 shows that the side chain of Asp11, which points away from the active site, reorients to coordinate with the metal. However, it should be pointed out that in other response regulators, such as CheY, the metal cavity is already present even in the absence of the metal. Side chains of three other residues relevant to the present discussion are also shown in Fig. 2. Lys104, a highly conserved residue, is suitably positioned to have critical interactions in the acidic pocket. Position 82 is occupied by a Thr residue and position 101 by a His residue in Spo0F, and the relevance of these two residues will be discussed in the next section.
FIG. 2.
A view of Spo0F from the top of the β-sheet, showing the overall fold. The five helices are marked α1 to α5. There are five β-strands (β1 to β5), and each one precedes the corresponding helix; that is, strand β1 is on the N-terminal side of helix α1. Shown in gray is a ribbon representation of the metal-free form of Spo0F depicting the location of six functionally critical residues. The conserved aspartates Asp 10, Asp 11, and Asp 54 form the acidic portion of the active site. A conserved lysine residue, Lys104, points towards the aspartates. Positions 82 and 101 are also normally conserved with a hydroxyl-containing Ser/Thr and an aromatic residue, respectively. In Spo0F, position 82 is a threonine, but 101 is a histidine. These two residues point away from the active site. The orientations of Asp 10, Asp 11, Asp 54, Thr82, His101, and Lys104 are shown in red for the metal-free structure. Upon metal coordination, Asp11 reorients to form the metal coordination site. The location of the metal ion and the new orientation of Asp11 in the metal-bound structures are shown in cyan.
Insights into the phosphorylated state of Spo0F and the importance of the residues at positions 82 and 101.
The structure of Spo0F-P has not been elucidated, and therefore, to gain insights into its conformational state, we would make use of the crystal structures of Spo0A-P (16), FixJ-P (4), and RcpA-P (2). Although these three crystals are grown under various conditions, the phosphorylation induces certain common changes in these structures. The major changes that occur upon phosphorylation in Spo0A and FixJ involve residues at positions 82 and 101 (Spo0F numbering). A sequence comparison (26) of the response regulators showed that positions 82 and 101 of Spo0F are conserved with a hydroxyl-containing residue, Ser/Thr, and with an aromatic residue, Phe/Tyr, respectively. Ser/Thr is located in the β4-α4 loop, and the aromatic residue is located on strand β5 at the α4-β5-α5 surface. In the unphosphorylated state, the side chain of the aromatic residue is generally positioned in the surface, and the side chain of Ser/Thr is pointed away from the active site. In Spo0A, these positions are occupied by residues Thr84 and Phe103. In FixJ, they are occupied by the residues Thr82 and Phe101. In Spo0A and FixJ, the Thr residue moves towards the active site to interact with a phosphoryl oxygen. In FixJ-P and Spo0A-P, the Phe residue moves to an internal orientation. In RcpA-P also, the Tyr residue (Tyr119) is in a buried orientation. The serine residue (Ser100) has two conformations, one interacting with the phosphoryl oxygen and the other pointing to the solvent. The coupled movements of these two residues have been observed in the BeF3− complex of CheY also (14). The solution structure of NtrC-P (13, 24) displays a different mode of reorientation; however, the available crystallographic data on phosphorylated response regulators or beryllofluoride complexes of response regulators are generally consistent with the pattern described above.
In CheY, the existence of mutations that lead to constitutively active and inactive phenotypes irrespective of the state of phosphorylation has given rise to the notion that the conformation of CheY itself, and not the state of phosphorylation, defines its interaction with the motor protein FliM (5, 8, 31). The constitutively active CheY mutants generally have Tyr106 in the inward orientation, and in the constitutively inactive mutants, Tyr106 is in the outward orientation (8, 30, 31). In CheY, the α4-β5-α5 surface interacts with the P2 domain of CheA and with FliM, the flagellar motor protein. Therefore, the existence of distinct conformational states of the receiver domain is a key factor in the two-component/phosphorelay signal transduction, and it is associated with the major movements of residues 82 and 101 (Spo0F numbering) (8, 31).
