Abstract
We characterized the role of catabolite control protein A (ccpA) in the physiology and virulence of Streptococcus pneumoniae. S. pneumoniae has a large percentage of its genome devoted to sugar uptake and metabolism, and therefore, regulation of these processes is likely to be crucial for fitness in the nasopharynx and may play a role during invasive disease. In many bacteria, carbon catabolite repression (CCR) is central to such regulation, influencing hierarchical sugar utilization and growth rates. CcpA is the major transcriptional regulator in CCR in several gram-positive bacteria. We show that CcpA functions in CCR of lactose-inducible β-galactosidase activity in S. pneumoniae. CCR of maltose-inducible α-glucosidase, raffinose-inducible α-galactosidase, and cellobiose-inducible β-glucosidase is unaffected in the ccpA strain, suggesting that other regulators, possibly redundant with CcpA, control these systems. The ccpA strain is severely attenuated for nasopharyngeal colonization and lung infection in the mouse, establishing its role in fitness on these mucosal surfaces. Comparison of the cell wall fraction of the ccpA and wild-type strains shows that CcpA regulates many proteins in this compartment that are involved in central and intermediary metabolism, a subset of which are required for survival and multiplication in vivo. Both in vitro and in vivo defects were complemented by providing ccpA in trans. Our results demonstrate that CcpA, though not a global regulator of CCR in S. pneumoniae, is required for colonization of the nasopharynx and survival and multiplication in the lung.
Carbon metabolism and its regulation are central to prokaryotic life. Sugars serve as the most facile source of carbon and energy, both of which are needed to replenish essential nucleotide cofactors and other metabolites in the cell. When faced with a wide variety of carbon and energy sources, a bacterium has to make metabolic decisions, opting for preferential use of one source over another in order to maintain optimal growth (70, 74). Simultaneous utilization of all available sugars would be metabolically inefficient and would lead to slower growth. The ability to utilize preferred sugars depends on a regulatory process called carbon catabolite repression (CCR) (69, 74, 79). CCR causes silencing of genes specific for the utilization of nonpreferred sugars until the cell has consumed the preferred sugar(s).
CCR has been studied in considerable detail in the model free-living, gram-positive bacterium Bacillus subtilis (71, 74, 79). The main global regulator of CCR in this organism is catabolite control protein A (CcpA) (10, 31). CcpA belongs to the LacI/GalR family of activator-repressor transcription factors and influences the expression of a wide range of catabolic operons in B. subtilis (4, 24, 25, 30, 33, 36, 67, 71, 78, 79). CcpA has also been identified to function in the regulation of catabolic operons and catabolite repression in many Streptococcus spp. (1, 17, 59, 77). CcpA has also shown to be required for biofilm formation in Streptococcus mutans (80). Candidate genes or operons that are subject to CcpA-dependent CCR are often identifiable by the presence of an operator sequence, called the catabolite-repressible element (cre), to which CcpA binds (1, 37, 48, 56).
The affinity of CcpA for cre sequences is enhanced by binding to another protein, the histidine phosphoprotein (HPr). HPr is an integral component of the phosphoenolpyruvate-dependent phosphotransferase system (PTS), where it normally functions in the transfer of high-energy phosphate from phosphoenolpyruvate to the enzyme II complex during sugar uptake (55, 57). The presence of a preferred sugar, such as glucose, in the medium activates phosphorylation of HPr on a conserved serine residue at position 46 by the Hpr kinase, which itself is activated by metabolites such as the high-energy glycolytic intermediate fructose-1,6-bisphosphate (8, 21, 54, 58, 73). CcpA interacts with the phosphoserine form of HPr, P∼Ser-HPr, to form a dimeric complex. This interaction increases the affinity of CcpA for the cre. Binding of this dimeric complex typically causes repression of promoters, facilitating CCR (1, 13, 14). CcpA residues involved in binding of P∼Ser-HPr (38) and those involved in binding of cre (37) have been characterized, and the crystal structure of the CcpA-P∼Ser-HPr complex has been recently solved (63).
CCR has also been shown to play a role in the regulation of virulence factors in some gram-positive pathogens (23, 46). The coordinate regulation of virulence genes with carbon utilization genes may be critical for fitness when pathogens compete with other microbes for niche colonization. CCR may be needed during infection of host compartments where multiple sugars are available to the pathogen. Streptococcus pneumoniae is, under normal conditions, a resident of the human nasopharynx. Mucosal immunity prevents extensive colonization and, together with serum antibodies, prevents invasive disease. However, under certain conditions that are not well understood, most commonly in the very young or the elderly, the bacteria spread to the lung and cause pneumonia with further potentially serious complications such as bacteremia and meningitis (27, 52). Middle ear infections by this bacterium are also fairly common and severe in young children (27, 52, 76).
S. pneumoniae has a large fraction of its transporters in the genome devoted to the uptake and metabolism of sugars, and these include classical PTS, ATP-binding cassette, and ion gradient-driven transporters (72). The ability of S. pneumoniae to metabolize a wide range of sugars may confer a fitness boost in certain host niches. For example, S. pneumoniae may be able to utilize a wide variety of host sugars from surface glycoproteins, thereby contributing to its effective growth and colonization of the nasopharynx, as well as during invasive disease (72). Indeed, CcpA from S. pneumoniae (also called RegM) shares ∼54% identity with B. subtilis CcpA and has been recently shown to contribute to the virulence of the D39 serotype 2 strain of S. pneumoniae in a murine bacteremia model (22). However, the role of CcpA during colonization or infection at mucosal surfaces in the host is not known. Given these observations, it is clear that a thorough understanding of the pathogenesis of S. pneumoniae will require study of its sugar metabolism and basic physiology.
In this work, we characterized the role of CcpA in more detail by studying its physiological role in vitro, as well as in thevirulence of a serotype 4 strain of S. pneumoniae in murine pneumonia and nasopharyngeal carriage models. In vitro, the ccpA strain showed increased total α-glucosidase activity, confirming an earlier report that CcpA acts as a repressor of the synthesis of this enzyme (22). CcpA is required for complete catabolite repression of the total cellular β-galactosidase. Deletion of ccpA severely compromised the virulence of S. pneumoniae in a pneumonia model and, in addition, severely compromised colonization of the nasopharynx. To begin to address the role of CcpA during colonization and infection, we compared the proteins that are differentially expressed on the surface of the cell in the wild-type and ccpA mutant strains. This analysis led to the identification of many surface-localized metabolic enzymes that were down-regulated in the ccpA strain; some of these enzymes have previously been shown to play a role in in vivo fitness and/or virulence. For two of these candidates (enolase and Hpr), loss of ccpA did not alter the synthesis of the respective mRNAs. This suggests a defect at the posttranscriptional level causing decreased translocation to the cell surface. This role for a carbon regulator in the control of genes associated with metabolism and in vivo fitness connects the physiological process of carbon and sugar metabolism with colonization and disease and unexpectedly reveals CcpA-dependent, surface-localized metabolic enzymes with a potential role in invasion and virulence in the lung.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers for DNA manipulations.
Refer to Table 1 for a list of relevant strains and plasmids and to Table 2 for the primers used in this study.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| S. pneumoniae | ||
| AC353 | Spontaneous Smr derivative of TIGR4 | 27 |
| RI1932 | AC353 ΔccpA::cat | This work |
| RI1933 | RI1932 ΔccpA::cat ΔSP0474-SP0478::aad9-ccpA | This work |
| E. coli | ||
| AC578 | DH5α λpir(pR412); Apr Spr | This work |
| AC1000 | DH5α λpir(pAC1000); Apr Cmr | This work |
| Plasmids | ||
| pAC1000 | S. pneumoniae suicide vector; Cmr | 28 |
| pR412 | Contains magellan5 minitransposon; Apr Spr | 45 |
TABLE 2.