The rearrangements of these residues also can take place due to protein-protein interactions, especially by dimerization, as illustrated by the crystal structures of YycF (3), Rcp1 (11), and RcpB (2), which form dimers in the crystal lattice by using the α4-β5-α5 surface. Displacement of the aromatic side chain from the surface is necessary for dimer formation. Bent et al. (3) have proposed that these conformations are concentration dependent rather than phosphorylation dependent. When the concentration reaches a critical value, the molecules form dimers, causing the displacements of these residues. Therefore, it appears that the correlated movements of these residues are induced by conditions that favor dimer formation in just the same manner as by phosphorylation. Figure 3 is a cartoon showing the correlated movements of Ser/Thr and Phe/Tyr residues upon phosphorylation, which lead in certain cases to dimerization of the response regulators.
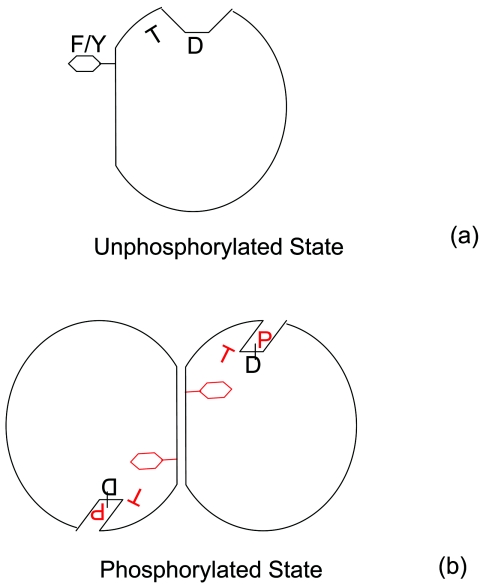
FIG. 3.
A cartoon showing the reorientation of two conserved side chains (corresponding to positions 82 and 101 of Spo0F) upon phosphorylation. D marks the sites of phosphorylation, T represents Thr/Ser residue, and F/Y represents the aromatic residue. (a) Unphosphorylated state. The residue at 82 is orientated away from the site of phosphorylation, and it is shown by the letter T tilted to the left. The side chain of residue 101 is positioned externally. (b) Phosphorylated state. Thr at 82 reorients (shown by a tilt to the right) and interacts with the phosphoryl group. The side chain at 101 gets internalized, clearing the way for dimerization. The phosphoryl moiety and the repositioned residues at position 82 and 101 are shown in red.
At this stage, it is difficult to assess the relevance of His 101 movement in Spo0F. The function of Spo0F does not involve dimerization as those of some response regulator transcription factors do. The interacting interface of Spo0F with Spo0B in the crystal structure does not include the α4-β5-α5 surface where His101 is situated. It is not known if phosphatases or kinases have any interactions with this region. On the other hand, the reorientation of Thr82 to interact with the phosphoryl oxygen is most likely an essential feature of the phosphorylated form. As these two residues are seen to move in a correlated fashion, it is likely that His101 is internalized upon phosphorylation. The mutation of His101 to Ala created an altered phenotype in sporulation, and the mutation of Thr82 to Ala completely shut down sporulation (21).
Interacting states.
Next, we should consider the conformations of unphosphorylated Spo0F when it interacts with KinA and when the phosphorylated Spo0F interacts with Spo0B. There are no crystal structures representing these states of interaction. These transient interactions are solely for phosphoryl transfer, and therefore it is more pertinent to consider the conformation of the molecule that allows phosphoryl transfer. Fortunately, considerable insights have been gained from the Spo0F:Spo0B structure (29) (Fig. 4a). As the phosphotransferase domains of histidine kinases are also four-helix bundles like Spo0B (23), the crystal structure of this complex is a reasonable model for kinase-response regulator interactions.
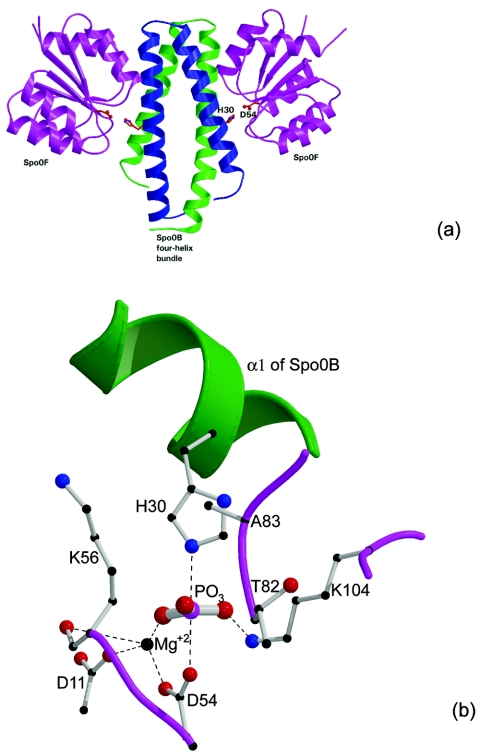
FIG. 4.
Spo0F:Spo0B association and phosphoryl transfer. (a) Crystal structure of the Spo0B:Spo0F complex viewed perpendicularly to the four-helix bundle. The residues His30 of Spo0B and Asp54 of Spo0F are in close proximity for phosphoryl transfer (reproduced, with permission, from reference 22). (b) A model for the transition state intermediate, produced using the crystal structure of the Spo0F:Spo0B complex by placing a phosphoryl group between the active His30 and Asp54. The P-to-O and P-to-N distances are 2.45 Å each. The model shows that the environment produced by the association of the two molecules stabilizes the transition state interactions through suitable electrostatic and hydrogen-bonding interactions. The phosphorus atom forms partial covalent bonds with Oδ of Asp and Nɛ of His and is in a pentacoordinated state. The negative charges on the phosphoryl oxygens are compensated through interactions with Mg2+ and Lys104 (reproduced, with permission, from reference 29).
The Spo0F in the crystal structure of the complex is not phosphorylated. Although the crystallization solution contained aluminum fluoride, the crystal structure does not show any density that would indicate its presence. The crystals did not grow in the absence of aluminum fluoride, but we do not know its role in the association of the molecules. The important feature of the structure of the complex is that it satisfies the geometrical and electrostatic requirements for transferring a phosphoryl group (Fig. 4b). The phosphoryl-transferring state should be considered the most active conformation for Spo0F.
OPEN AND CLOSED STATES
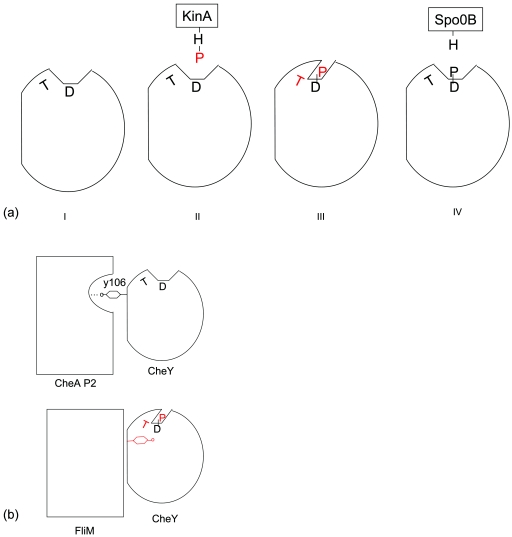
A number of regulatory processes have been described in terms of “closed” and “open” states of certain molecules, and the function of Spo0F will also be presented herein in relation to two conformational states. The first one is an open state, where the active site is accessible to accept or donate the phosphoryl group. The second one is a protected state, where Spo0F is phosphorylated and the phosphoryl group is stabilized through rearrangements of loops and side chains. We call this state a closed state. The crystal structures of the calcium complex (18), the manganese complex (19), and the metal-free form (17) are in the open states where Asp54 is accessible. In the metal-free Spo0F structure, the side chain of an Arg residue from a neighboring molecule enters the active site to form a strong salt bridge with the site of phosphorylation (17). Phosphorylation is accompanied by conformational alterations around the active site, and of special significance are the changes in the β4-α4 loop which facilitate the interaction of Thr82 with the phosphoryl group. The rearrangements reduce the solvent accessibility of the phosphoryl group and provide stabilizing interactions. The unphosphorylated state where Asp54 is accessible to solvent or ligands is an open state. The interacting state has to be an open state where Asp54 can interact either with His30 of Spo0B or with His405 of KinA. In the cocrystal, we observe the interacting state where Spo0F interacts with Spo0B in order to transfer the phosphoryl group. A simplified view of conformational changes of Spo0F along the signaling pathway by use of the discrete open and closed states is depicted in Fig. 5a.