Sequences of primers used in this study
| Primer | Contig no.; coordinates or source | Sequencea (5′ to 3′) |
|---|---|---|
| ccpupF | AE007488; 11641-11660 | GCTGCCAAGTATGTCACCAA |
| ccpupR | AE007489; 358-339 | GAAGAAGGTTTTTATATTACAGCTCCAAAAAAATCAGGGAATCGAGA |
| ccpdnF | AE007489; 1370-1390 | CATCAAGCTTATCGATACCGTCCTTTTCCTGTCCTTTCTAT |
| ccpdnR | AE007489; 2390-2368 | AAAGTATTAGTCGCAGAAGATCA |
| ccpF | AE007489; 487-504 | GAGGTTGGGCAATCGTTG |
| ccpR | AE007489; 1069-1049 | AATAGCGATGAAGATAACGAG |
| ccpF0 | AE007488; 11593-11610 | AGGGCTGCTGACAAAGGA |
| ccpR0 | AE007489; 2430-2411 | AGTGCAGGTTCGACTACTTT |
| catF1 | pAC1000 | CGGTATCGATAAGCTTGATG |
| NcatR1 | pAC1000 | TGGAGCTGTAATATAAAAACCTTC |
| lacEF1 | AE007358; 10181-10200 | TTTAGAGGCTCCTATTTTTT |
| lacER1 | AE007359; 96-114 | CACCGGAACTCCTTTTTTT |
| lacER2 | AE007359; 7860-7841 | CCTATCTGGTCAGTATCGGA |
| lacESp | AE007359; 114-96 | AAAAAAAGGAGTTCCGGTGCCCAGATCTACCGCTCTAGAACTAGTGGATCCC |
| SpcR1 | pR412 | CCCAGATCTCAATTTTTTTATAATTTTTTTAATCTG |
| SpcF1 | pR412 | CCCAGATCTACCGCTCTAGAACTAGTGGATCCC |
| Ccp1 | AE007489; 1390-1370 | ATAGAAAGGACAGGAAAAGGA |
| Ccp2 | AE007489; 339-358 | TCTCGATTCCCTGATTTTTT |
| Spccp1 | AE007489; 1390-1370 | CAGATTAAAAAAATTATAAAAAAATTGAGATCTGGGATAGAAAGGACAGGAAAAGGA |
| CplacE | AE007489; 358-339 | AAAAAATCAGGGAATCGAGAGCTGTGTAGTAAGTTTTTCCA |
| NlacEF | AE007358; 10261-10283 | GGGTATTGTGTGGATTAAAAAGG |
| NlacER | AE007359; 7736-7713 | ACTGGTTTCTACAGGCTTGATTAG |
| 76rpa | AE007418; 8260-8277 | TAATACGACTCACTATAGGGAGATCACCACACATACCAGCC |
| Sal76 | AE007418; 8051-8071 | AGCGTCGACTCAATCAAAATTAACGTATTCTT |
| 28rpa | AE007414; 1192-1173 | TAATACGACTCACTATAGGGAGAGCCATTTCGATAGCTTCAA |
| Sal128 | AE007414; 1417-1396 | AGCGTCGACTTATTTTTTAAGGTTGTAGAAT |
| 1414F | AE007438; 6294-6276 | ATGTCTAAAACAGTAGTAC |
| 1414R | AE007438; 6118-6140 | TAATACGACTCACTATAGGGAGTTAGAATTTTTTACGTTTACGAG |
Underlined sequences are complementary to the S. pneumoniae genomic DNA. Coordinates are provided in the middle column.
Construction of the ccpA mutant.
The ccpA mutant was constructed by replacing the entire 1,011-bp coding sequence with a chloramphenicol (CM) resistance cassette, cat. The cat cassette, which has its own promoter and conferred CM resistance on Escherichia coli and S. pneumoniae, was PCR amplified from pAC1000 by using primers catF1 and NcatR1. One-kilobase-pair DNA fragments containing the regions 5′ and 3′ of the ccpA gene were PCR amplified from AC353 genomic DNA by using two primer pairs, ccpupF-ccpupR and ccpdnF-ccpdnR. One of the primers in each of the pairs was designed to have a 20-bp overlap with the 5′ and 3′ sequences of the cat cassette. The cat cassette was fused to these two PCR products by splicing by overlap extension (SOE) (32). The final PCR product was introduced into S. pneumoniae TIGR4 by natural transformation as previously described (7). The double-recombination event was selected for by plating on medium containing 4 μg/ml CM and was confirmed by PCR and DNA sequencing.
Construction of the ccpA complementation strain.
For complementation of the ccpA mutation, ccpA, along with its native ribosome-binding site, was amplified by PCR with primers Spccp1 and ccp2. Spccp1 contained a 36-bp sequence that overlaps the 3′ end of the aad9 (spectinomycin [SP resistance) gene (29). aad9 and its promoter were amplified by PCR with primers spcF1 and spcR1 from plasmid pR412 (45). The product of the Spccp1-and-ccp2 PCR was joined to aad9 by using SOE to generate the aad9-ccpA cassette. This PCR construct was flanked by 800-bp DNA identical to the regions 5′ and 3′ of the lacE PTS operon (SP0474 to SP0478) to target the cassette to this region of the chromosome. The lacE 5′ region, LacE(up), was amplified from AC353 genomic DNA by using the primer pair lacEF1-lacER1. In a separate PCR, the aad9-ccpA cassette was amplified by using primers lacESp and ccp2, creating a fragment that has a sequence overlap with the 3′ end of the lacEF1-lacER1 product. Both of these two fragments were fused by using SOE to create LacE(up)-aad9-ccpA. In a similar manner, the lacE downstream region, LacE(down), was PCR amplified by using the primer pair CplacE-lacER2, in which the former has a sequence overlap with the 3′ end of ccpA. This piece was fused to the LacE(up)-aad9-ccpA cassette by using SOE to create the final complementation cassette LacE(up)-aad9-ccpA-LacE(down). The complementation construct completely replaced the coding region of the lacE operon and expressed aad9 and ccpA constitutively from the aad9 promoter. The ccpA mutant was transformed with the complementation cassette, and the double-recombination event was selected for by plating on medium containing 100 μg/ml SP and was confirmed by PCR and DNA sequencing. We have previously determined that a deletion-insertion in this locus affected neither in vitro growth nor virulence (data not shown).
Bacterial growth and media.
S. pneumoniae was routinely grown in Todd-Hewitt broth (THB) or on blood agar plates containing 100 μg/ml streptomycin (SM) at 37°C in a 5% CO2 atmosphere. For broth cultures, 1.5 μl/ml Oxyrase (Oxyrase, Inc.) was added in addition and cultures were typically grown to a final optical density at 600 nm (OD600) of 0.5 to 0.6. For the sugar hydrolysis assays, S. pneumoniae was grown in a semidefined minimal medium (SDMM) without BSA (39) containing 100 μg/ml SM for the wild type, 100 μg/ml SM and 4 μg/ml CM for the ccpA mutant, and 100 μg/ml SM and 100 μg/ml SP for the ΔccpA/ccpA+ complemented strain. Fresh catalase (3 U/ml; Sigma-Aldrich) was added to the SDMM before inoculation with S. pneumoniae in order to facilitate optimal growth. Wild-type or ccpA mutant cells were grown in SDMM containing the appropriate sugar(s) as the primary carbon source for growth curve and generation time determinations. Bacteria were grown in SDMM in the presence of an inducing sugar (lactose, maltose, cellobiose, or raffinose) either alone or in combination with a repressing sugar (glucose or sucrose) to create catabolite-repressing conditions. The concentration of sugar was 10 mM in all cases. There was negligible growth in SDMM in the absence of added sugar.
Animal infections.
All animal infections were done with 8- to 10-week-old female Swiss-Webster mice (Taconic Laboratories). Mice provided with continuous food and water were housed in accordance with guidelines provided by the Tufts University Department of Laboratory Animal Medicine. Bacteria were recovered from plates of tryptic soy agar (TSA) with 5% sheep’s blood, subcultured in prewarmed THB containing Oxyrase, and grown at 37°C for 3 to 4 h to an OD600 of 0.4. One milliliter of cultured cells was centrifuged at 10,000 rpm for 2 min in a Microfuge, and the cells were resuspended in 500 μl of prewarmed THB. For competition assays, mutant and wild-type bacteria were mixed 1:1 and inoculated intranasally at the following doses: 107 CFU in a 40-μl volume for lung infections and 105 CFU in a 10-μl volume for nasopharyngeal colonization. Prior to inoculation, mice were anesthetized by isoflurane inhalation. Similar volumes and concentrations of bacteria were used for single-strain infections. Immediately after inoculation of mice, 106 CFU of the input mixture was also used to inoculate 10 ml of prewarmed THB containing 100 μg/ml SM and 1.5 μl/ml Oxyrase. Growth of this in vitro competition culture was carried out at 37°C in 5% CO2 for 5 h, after which the cells were diluted and plated on blood agar plates supplemented with 100 μg/ml SM. Lung infection was carried out for 42 h and nasopharyngeal colonization for 7 days, after which the mice were sacrificed. Lungs were removed and placed in 3 ml of THB containing 20% glycerol and mechanically homogenized. The homogenates were appropriately diluted and spread on blood agar plates supplemented with 100 μg/ml SM. Bacteria were recovered from the mouse nasopharynx by allowing 1 ml of phosphate-buffered saline to flow into the trachea and out through the nasal passages with collection of the flowthrough on Parafilm. Appropriate dilutions of the flowthrough were plated on blood agar supplemented with 100 μg/ml SM. For competition assays, the ratio of the ccpA mutant or the ccpA-complemented strain to the wild type in the output from each mouse was determined by replica plating on blood agar plates supplemented with 100 μg/ml SM and 4 μg/ml CM or 100 μg/ml SM and 100 μg/ml SP, respectively.
Colony size measurements.
Individual colony sizes of wild-type and ccpA cells were magnified with the 10× Plan-NEOFLUAR lens on an Axioplan 2 microscope (Zeiss) and an Orca cooled, charge-coupled device camera (Hamamatsu Photonics). Images were recorded by Openlab 3.1.7 software (Improvision).
RNase protection assays (RPAs).
Total RNA was isolated from 20 ml of S. pneumoniae wild-type and ccpA mutant cells grown in THB supplemented with streptomycin (100 μg/ml) and Oxyrase (1.5 μl/ml). Cells were pelleted at 4,000 rpm for 10 min. RNA was isolated from the cell pellet with a QIAGEN RNeasy kit in accordance with the recommendations of the manufacturer (QIAGEN). Template DNA for the generation of riboprobes was PCR amplified with primersets 76rpa-Sal76, 28rpa-Sal128, and 1414F-1414R. One of the primers in eachpair had the T7 promoter sequence (5′-TAATACGACTCACTATAGGGAG-3′) so that the resultant PCR piece could be directly used as a template for the generation of riboprobes with a MaxiScript III in vitro transcription kit (Ambion). Synthesized probes were purified on a 4% denaturing polyacrylamide gel containing 8 M urea. RPAs were carried out as described by the manufacturer with an RPAIII kit (Ambion). The protected fragments were visualized by exposing each gel to a phosphorimaging screen (Kodak) and analyzed with a Storm 860 scanner and IQMac V1.2 imaging software. The relative amount of each protected fragment in each assay was normalized to the amount of SP1414 (rpsU) protected RNA in each lane.
Enzyme assays. (i) α-Glucosidase.
α-Glucosidase activity was assayed as previously described (9). Enzyme activity was monitored by following the increase in absorbance at 420 nm due to enzymatic release of p-nitrophenol from p-nitrophenyl-α-d-glucoside. The reaction mixture contained 0.2 ml of p-nitrophenylglucoside (2 mg/ml) in 250 mM potassium phosphate buffer (pH 7.0). To this, 0.8 ml of 50 mM potassium phosphate buffer (pH 7.0) was added to bring the final volume to 1 ml. One milliliter of growing bacteria at an OD600 of 0.4 was pelleted in a Microfuge at 10,000 rpm for 1 min and resuspended in 100 μl of 50 mM potassium phosphate buffer (pH 7.0). One microliter of a 10% Triton X-100 stock was added to 100 μl of mid-log-phase cells of S. pneumoniae, and the cells were incubated at 37°C for 10 min to lyse the cells. The reaction was initiated by addition of 50 μl of cell extract. Enzyme activity was expressed as nanomoles of p-nitrophenol released per unit of time per unit of volume of cell extract.
(ii) β-Glucosidase.
β-Glucosidase activity was assayed as previously described (49). Fifty-microliter aliquots of mid-log-phase S. pneumoniae cells were washed and resuspended in 50 μl of 50 mM Tris-HCl (pH 7.4). Five microliters of 1 M MgCl2 and 50 μl of 100 mM p-nitrophenyl-β-d-glucopyranoside were added to 0.895 ml of 50 mM Tris-HCl (pH 7.4) to get a final volume of 0.95 ml. The reaction was then initiated by addition of 50 μl of the cell suspension. Enzymatic release of p-nitrophenol was monitored at 405 nm, and activity was expressed as nanomoles of p-nitrophenol released per unit of time per unit of volume of cell suspension.
(iii) α-Galactosidase.
α-Galactosidase activity was assayed as previously described (60). One milliliter of 1 M MgCl2, 3.1 μl of 1.43 M β-mercaptoethanol, and 90 μl of a 1 mg/ml solution of p-nitrophenyl-α-d-galactopyranoside were added to 0.896 ml of 100 mM sodium phosphate (pH 7.5). Ten microliters of Triton X-100 cell extract of S. pneumoniae was added to the reaction mixture. Enzymatic release of p-nitrophenol was monitored at 405 nm, and activity was expressed as nanomoles of p-nitrophenol released per unit of time per unit of volume of cell extract.
(iv) β-Galactosidase.
β-Galactosidase activity was assayed as previously described (47). One microliter of 1 M MgCl2 and 3.5 μl of 14.3 M β-mercaptoethanol were added to 0.945 ml of 100 mM sodium phosphate buffer (pH 7.5) containing 0.8 mg/ml o-nitrophenyl-β-d-galactopyranoside. Fifty microliters of a Triton X-100 cell extract of S. pneumoniae was added to the reaction mixture. The enzymatic release of o-nitrophenol was monitored at 420 nm, and activity was expressed as nanomoles of o-nitrophenol released per unit of time per unit of volume of cell extract.
Cell wall fractionation and two-dimensional (2-D) gels.
Cell wall fraction was prepared by overnight digestion with mutanolysin enzyme as previously described (5). The protein concentration in the sample was estimated with the bicinchoninic acid protein assay kit (Pierce) before loading of isoelectric focusing strips. One hundred micrograms of total protein was resuspended in 180 μl of buffer containing pH 4 to 7 range ampholytes from Invitrogen and loaded directly onto the focusing strips. 2-D gels were run with the Zoom IPG runner system and subsequently 4 to 12% Bis-Tris Zoom polyacrylamide gels to separate proteins in the second dimension. Proteins in the gel were stained with Coomassie brilliant blue.
Protein identification.
Protein spots were excised from Coomassie-stained gels and dehydrated in 100% acetonitrile for 10 min. The acetonitrile was removed, and the tube with the gel piece was vacuum centrifuged until dry. The gel was rehydrated at 4°C for 45 min in 5 μl of buffer containing trypsin (12 ng/μl of buffer) and 50 mM NH4HCO3. The buffer was replaced with 10 μl of 20 mM NH4HCO3, and the digestion was carried out for 18 h at 37°C. Peptides were then extracted from the gel by three exchanges of 50 μl of 5% formic acid in 50% acetonitrile (20 min of incubation each time) at room temperature. The supernatants were pooled and vacuum centrifuged to a dry pellet. The proteins wereresuspended in 100 μl of 0.4% acetic solution prior to mass spectrometer analysis.
Tandem mass spectrometry.