FIG. 5.
Discrete conformational states in the functional pathways of Spo0F and CheY. (a) Conformational fluctuations of Spo0F in the phosphotransfer pathway visualized in terms of closed and open states. (I) Unphosphorylated state; (II) Spo0F interacting with KinA-P; (III) phosphorylated state; (IV) Spo0F-P interacting with Spo0B. (b) The two conformational states of CheY. (Top) The external orientation of Y106 favors interaction with P2 domain; (bottom) the internal orientation of Y106 paves the way for FliM to associate with CheY. The phosphoryl moiety and the repositioned residues are shown in red.
The distinct conformational states of CheY, when it interacts with the P2 domain of CheA and the N-terminal peptide of FliM, illustrate the biological relevance of the closed and open states and the correlated motions of Ser/Thr and Phe/Tyr residues. In the crystal structure of apo-CheY (27), the side chain of Tyr106 exists in two orientations, external and internal. In the CheY:CheA P2 domain complex (28), Tyr106 exclusively adopts the external conformation stabilized by a hydrogen bond to CheA, and T87 is oriented away from the active site. The interaction therefore stabilizes the open state of CheY, where it is ready to accept the phosphoryl group (Fig. 5b). In the structure of CheY:BeF3− in complex with the N-terminal peptide of FliM, Thr87 forms a hydrogen bond with one of the fluorines, and Tyr106 takes up the internal orientation and makes a hydrogen bond with carbonyl of Glu89 (15); therefore, it is in a closed state (Fig. 5b). The internalization of Tyr106 paves the way for association with FliM. One could think of a mechanism where unphosphorylated CheY binds to CheA with the external orientation for Tyr106 suitable for a hydrogen-bonding interaction with CheA. Upon phosphorylation, Thr87 moves towards the Asp57-P accompanied by internalization of Tyr106, eliminating its interaction with CheA, which reduces the binding affinity between CheY and CheA, resulting in the release of CheY-P. Similarly, when the phosphorylated CheY bound to FliM loses the phosphoryl group, Thr87 and Tyr106 could move in a coupled manner, pushing the Tyr106 to the external orientation and thereby promoting the dissociation of CheY and FliM.
The constitutively active CheY mutants D13K (12), D13K/Y106W (6), and I95V (20) have the side chain of residue 106 in the external position, contradicting the prevailing notions about the active state. When the D13W/Y106W mutant binds to the FliM peptide, however, the W106 side chain takes up an internal orientation (6). These three mutants must have a lower energy barrier between the ground state and the activated state, increasing their affinities to FliM. Protein-protein interactions in turn will drive the side chain of residue 106 to the internal location.
A DIFFERENT PERCEPTION ON THE ACTIVE STATE OF Spo0F
Recently, Gardino et al. (9) reported the NMR structure of Spo0F in the presence of BeF3−, and they concluded that the structure represents the activated state of the molecule by virtue of its interactions with BeF3−. By stating that the molecule is activated, it appears that the authors imply that the molecule has the conformation of the phosphorylated state. When phosphorylation causes an inactive molecule to become active, the phosphorylated state should be called the activated state. The activity of Spo0F, however, is to transfer the phosphoryl moiety from kinases to Spo0B or from Spo0B to kinases, and therefore the phosphoryl-transferring state and not the phosphorylated state is the most active state of Spo0F. Gardino et al. (9) state that the Spo0F in the cocrystal of Spo0F:Spo0B (29) is in the inactive form. It must be pointed out that the environment in the crystal structure satisfies the requirements of phosphoryl transfer (Fig. 4b) (29), and therefore it represents the most active state of Spo0F. It is illogical to expect the transferring state to have the same conformation as the isolated phosphorylated state.