In brief, tryptic peptides from in-gel-digested spots were analyzed by μLC-ESI-MS/MS with an LCQ-DECA mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with a C18 trap and an analytical nano-LC column-emitter. To prevent carryover between runs, a fresh trap and a fresh column-emitter were used for every run. Mass spectra were searched against both of the published S. pneumoniae TIGR4 and R6 genomes to match fragment ion peaks to theoretical peaks. Probability of peptide identification was determined with PeptideProphet, and assignment of peptides to proteins was assessed by ProteinProphet (38).
RESULTS
Growth characteristics of the ccpA strain.
We generated a ccpA deletion-insertion mutation (ΔccpA::cat) in the encapsulated serotype 4 S. pneumoniae TIGR4 strain. The resultant ccpA strain produced smaller colonies on blood agar plates than did the parent strain. The average colony size of the wild type (0.58 ± 0.03 mm) was ∼22% larger than that of the ccpA mutant (0.46 ± 0.05 mm). This is similar to the observation made for a ccpA strain in the D39 (serotype 2) S. pneumoniae background (22). In that report, the small-colony phenotype was attributed to the lowered transcription from the capsule locus, causing closer packing of bacterial cells within colonies. To test if a similar phenomenon was occurring in the TIGR4 ccpA strain, the Quellung reaction was used to qualitatively measure capsule expression. There was no detectable difference in the Quellung reaction between ccpA and wild-type cells (data not shown).
An alternative explanation for the small-colony phenotype is a reduced growth rate. It is known that loss of ccpA in B. subtilis causes a general growth defect that arises from amino acid auxotrophy, specifically, Glu, Met, and the branched-chain amino acids Ile, Leu, and Val (41). We therefore tested for growth defects of the S. pneumoniae ccpA strain in SDMM with a variety of sugars as the sole carbon source. However, there was no statistically significant difference in generation time on most of the carbon sources tested, except raffinose (Table 3). The ccpA strain was mildly outcompeted (twofold) in in vitro competitions with wild-type cells in rich broth (∼10 generations of growth), suggesting a mild decrease in fitness in complex media. Consistent with this, when grown separately, the generation time of the ccpA strain (55 ± 0.7 min) was increased in rich broth compared to that of the wild type (47 ± 2 min). Finally, we measured the numbers of CFU per colony of the wild-type and ccpA strains. A single ccpA colony had approximately 30% fewer CFU compared to the wild type (4.8× 105 ± 0.7 × 105 and 7.0 × 105 ± 0.6 × 105 for ccpA and thewild type, respectively). This is consistent with the mild competitive defect observed in complex broth that we propose accounts for the small-colony phenotype.
TABLE 3.
Generation times of wild type and ccpA mutant in SDMM with different sugars
| Sugar (class)a | Mean generation time (min) ± SD
|
|
|---|---|---|
| Wild type (n = 3) | ccpA (n = 3) | |
| Glucose (monosaccharide) | 46 ± 4 | 53 ± 8 |
| Sucrose (disaccharide) | 40 ± 3 | 47 ± 9 |
| Lactose (disaccharide) | 61 ± 2 | 62 ± 5 |
| Raffinose (trisaccharide) | 59 ± 8 | 76 ± 11b |
| N-Acetylglucosamine (amino sugar) | 54 ± 1 | 53 ± 4 |
| Mannitol (sugar alcohol) | 104 ± 17c | 103 ± 11c |
Each sugar was used at 10 mM.
Significant at P < 0.05.
Average of two independent determinations.
CcpA in sugar metabolism.
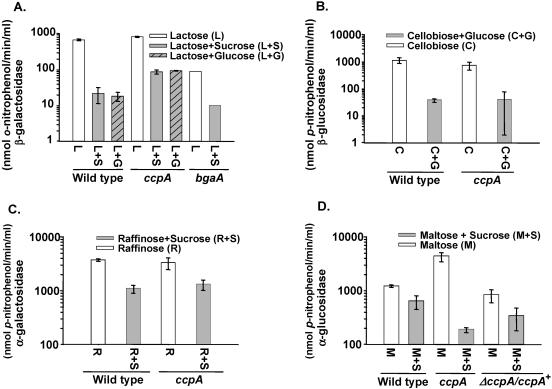
S. pneumoniae can grow on many sugars, including maltose, cellobiose, raffinose, and lactose, when provided as the sole carbon source (Table 3; data not shown). The presence of these sugars induces the enzymes α-glucosidase (9, 26, 62), β-glucosidase (34, 43, 49), α-galactosidase (2, 3, 60), and β-galactosidase (15, 66), respectively. While these activities were chosen as representative candidates to investigate the role of ccpA in CCR of sugar metabolism, we do not know the identities of the genes that are specifically responsible for these enzymes, with the exception of bgaA, which encodes the cell wall-localized β-galactosidase. The wild-type and ccpA strains were grown in the presence of the inducing sugar either alone or in combination with a repressing sugar, glucose or sucrose, and CCR was measured. The presence of either glucose or sucrose in the growth medium repressed lactose-inducible β-galactosidase in wild-type cells 30-fold (Fig. 1A, compare bar 1 with bars 2 and 3) and was taken as an indication of CCR. CCR was relieved about threefold in the ccpA strain (Fig. 1A, bars 5 and 6), indicating that CcpA mediates part of the CCR of β-galactosidase activity in the presence of glucose or sucrose.
FIG. 1.
CCR of sugar-degrading enzymes in S. pneumoniae. (A) β-Galactosidase activity in wild-type and ccpA and bgaA mutant cells in the presence of the inducer lactose (L) alone compared to activity in the presence of the repressing sugar glucose (L+G) or sucrose (L+S). (B) β-Glucosidase activity in wild-type and ccpA mutant cells in the presence of the inducer cellobiose (C) compared to activity in the presence of the repressing sugar glucose (C+G). (C) α-Galactosidase activity in wild-type and ccpA mutant cells in the presence of the inducer raffinose (R) alone compared to activity in the presence of the repressing sugar sucrose (R+S). (D) α-Glucosidase activity in wild-type and ccpA mutant cells in the presence of the inducer maltose (M) alone compared to activity in the presence of the repressing sugar sucrose (M+S).
In contrast, loss of ccpA in D39 led to an increase in lactose-induced β-galactosidase in the absence of a repressing sugar (22). This implies inherent differences in CcpA-mediated regulation of β-galactoside metabolism between serotype 2 and 4 strains. A major portion (∼85%) of the total cellular β-galactosidase activity measured in TIGR4 above arises from the bgaA gene (82) (Fig. 1A, compare bars 1 and 7). Deleting bgaA allowed us to monitor the remaining β-galactosidase activity. The BgaA-independent β-galactosidase activity is about sevenfold lower than that of the wild type (Fig. 1A, compare bars 1 and 7). It is repressed about 10-fold in the presence of the repressing sugar (Fig. 1A, compare bars 7 and 8), which is ∼3-fold less than the repression seen in the wild type.
In contrast to β-galactosidase activity, β-glucosidase and α-galactosidase activities were still subject to CCR in the absence of CcpA (Fig. 1B and C). This suggests that another regulator(s), which may or may not be redundant with CcpA, mediates CCR of these genes. The possibility that CcpA could still regulate the expression of these genes in the absence of the CcpA-independent mechanism cannot be excluded. Indeed, redundancy in CCR and regulation of carbon metabolism is not unprecedented since this has been observed in the regulation of the B. subtilis glycerol operon (12, 14).
Loss of CcpA leads to significantly increased CCR of α-glucosidase activity in the presence of the repressing sugar compared to the wild type (Fig. 1D, bars 2 and 4). This indicates the existence of an alternative regulator that mediates CCR of α-glucosidase in TIGR4 in the absence of CcpA. We also observed that loss of ccpA produced a significant increase in total cellular α-glucosidase activity under inducing conditions (Fig. 1D, compare bars 1 and 3). This indicates that CcpA represses this system in the wild type when an inducer is present as the sole carbon source. This defect was restored to the wild type by complementation of ccpA in trans (Fig. 1D, bars 5 and 6). Thus, CcpA can also function as a negative regulator in S. pneumoniae.
Role of CcpA in virulence.
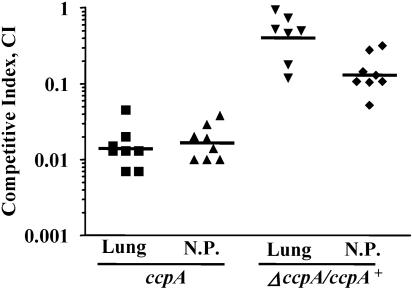
CcpA has been shown to contribute to the growth of strain D39 in a murine model of bacteremia (22). The D39 ccpA strain also showed a significant decrease in transcription of the capsular locus, and the authors attributed some of the attenuation of virulence to this defect. In order to investigate the role of CcpA in the colonization and infection of mucosal surfaces, we tested the role of ccpA in the TIGR4 strain in murine models of pneumonia and nasopharyngeal colonization. The ccpA strain was outcompeted by the wild type in both the pneumonia (competitive index [CI] of0.015) and nasopharyngeal carriage (CI of 0.02) models (Fig. 2). Competition assays in vitro in THB revealed a mild growth defect in the ccpA strain (CI = 0.5). Attenuation of virulence in the lung was almost completely complemented by constitutive expression of ccpA in trans; however, the defect in the nasopharynx was only partially complemented (Fig. 2). It is possible that constitutive expression of ccpA in trans is not able to fully complement due to slight over- or underexpression or, alternatively, due to incorrect temporal expression. The sum of these results demonstrates that significant attenuation of virulence and colonization results from the loss of CcpA.
FIG. 2.
Role of ccpA in pneumonia and nasopharyngeal colonization in mice. Loss of ccpA causes attenuation of the ccpA mutant (ccpA) in an in vivo competition with the wild type in the pneumonia (Lung) and nasopharyngeal carriage (N.P.) models of infection. Provision of a single copy of ccpA complements both defects (ΔccpA/ccpA+). Horizontal bars represent geometric means.
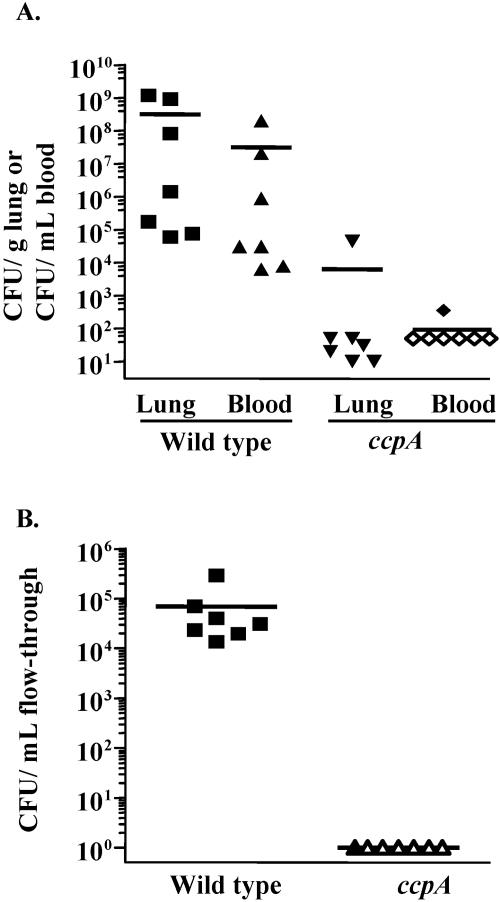
To test the possibility that the attenuation seen in the ccpA strain was a result of competition by the wild type for limited resources, we also performed single-strain infections. Forty-two hours after inoculation of the lungs, the wild type was recovered in large numbers from the lung (3 × 108 CFU/g) and the bloodstream (3 × 107 CFU/ml), whereas the ccpA mutant was recovered at levels approximately 5 orders of magnitude lower from either site (Fig. 3A). Similar results were obtained from experiments to test colonization of the nasopharynx: The wild type was recovered in large numbers (7 × 104 CFU/ml), whereas the ccpA mutant was recovered at or below the limit of detection (Fig. 3B). It is formally possible that the wild-type cells might be able to complement some of the ccpA deficits in vivo, and this could explain the more severe attenuation in the single-strain infections compared to the competition experiments. These results show that CcpA plays an important role in lung infection and colonization of the mouse nasopharynx.
FIG. 3.
Single-strain pneumonia and nasopharyngeal colonization of mice with the wild type and the ccpA mutant. The ccpA strain (ccpA) is attenuated in both pneumonia (A) and nasopharyngeal colonization (B) compared to the wild type. Open symbols represent data at or below the limit of detection (∼100 per mouse). Horizontal bars represent arithmetic means.
Identification of CcpA-regulated surface proteins.
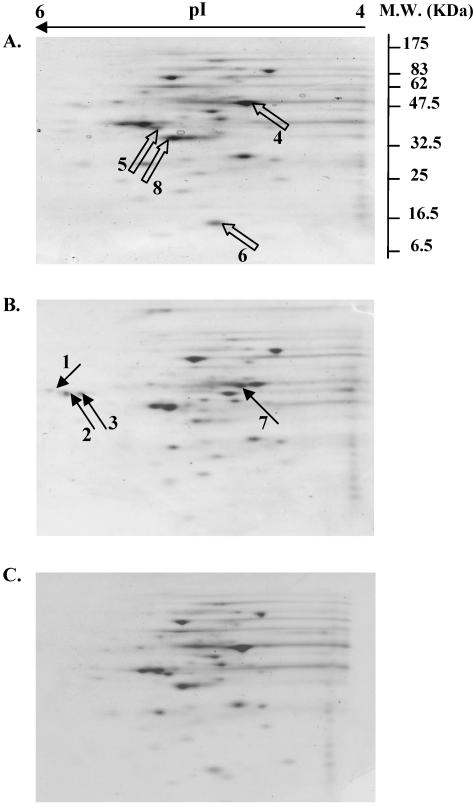
CcpA is a global regulator of several catabolic operons in B. subtilis. However, the nature of the genes regulated by CcpA in serotype 4 S. pneumoniae is unclear. Given the minimal growth defects of the ccpA strain observed in vitro but the substantial defects exhibited in the mouse lung and nasopharynx, we wanted to identify ccpA-regulated factors that could underlie the observed lack of fitness in vivo. We looked for the subset of ccpA-dependent proteins that are cell wall associated, since such factors may mediate important interactions with the host to facilitate colonization and multiplication. Figure 4 shows representative 2-D gel profiles of the cell wall fractions of the wild-type and ccpA strains. Some prominent differences in protein spots between the wild-type and ccpA strains are highlighted with arrows. While some spots were up-regulated in the ccpA strain (arrows in panel B), others were absent or reduced in the ccpA strain (open arrows in panel A). Provision of a wild-type copy of ccpA in trans restored the cell wall protein profile to that of the wild type (Fig. 4C), showing that the altered expression of proteins in the cell wall arose from the lack of CcpA. These results are consistent with a dual function of CcpA as both an activator and a repressor. Table 4 lists a subset of proteins identified in this analysis by peptide fingerprinting by mass spectrometry.
FIG. 4.
2-D gels of cell wall fraction of S. pneumoniae. Panels: A, wild type; B, ccpA mutant (ccpA)−; C, ccpA-complemented strain (ΔccpA/ccpA+). Arrows indicate differences in the protein profiles. M.W., molecular mass.
TABLE 4.
CcpA-dependent cell wall-associated proteins
| Protein class and name | Function | Spot(s)a |
|---|---|---|
| Up-regulated in ccpA strain | ||
| SP1580 | MsmK ATP-binding protein | 1 |
| SP0715 | Lactate oxidase | 2, 3 |
| SP1588 | Pyruvate-2-oxoglatarate and dihydrolipoamide dehydrogenase (E3) complex | 7 |
| SP2108 | Maltose-binding protein | 7 |
| Down-regulated in ccpA strain | ||
| SP1128 | Enolase | 4 |
| SP1220 | Malate/lactate dehydrogenase | 5 |
| SP1177 | HPr component of PTS | 6 |
| SP0605 | Fructose-1,6-bisphosphate aldolase | 8 |
| SP0764 | Dihydroorotate dehydrogenase | 8 |
Spots in Fig. 4.