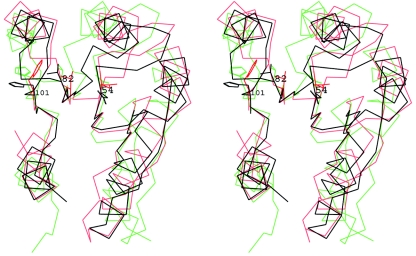
Additionally, Spo0F:BeF3− does not appear to fully represent a phosphorylated state. The crystal structure of Spo0F-P has not been determined, but certain common features are observed in the crystal structures of the phosphorylated forms with regard to the side chain orientation of residues at positions 82 and 101. Figure 6 is a stereoscopic view of the superposition of Spo0F:BeF3− (9) with Spo0A-P (16) and the unphosphorylated Spo0F (17). The other two crystal structures, FixJ-P and Rcp-P, exhibit the same pattern of changes as Spo0A-P, and therefore they are excluded from this figure for clarity. The positions occupied by residues Thr82 and His101 in the beryllofluoride structure are midway between those of the phosphorylated states and that of unphosphorylated Spo0F (17). The distance of the Oγ of Thr82 from the closer Oδ of Asp54 is 6.5 Å in the unphosphorylated Spo0F (17), while the corresponding distances are 4.2 Å in Spo0A-P and 5.4 Å in Spo0F:BeF3− (9). Therefore, it appears that the beryllofluoride interactions of Spo0F take the molecule towards the phosphorylated state, but not fully. As Spo0F differs from other response regulators by having a His residue at position 101 in place of an aromatic residue, it is possible that it behaves differently upon phosphorylation, but taking the external orientation of His101 in the crystal structures as the resting state, beryllofluoride is causing it to move to a partly buried position en route to an internalized state. The author would like to comment on two additional issues from the paper by Gardino et al. (9).
FIG. 6.
A stereoscopic view of the superposition of the unphosphorylated Spo0F structure (17) (black) with Spo0F:BeF3− (9) (cyan) and the Spo0A-P structure (16) (red). Side chains of Asp54, Thr82, and His101 for the Spo0F structure and of Asp55, Thr84, and Phe103 for Spo0A-P are shown. The superposition was carried out using the β-sheet residues 5 to 11, 19 to 22, 50 to 53, 77 to 82, and 101 to 104.
In the NMR structure of the unphosphorylated Spo0F (7), helix α4 starts only at residue 90, while in the Spo0F:BeF3− structure (9), it starts at residue 87. It should be pointed out that helix α4 has been found to be unstable in some response regulators—CheY (1) and NtrC (25). Therefore, it is possible that the presence of BeF3− causes the extension of the helix. Thr82 is located in the β4-α4 loop, and its interaction with the phosphoryl group or BeF3− could increase the stability of this region and may cause an extension of the N-terminal portion of helix α4. On the other hand, X-ray studies (17-19) indicate that the longer helix is the ground state of the molecule because, despite the difference in crystallization conditions, in all the crystal structures helix α4 starts at residue 87. Additionally, in the Spo0F:BeF3− structure, Thr82 does not appear to be positioned for strong interactions with BeF3−. Therefore, the extended helix appears to be the resting state, and the shorter helix observed in the solution structure (7) may have resulted from the experimental conditions.
In the solution structure of Spo0F (7), the side chain of His101 is buried inside, and it is hydrogen bonded to the amide of Thr 82. In the Ca2+ complex (18) and the Mn2+ complex (19), the His101 side chain is positioned in the exterior surface of the molecule. Gardino et al. (9) attribute the orientation of His101 in the crystal lattice to the low pH (4.5) at which crystals were grown. At this pH, the histidine is protonated and therefore cannot act as an acceptor of hydrogen bonds. It should be pointed out that the wild-type Spo0F (17) crystallized at pH 7.8 also exhibits the same conformation for His101. Additionally, the exterior positioning of this side chain is the expected orientation of this side chain for the unphosphorylated state.
CONCLUSION
Response regulators undergo conformational changes in order to transduce signals, and, generally, two conserved residues, Thr/Ser and Tyr/Phe (corresponding to positions 82 and 101 of Spo0F), reorient in a correlated fashion upon phosphorylation. The rearrangement of Ser/Thr results in the stabilization of the phosphoryl group, and it appears to be the characteristic pattern for phosphorylation. Various conformational states are required for the activity of Spo0F. As the role of the molecule is the exchange of the phosphoryl group, the interacting mode where it transfers the phosphoryl group is the most active state of the molecule.
Acknowledgments
This research was supported by grant GM54246 from the National Institutes of General Medical Sciences, National Institute of Health, USPHS.
REFERENCES
- 1.Bellsolell, L., J. Prieto, L. Serrano, and M. Coll. 1994. Magnesium binding to the bacterial chemotaxis protein CheY results in large conformational changes involving its functional surface. J. Mol. Biol. 238:489-495. [DOI] [PubMed] [Google Scholar]
- 2.Benda, C., C. Scheufler, D. M. Tandeau, and W. Gartner. 2004. Crystal structures of two cyanobacterial response regulators in apo- and phosphorylated form reveal a novel dimerization motif of phytochrome-associated response regulators. Biophys. J. 87:476-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bent, C. J., N. W. Isaacs, T. J. Mitchell, and A. Riboldi-Tunnicliffe. 2004. Crystal structure of the response regulator 02 receiver domain, the essential YycF two-component system of Streptococcus pneumoniae in both complexed and native states. J. Bacteriol. 186:2872-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birck, C., L. Mourey, P. Gouet, B. Fabry, J. Schumacher, P. Rousseau, D. Kahn, and J.-P. Samama. 1999. Conformational changes induced by phosphorylation of the FixJ receiver domain. Structure 7:1505-1515. [DOI] [PubMed] [Google Scholar]
- 5.Djordjevic, S., and A. M. Stock. 1998. Structural analysis of bacterial chemotaxis proteins: components of a dynamic signaling system. J. Struct. Biol. 124:189-200. [DOI] [PubMed] [Google Scholar]
- 6.Dyer, C. M., M. L. Quillin, A. Campos, J. Lu, M. M. McEvoy, A. C. Hausrath, E. M. Westbrook, P. Matsumura, B. W. Matthews, and F. W. Dahlquist. 2004. Structure of the constitutively active double mutant CheYD13K Y106W alone and in complex with a FliM peptide. J. Mol. Biol. 342:1325-1335. [DOI] [PubMed] [Google Scholar]
- 7.Feher, V. A., J. W. Zapf, J. A. Hoch, J. M. Whiteley, L. P. McIntosh, M. Rance, N. J. Skelton, F. W. Dahlquist, and J. Cavanagh. 1997. High-resolution NMR structure and backbone dynamics of the Bacillus subtilis response regulator, Spo0F: implications for phosphorylation and molecular recognition. Biochemistry 36:10015-10025. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli, S., H. Wang, P. Matsumura, and K. Volz. 1995. Uncoupled phosphorylation and activation in bacterial chemotaxis. The 2.1-Å structure of a threonine to isoleucine mutant at position 87 of CheY. J. Biol. Chem. 270:17386-17393. [PubMed] [Google Scholar]
- 9.Gardino, A. K., B. F. Volkman, H. S. Cho, S. Y. Lee, D. E. Wemmer, and D. Kern. 2003. The NMR solution structure of BeF(3)(-)-activated Spo0F reveals the conformational switch in a phosphorelay system. J. Mol. Biol. 331:245-254. [DOI] [PubMed] [Google Scholar]
- 10.Hoch, J. A. 1995. Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system, p. 129-144. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 11.Im, Y. J., S. H. Rho, C. M. Park, S. S. Yang, J. G. Kang, J. Y. Lee, P. S. Song, and S. H. Eom. 2002. Crystal structure of a cyanobacterial phytochrome response regulator. Protein Sci. 11:614-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, M., R. B. Bourret, M. I. Simon, and K. Volz. 1997. Uncoupled phosphorylation and activation in bacterial chemotaxis. The 2.3 A structure of an aspartate to lysine mutant at position 13 of CheY. J. Biol. Chem. 272:11850-11855. [DOI] [PubMed] [Google Scholar]
- 13.Kern, D., B. F. Volkman, P. Luginbühl, M. J. Nohaile, S. Kustu, and D. E. Wemmer. 1999. Structure of a transiently phosphorylated switch in bacterial signal transduction. Nature 402:894-898. [DOI] [PubMed] [Google Scholar]