Among the proteins that were down-regulated in the ccpA strain are ones associated with PTSs (SP1177, HPr) (57), glycolysis (SP0605, aldolase; SP1128, enolase), pyrimidine nucleotide biosynthesis (SP0764) (61), and lactate-to-pyruvate interconversion (SP1220) (1). SP1128 has also been shown to bind plasminogen, thereby facilitating host invasion, and contributes to virulence potential (6, 20). Some of the proteins that are derepressed in the ccpA strain are associated with sugar uptake (SP1580, SP2108) (50, 60) and organic acid metabolism (SP0715, SP1588) (64, 68).
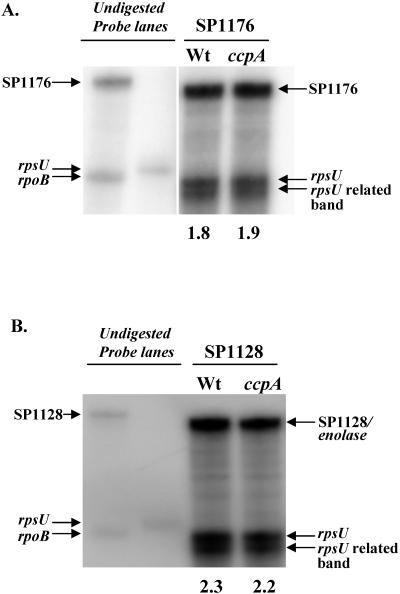
To see if the down-regulation of proteins in the cell wall in the ccpA strain was due to a general decreased transcription of these genes versus defects in translocation of the proteins, we carried out RPAs. We used [32P]UTP-labeled riboprobes to quantify the amounts of the SP1128 (enolase) and SP1176 (ptsI) transcripts. SP1176 is located immediately downstream of SP1177 (ptsH/hpr) and is predicted to be in an operon with this gene. Both SP1176 (ptsI) (Fig. 5A) and SP1128 (enolase) (Fig. 5B) were transcribed to the same extent in the wild-type and ccpA strains. The absence of a difference at the level of transcription suggests that the underlying cause for the decreased levels of HPr and the enolase in the cell wall is posttranscriptional in nature.
FIG. 5.
RPA to analyze mRNA levels of SP1176 (ptsI) (A) and SP1128 (enolase) (B) in wild-type (Wt) strain AC353 and ccpA strain RI1932. Riboprobes for SP1176 (ptsI), SP1128 (enolase), and SP1414 (rpsU loading control) were generated and hybridized to 3 μg of RNA from the three S. pneumoniae strains. RNA was harvested from cells grown in THB to an OD600 of 0.3. Undigested riboprobes of SP1176, SP1414, and SP1128 in the absence of S. pneumoniae RNA are indicated. An rpoB riboprobe is also indicated but was not used in the experiments. The value below a lane is the relative abundance of the SP1176 or SP1128 transcript in the wild-type or ccpA strain normalized to SP1414 (rpsU), as calculated by densitometry with ImageQuant TL software (Amersham Biosciences).
DISCUSSION
Given its lack of a functional electron transport chain and tricarboxylic acid cycle, S. pneumoniae is predicted to be highly dependent on external sugars for its energy requirements (72). In this study, we tested the role of CcpA as a central player in mediating the CCR and virulence of this bacterium.
Loss of CcpA affects the catabolite repression of only one of the four sugar-metabolizing enzymes tested. Even this is only a partial effect. The result suggests the presence of alternative regulatory systems that are operative independently of ccpA, implying that CcpA may not be a universal regulator of CCR in S. pneumoniae. Unexpectedly, loss of CcpA led to greatly increased catabolite repression of α-glucosidase activity in the presence of sucrose, pointing to the existence of perhaps another transcription factor that is capable of relaying carbon catabolite regulatory signals.
B. subtilis has been shown to possess, in addition to CcpA, two other functionally related regulators, CcpB (11), which is involved in catabolite repression of the gluconate and xylose operons, and CcpC (35), which regulates catabolite repression of the aconitase and citrate synthase genes. Although searching the S. pneumoniae genome for CcpA paralogs and for orthologs of B. subtilis CcpB and CcpC identified potential relatives, their role in CCR, if any, remains untested. Deleting these genes individually or in combination with ccpA may reveal parallel CcpA-like catabolite repression pathways in S. pneumoniae. Searching the TIGR4 genome for orthologs of the B. subtilis glycolytic regulator CggR (16) and the gluconeogenic gene CCR regulator CcpN (65) revealed a putative ortholog for CggR (SP0247), while CcpN had no matches. While the existence of such parallel pathways remains a viable explanation, inducer exclusion (74) via the phospho-Ser HPr protein could also account for the CcpA-independent CCR observed for the β-glucosidase and α-galactosidase activities.
Because S. pneumoniae is an obligate commensal and clinically important pathogen of humans, we also wanted to examine if CcpA influences the colonization and virulence characteristics of this organism in a murine model. In contrast with the dispensability of CcpA for growth in vitro, the ccpA strain was drastically attenuated for infection of the lung and colonization of the nasopharynx. While providing ccpA in trans to the mutant restores virulence in the lung, the rescue of nasopharyngeal colonization is only partial, albeit significant. This could be due to aberrant expression of ccpA from the complementation construct. CcpA naturally autoregulates its own expression in some bacteria due to the presence of a cre sequence upstream of the gene (19, 42). Inspection of the region upstream of ccpA in S. pneumoniae reveals the presence of a candidate cre about 140 bp upstream of the start codon. CcpA autoregulation might be required for the optimal regulation of target genes in vivo.
The large attenuation of colonization and virulence of the ccpA strain suggested that CcpA is directly or indirectly regulating genes that are required for in vivo fitness. Our characterization of the cell wall proteome revealed that CcpA serves to activate the expression of some proteins while negatively regulating others. Since CcpA has been shown to be important in central metabolism in other bacteria, the presence of metabolic enzymes in the list of up- and down-regulated cell wall proteins is not entirely surprising. Among the former class is a metabolic protein, enolase, which is known to be required for survival and multiplication of S. pneumoniae in vivo (6). Enolase (SP1128) is a glycolytic enzyme that normally converts 2-phosphoglycerate to generate phosphoenolpyruvate. However, some enolase has been shown to be cell wall associated and to bind host plasminogen, which, when converted to plasmin, facilitates invasion of host tissues by degrading the extracellular matrix (6, 20, 81). Another metabolic enzyme, dihydroorotate dehydrogenase (SP0764), is down-regulated in the ccpA strain. Dihydroorotate dehydrogenase is normally involved in pyrimidine biosynthesis (61) and is induced in vivo (44). In addition, microarray analysis revealed that a dihydroorotate dehydrogenase gene is up-regulated in bacteria attached to a pharyngeal epithelial cell line in vitro (51). Fructose-1,6-bisphosphate aldolase (SP0605), a cytoplasmic glycolytic enzyme, splits fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. This enzyme has also been shown to be cell wall associated and antigenic in mice (40), although a possible role in infection has not been investigated. CcpA has been shown to positively regulate glycolysis in B. subtilis (75). The involvement of CcpA as a positive regulator of these potentially dual-role metabolic enzymes in S. pneumoniae is a novel observation that warrants further investigation.
HPr, which is one of the chief players in PTS-mediated sugar uptake, is normally a cytoplasmic protein. It has been shown to also localize to the surface in Streptococcus suis cells (18). Cell surface-associated HPr, although retaining function, was shown to be devoid of the N-terminal methionine residue, a modification that may lead to its altered location. The down-regulation of HPr (SP1177) and enolase (SP1128) in the ccpA strain cell wall could reflect an overall decrease in the expression of these proteins in the cell due to decreased transcription of these genes or a defect in the efficiency of presentation on the cell surface. Our RPA results reveal that SP1176 (ptsI), SP1177 (ptsH/hpr), and SP1128 (enolase) are transcribed to the same extent in the ccpA strain as in the wild type, suggesting that the differences are likely to arise from changes at the posttranscriptional level.
We additionally found that CcpA functions as a repressor of some cell wall proteins in S. pneumoniae. Among the derepressed proteins observed is lactate oxidase (SP0715) (64), which is involved in metabolic processes resulting in the formation of acetyl phosphate (53), a high-energy precursor in the cell. The two protein spots corresponding to lactate oxidase on 2-D gels differed in pI but not molecular weight, indicating a possible posttranslational modification. It is unknown how this enzyme is exported and retained in the cell wall compartment in S. pneumoniae. Other cell wall proteins subject to derepression in the ccpA strain include components of sugar binding and uptake systems like MsmK (SP1580), a maltose-binding periplasmic protein (SP2108), and a member of the dihydrolipamide dehydrogenase family (SP1588). Among these candidates, dihydrolipamide dehydrogenase has been shown to be required for survival and multiplication of S. pneumoniae in vivo (68). However, it is not clear if derepression of these factors has a role in the observed attenuation in colonization and virulence by the ccpA strain.
Our results highlight an unappreciated role for CcpA as a transcriptional regulator that controls the expression of genes with varied functions in metabolism in S. pneumoniae. Prominent on the list of altered CcpA-regulated candidates are proteins associated with functions in central and intermediary cellular metabolism. These changes in basic cellular metabolism could be responsible for the observed defects in vivo. Indeed, the loss of CcpA also causes down-regulation of glycolytic enzymes like enolase and other metabolic enzymes. Some of these are known to contribute to virulence and might meet specific metabolic needs during infection. It is conceivable that misregulation of these factors adversely affects colonization of the nasopharynx and survival in the lungs during infection, leading to rapid clearance. The requirement for CcpA as a regulator in determining colonization and infection of mucosal surfaces by S. pneumoniae, and the potential for a similar role for CcpA-independent CCR mechanisms, opens up new regulatory roles for carbon regulators in the control of CCR and sugar metabolism in the context of pathogenesis.
Acknowledgments
We are grateful to A. L. Sonenshein for critically reading the manuscript and for helpful discussions to C. Squires and the Camilli lab for suggestions, to S. Donahue for help with proteomic analysis, to A. Tischler for assistance with RPAs, and to Y. Yamaichi, H. Kimsey, and the Waldor lab for help with experiments.
A.C. is an investigator of the Howard Hughes Medical Institute (HHMI). This work was supported by the HHMI. N.S.B. was supported by NSF grant EF-0313754.
REFERENCES
- 1.Asanuma, N., T. Yoshii, and T. Hino. 2004. Molecular characterization of CcpA and involvement of this protein in transcriptional regulation of lactate dehydrogenase and pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis. Appl. Environ. Microbiol. 70:5244-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslanidis, C., K. Schmid, and R. Schmitt. 1989. Nucleotide sequences and operon structure of plasmid-borne genes mediating uptake and utilization of raffinose in Escherichia coli. J. Bacteriol. 171:6753-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barletta, R. G., and R. Curtiss, 3rd. 1989. Impairment of melibiose utilization in Streptococcus mutans serotype c gtfA mutants. Infect. Immun. 57:992-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belitsky, B. R., H. J. Kim, and A. L. Sonenshein. 2004. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J. Bacteriol. 186:3392-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, M. H., R. T. Cartee, and J. Yother. 2003. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J. Bacteriol. 185:6057-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. [DOI] [PubMed] [Google Scholar]
- 7.Bricker, A. L., and A. Camilli. 1999. Transformation of a type 4 encapsulated strain of Streptococcus pneumoniae. FEMS Microbiol. Lett. 172:131-135. [DOI] [PubMed] [Google Scholar]
- 8.Brochu, D., and C. Vadeboncoeur. 1999. The HPr(Ser) kinase of Streptococcus salivarius: purification, properties, and cloning of the hprK gene. J. Bacteriol. 181:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasin, L. A., and B. Magasanik. 1968. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J. Biol. Chem. 243:5165-5178. [PubMed] [Google Scholar]
- 10.Chauvaux, S. 1996. CcpA and HPr(ser-P): mediators of catabolite repression in Bacillus subtilis. Res. Microbiol. 147:518-522. [DOI] [PubMed] [Google Scholar]
- 11.Chauvaux, S., I. T. Paulsen, and M. H. Saier, Jr. 1998. CcpB, a novel transcription factor implicated in catabolite repression in Bacillus subtilis. J. Bacteriol. 180:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darbon, E., P. Servant, S. Poncet, and J. Deutscher. 2002. Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression. Mol. Microbiol. 43:1039-1052. [DOI] [PubMed] [Google Scholar]
- 13.Deutscher, J., E. Kuster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049-1053. [DOI] [PubMed] [Google Scholar]
- 14.Deutscher, J., J. Reizer, C. Fischer, A. Galinier, M. H. Saier, Jr., and M. Steinmetz. 1994. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J. Bacteriol. 176:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vos, W. M., I. Boerrigter, R. J. van Rooyen, B. Reiche, and W. Hengstenberg. 1990. Characterization of the lactose-specific enzymes of the phosphotransferase system in Lactococcus lactis. J. Biol. Chem. 265:22554-22560. [PubMed] [Google Scholar]
- 16.Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic genes in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 47:1709-1721. [DOI] [PubMed] [Google Scholar]
- 17.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubreuil, J. D., M. Jacques, D. Brochu, M. Frenette, and C. Vadeboncoeur. 1996. Surface location of HPr, a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus suis. Microbiology 142(Pt. 4):837-843. [DOI] [PubMed] [Google Scholar]
- 19.Egeter, O., and R. Bruckner. 1996. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol. Microbiol. 21:739-749. [DOI] [PubMed] [Google Scholar]
- 20.Ehinger, S., W. D. Schubert, S. Bergmann, S. Hammerschmidt, and D. W. Heinz. 2004. Plasmin(ogen)-binding α-enolase from Streptococcus pneumoniae: crystal structure and evaluation of plasmin(ogen)-binding sites. J. Mol. Biol. 343:997-1005. [DOI] [PubMed] [Google Scholar]
- 21.Frey, N., S. Nessler, S. Fieulaine, K. Vaillancourt, M. Frenette, and C. Vadeboncoeur. 2003. The HPr(Ser) kinase of Streptococcus salivarius: a hexameric bifunctional enzyme controlled by glycolytic intermediates and inorganic phosphate. FEMS Microbiol. Lett. 224:67-72. [DOI] [PubMed] [Google Scholar]
- 22.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbreth, S. E., A. K. Benson, and R. W. Hutkins. 2004. Catabolite repression and virulence gene expression in Listeria monocytogenes. Curr. Microbiol. 49:95-98. [DOI] [PubMed] [Google Scholar]
- 24.Grundy, F. J., A. J. Turinsky, and T. M. Henkin. 1994. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 176:4527-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy, F. J., D. A. Waters, T. Y. Takova, and T. M. Henkin. 1993. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol. Microbiol. 10:259-271. [DOI] [PubMed] [Google Scholar]
- 26.Guffanti, A. A., and W. A. Corpe. 1975. Maltose metabolism of Pseudomonas fluorescens. J. Bacteriol. 124:262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 28.Hava, D. L., C. J. Hemsley, and A. Camilli. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J. Bacteriol. 185:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemsley, C., E. Joyce, D. L. Hava, A. Kawale, and A. Camilli. 2003. MgrA, an orthologue of Mga, acts as a transcriptional repressor of the genes within the rlrA pathogenicity islet in Streptococcus pneumoniae. J. Bacteriol. 185:6640-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henkin, T. M. 1996. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 135:9-15. [DOI] [PubMed] [Google Scholar]
- 31.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol. Microbiol. 5:575-584. [DOI] [PubMed] [Google Scholar]
- 32.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 33.Hueck, C. J., and W. Hillen. 1995. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 15:395-401. [DOI] [PubMed] [Google Scholar]