- 14.Lee, S.-Y., H. S. Cho, J. G. Pelton, D. Yan, E. A. Berry, and D. E. Wemmer. 2001. Crystal structure of activated CheY. J. Biol. Chem. 276:16425-16431. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S.-Y., H. S. Cho, J. G. Pelton, D. Yan, R. K. Henderson, D. S. King, L.-S. Huang, S. Kustu, E. A. Berry, and D. E. Wemmer. 2001. Crystal structure of an activated response regulator bound to its target. Nat. Struct. Biol. 8:52-56. [DOI] [PubMed] [Google Scholar]
- 16.Lewis, R. J., J. A. Brannigan, K. Muchova, I. Barak, and A. J. Wilkinson. 1999. Phosphorylated aspartate in the structure of a response regulator protein. J. Mol. Biol. 294:9-15. [DOI] [PubMed] [Google Scholar]
- 17.Madhusudan, M., J. Zapf, J. A. Hoch, J. M. Whiteley, N. H. Xuong, and K. I. Varughese. 1997. A response regulatory protein with the site of phosphorylation blocked by an arginine interaction: crystal structure of Spo0F from Bacillus subtilis. Biochemistry 36:12739-12745. [DOI] [PubMed] [Google Scholar]
- 18.Madhusudan, J. Zapf, J. M. Whiteley, J. A. Hoch, N. H. Xuong, and K. I. Varughese. 1996. Crystal structure of a phosphatase-resistant mutant of sporulation response regulator Spo0F from Bacillus subtilis. Struct. Folding Design 4:679-690. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay, D., U. Sen, J. Zapf, and K. I. Varughese. 2004. Metals in the sporulation phosphorelay: manganese binding by the response regulator Spo0F. Acta Crystallogr. Sect. D 60:638-645. [DOI] [PubMed] [Google Scholar]
- 20.Schuster, M., R. Zhao, R. B. Bourret, and E. J. Collins. 2000. Correlated switch binding and signaling in bacterial chemotaxis. J. Biol. Chem. 275:19752-19758. [DOI] [PubMed] [Google Scholar]
- 21.Tzeng, Y.-L., and J. A. Hoch. 1997. Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis. J. Mol. Biol. 272:200-212. [DOI] [PubMed] [Google Scholar]
- 22.Varughese, K. I. 2002. Molecular recognition of bacterial phosphorelay proteins. Curr. Opin. Microbiol. 5:142-148. [DOI] [PubMed] [Google Scholar]
- 23.Varughese, K. I., Madhusudan, X.-Z. Zhou, J. M. Whiteley, and J. A. Hoch. 1998. Formation of a novel four-helix bundle and molecular recognition sites by dimerization of a response regulator phosphotransferase. Mol. Cell 2:485-493. [DOI] [PubMed] [Google Scholar]
- 24.Volkman, B. F., D. Lipson, D. E. Wemmer, and D. Kern. 2001. Two-state allosteric behavior in a single-domain signaling protein. Science 291:2429-2433. [DOI] [PubMed] [Google Scholar]
- 25.Volkman, B. F., M. J. Nohaile, N. K. Amy, S. Kustu, and D. E. Wemmer. 1995. Three-dimensional solution structure of the N-terminal receiver domain of NTRC. Biochemistry 34:1413-1424. [DOI] [PubMed] [Google Scholar]
- 26.Volz, K. 1993. Structural conservation in the CheY superfamily. Biochemistry 32:11741-11753. [DOI] [PubMed] [Google Scholar]
- 27.Volz, K., and P. Matsumura. 1991. Crystal structure of Escherichia coli CheY refined at 1.7-Å resolution. J. Biol. Chem. 266:15511-15519. [DOI] [PubMed] [Google Scholar]
- 28.Welch, M., N. Chinardet, L. Mourey, C. Birck, and J.-P. Samama. 1998. Structure of the CheY-binding domain of histidine kinase CheA in complex with CheY. Nat. Struct. Biol. 5:25-28. [DOI] [PubMed] [Google Scholar]
- 29.Zapf, J., U. Sen, Madhusudan, J. A. Hoch, and K. I. Varughese. 2000. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Struct. Folding Design 8:851-862. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, X., C. D. Amsler, K. Volz, and P. Matsumura. 1996. Tyrosine 106 of CheY plays an important role in chemotaxis signal transduction in Escherichia coli. J. Bacteriol. 178:4208-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu, X., J. Rebello, P. Matsumura, and K. Volz. 1997. Crystal structures of CheY mutants Y106W and T87I/Y106W. J. Biol. Chem. 272:5000-5006. [DOI] [PubMed] [Google Scholar]