- 34.Jiresova, M., Z. Dobrova, J. Naprstek, P. Rysavy, and J. Janecek. 1983. Induction of β-d-glucosidase in Streptomyces granaticolor. Folia Microbiol. 28:379-385. [DOI] [PubMed] [Google Scholar]
- 35.Jourlin-Castelli, C., N. Mani, M. M. Nakano, and A. L. Sonenshein. 2000. CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J. Mol. Biol. 295:865-878. [DOI] [PubMed] [Google Scholar]
- 36.Kim, H. J., A. Roux, and A. L. Sonenshein. 2002. Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol. Microbiol. 45:179-190. [DOI] [PubMed] [Google Scholar]
- 37.Kim, J. H., and G. H. Chambliss. 1997. Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site. Nucleic Acids Res. 25:3490-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus, A., E. Kuster, A. Wagner, K. Hoffmann, and W. Hillen. 1998. Identification of a co-repressor binding site in catabolite control protein CcpA. Mol. Microbiol. 30:955-963. [DOI] [PubMed] [Google Scholar]
- 39.Lacks, S. 1966. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics 53:207-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling, E., G. Feldman, M. Portnoi, R. Dagan, K. Overweg, F. Mulholland, V. Chalifa-Caspi, J. Wells, and Y. Mizrachi-Nebenzahl. 2004. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 138:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig, H., C. Meinken, A. Matin, and J. Stulke. 2002. Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J. Bacteriol. 184:5174-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahr, K., W. Hillen, and F. Titgemeyer. 2000. Carbon catabolite repression in Lactobacillus pentosus: analysis of the ccpA region. Appl. Environ. Microbiol. 66:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marasco, R., C. T. Lago, and M. De Felice. 1995. Utilization of cellobiose and other β-d-glucosides in Agrobacterium tumefaciens. Res. Microbiol. 146:485-492. [DOI] [PubMed] [Google Scholar]
- 44.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 70:1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38:867-878. [DOI] [PubMed] [Google Scholar]
- 46.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 47.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 48.Miwa, Y., K. Nagura, S. Eguchi, H. Fukuda, J. Deutscher, and Y. Fujita. 1997. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol. Microbiol. 23:1203-1213. [DOI] [PubMed] [Google Scholar]
- 49.Monedero, V., O. P. Kuipers, E. Jamet, and J. Deutscher. 2001. Regulatory functions of serine-46-phosphorylated HPr in Lactococcus lactis. J. Bacteriol. 183:3391-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nieto, C., M. Espinosa, and A. Puyet. 1997. The maltose/maltodextrin regulon of Streptococcus pneumoniae. Differential promoter regulation by the transcriptional repressor MalR. J. Biol. Chem. 272:30860-30865. [DOI] [PubMed] [Google Scholar]
- 51.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paton, J. C., and P. Giammarinaro. 2001. Genome-based analysis of pneumococcal virulence factors: the quest for novel vaccine antigens and drug targets. Trends Microbiol. 9:515-518. [DOI] [PubMed] [Google Scholar]
- 53.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poncet, S., I. Mijakovic, S. Nessler, V. Gueguen-Chaignon, V. Chaptal, A. Galinier, G. Boel, A. Maze, and J. Deutscher. 2004. HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in gram-positive bacteria. Biochim. Biophys. Acta 1697:123-135. [DOI] [PubMed] [Google Scholar]
- 55.Postma, P. W., and J. W. Lengeler. 1985. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol. Rev. 49:232-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramseier, T. M., J. Reizer, E. Kuster, W. Hillen, and M. H. Saier. 1995. In vitro binding of the CcpA protein of Bacillus megaterium to cis-acting catabolite responsive elements (CREs) of gram-positive bacteria. FEMS Microbiol. Lett. 129:207-213. [DOI] [PubMed] [Google Scholar]
- 57.Reizer, J., S. Bachem, A. Reizer, M. Arnaud, M. H. Saier, Jr., and J. Stulke. 1999. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology 145(Pt. 12):3419-3429. [DOI] [PubMed] [Google Scholar]
- 58.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stulke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 27:1157-1169. [DOI] [PubMed] [Google Scholar]
- 59.Rogers, J. D., and F. A. Scannapieco. 2001. RegG, a CcpA homolog, participates in regulation of amylase-binding protein A gene (abpA) expression in Streptococcus gordonii. J. Bacteriol. 183:3521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenow, C., M. Maniar, and J. Trias. 1999. Regulation of the α-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 9:1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowland, P., O. Bjornberg, F. S. Nielsen, K. F. Jensen, and S. Larsen. 1998. The crystal structure of Lactococcus lactis dihydroorotate dehydrogenase A complexed with the enzyme reaction product throws light on its enzymatic function. Protein Sci. 7:1269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schonert, S., T. Buder, and M. K. Dahl. 1998. Identification and enzymatic characterization of the maltose-inducible α-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis. J. Bacteriol. 180:2574-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schumacher, M. A., G. S. Allen, M. Diel, G. Seidel, W. Hillen, and R. G. Brennan. 2004. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell 118:731-741. [DOI] [PubMed] [Google Scholar]
- 64.Seki, M., K. Iida, M. Saito, H. Nakayama, and S. Yoshida. 2004. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate. J. Bacteriol. 186:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Servant, P., D. Le Coq, and S. Aymerich. 2005. CcpN (YqzB), a novel regulator for CcpA-independent catabolite repression of Bacillus subtilis gluconeogenic genes. Mol. Microbiol. 55:1435-1451. [DOI] [PubMed] [Google Scholar]
- 66.Shaw, G. C., H. S. Kao, and C. Y. Chiou. 1998. Cloning, expression, and catabolite repression of a gene encoding β-galactosidase of Bacillus megaterium ATCC 14581. J. Bacteriol. 180:4734-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, A. W., H. Roche, M. C. Trombe, D. E. Briles, and A. Hakansson. 2002. Characterization of the dihydrolipoamide dehydrogenase from Streptococcus pneumoniae and its role in pneumococcal infection. Mol. Microbiol. 44:431-448. [DOI] [PubMed] [Google Scholar]
- 69.Stulke, J., and W. Hillen. 1999. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 70.Stulke, J., and W. Hillen. 1998. Coupling physiology and gene regulation in bacteria: the phosphotransferase sugar uptake system delivers the signals. Naturwissenschaften 85:583-592. [DOI] [PubMed] [Google Scholar]
- 71.Stulke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54:849-880. [DOI] [PubMed] [Google Scholar]
- 72.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 73.Thevenot, T., D. Brochu, C. Vadeboncoeur, and I. R. Hamilton. 1995. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius. J. Bacteriol. 177:2751-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek 82:59-71. [PubMed] [Google Scholar]
- 75.Tobisch, S., D. Zuhlke, J. Bernhardt, J. Stulke, and M. Hecker. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J. Bacteriol. 181:6996-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuomanen, E. 1999. Molecular and cellular biology of pneumococcal infection. Curr. Opin. Microbiol. 2:35-39. [DOI] [PubMed] [Google Scholar]
- 77.van den Bogaard, P. T., M. Kleerebezem, O. P. Kuipers, and W. M. de Vos. 2000. Control of lactose transport, β-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar. J. Bacteriol. 182:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warner, J. B., B. P. Krom, C. Magni, W. N. Konings, and J. S. Lolkema. 2000. Catabolite repression and induction of the Mg2+-citrate transporter CitM of Bacillus subtilis. J. Bacteriol. 182:6099-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whiting, G. C., J. T. Evans, S. Patel, and S. H. Gillespie. 2002. Purification of native α-enolase from Streptococcus pneumoniae that binds plasminogen and is immunogenic. J. Med. Microbiol. 51:837-843. [DOI] [PubMed] [Google Scholar]
- 82.Zahner, D., and R. Hakenbeck. 2000. The Streptococcus pneumoniae β-galactosidase is a surface protein. J. Bacteriol. 182:5919-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]