Abstract
Sinorhizobium meliloti is a gram-negative soil bacterium, capable of establishing a nitrogen-fixing symbiosis with its legume host, alfalfa (Medicago sativa). Quorum sensing plays a crucial role in this symbiosis, where it influences the nodulation process and the synthesis of the symbiotically important exopolysaccharide II (EPS II). S. meliloti has three quorum-sensing systems (Sin, Tra, and Mel) that use N-acyl homoserine lactones as their quorum-sensing signal molecule. Increasing evidence indicates that certain eukaryotic hosts involved in symbiotic or pathogenic relationships with gram-negative bacteria produce quorum-sensing-interfering (QSI) compounds that can cross-communicate with the bacterial quorum-sensing system. Our studies of alfalfa seed exudates suggested the presence of multiple signal molecules capable of interfering with quorum-sensing-regulated gene expression in different bacterial strains. In this work, we choose one of these QSI molecules (SWI) for further characterization. SWI inhibited violacein production, a phenotype that is regulated by quorum sensing in Chromobacterium violaceum. In addition, this signal molecule also inhibits the expression of the S. meliloti exp genes, responsible for the production of EPS II, a quorum-sensing-regulated phenotype. We identified this molecule as l-canavanine, an arginine analog, produced in large quantities by alfalfa and other legumes.
Bacterial populations coordinately regulate gene expression by producing diffusible signal molecules. These signal molecules, known as autoinducers, accumulate extracellularly and interact specifically with a receptor protein to regulate important bacterial phenomena such as exopolysaccharide synthesis, virulence, motility, sporulation, and antibiotic production (16, 20, 31, 43, 46). Production and accumulation of autoinducers typically result in a concerted response upon reaching a critical concentration. These diffusible signals frequently act to induce gene expression in response to bacterial population density in a process often described as quorum sensing (1, 9, 14, 15, 22, 24, 37, 38, 46, 52-54). The best-characterized quorum-sensing mechanism is found in gram-negative organisms and involves the use of acylated homoserine lactones (AHLs) as signal molecules (1, 14-16, 22, 38, 43, 46, 52-54).
Quorum sensing has been implicated as a key player in the symbiotic relationships formed between the nitrogen-fixing rhizobia and their legume hosts (5, 6, 28, 31). The invasion of plant root nodules by Sinorhizobium meliloti requires the presence of at least one of the two exopolysaccharides (succinoglycan and exopolysaccharide II [EPS II]), made by the bacteria. Mutants that are unable to synthesize either exopolysaccharide form empty nodules that lack bacteria and bacteroids (12, 26, 27, 55). External addition of purified low-molecular-weight forms of either succinoglycan or EPS II is sufficient to rescue the symbiotic defects of exopolysaccharide-deficient mutants (21). Interestingly, the synthesis of EPS II was recently shown to be regulated by quorum sensing in S. meliloti (31).
S. meliloti harbors at least three quorum-sensing systems (Sin, Tra, and Mel) (33). The Sin quorum-sensing system is comprised of the response regulator sinR and the autoinducer synthase sinI, which is responsible for the production of several novel AHLs, ranging in size from C12-HL to C18-HL (33). Disruption of the Sin system correlates with a delay in the appearance of nitrogen-fixing nodules, as well as an overall decrease in the number of pink nodules, suggesting a role for quorum sensing in establishing a successful symbiosis with Medicago sativa (alfalfa) (33). A recently published microarray analysis of the Sin quorum-sensing mutants of S. meliloti shows that this system is involved in the regulation of a variety of symbiotically important phenotypes in S. meliloti, including production of exopolysaccharides, motility, nodulation, and nitrogen fixation (23).
Work from our laboratory has shown that the sinR, sinI, and expR genes are required for synthesis of EPS II by a strain proficient in the production of this exopolysaccharide (31). Regulation of EPS II production by sin/expR genes was shown to be important for nodule invasion, since a strain that exclusively produces EPS II, combined with a sinI mutation, is no longer capable of forming nitrogen-fixing nodules (31).
A wide variety of soil- and plant-associated bacteria produce AHLs (3), and it has been suggested that AHL production is more common in plant-associated than in soilborne pseudomonads (10). Therefore, the potential exists for the eukaryotic hosts to disrupt this regulatory system by producing compounds that interfere with bacterial quorum sensing and thus protect themselves from pathogens or encourage symbionts by modifying bacterial behavior. Interestingly, recent work has shown that host plants produce AHL-like signals capable of interacting with the quorum-sensing system of a variety of bacterial reporter strains (17, 20, 39, 48, 49). An example of a plant signal affecting quorum sensing in an associated bacterium is that of the Australian red alga, Delisea pulchra, which interferes with the swarming motility of Serratia liquefaciens, a gram-negative marine bacterium. D. pulchra produces halogenated furanones that are structurally similar to the AHL signals produced by S. liquefaciens. The furanones successfully inhibit swarming motility, a quorum-sensing-regulated phenotype in S. liquefaciens (20). It was recently demonstrated that the halogenated furanones bind the AHL receptor protein and reduce the half-life of the protein up to 100-fold and thus destabilize the transcriptional activator (30). Teplitski et al. have shown that various species of higher plants, including pea seedlings, secrete a series of unidentified signals that are capable of interfering with the quorum-sensing behavior of several bacterial reporter strains (49). This would suggest that plants may have evolved mechanisms to interfere with or respond to bacterial quorum sensing and thereby have the potential to manipulate the behavior of the associated bacteria to their advantage (8, 20, 34, 49).
We decided to investigate the possibility that alfalfa, the plant host of S. meliloti, could make quorum-sensing-interfering (QSI) compounds that could potentially have an effect on the S. meliloti quorum-sensing systems. This analysis could reveal another level of communication between alfalfa and S. meliloti. We observed that alfalfa seed and seedling exudates inhibited violacein production in Chromobacterium violaceum in a manner similar to that described by Teplitski et al. In their work, they isolated unidentified quorum-sensing mimics from exudates of pea seedlings that inhibited violacein production in C. violaceum strain CV026. They also observed that this compound partitioned into aqueous phase when extracted with organic solvents. In addition to this, our analysis also shows that alfalfa seed exudates inhibit quorum-sensing-regulated gene expression in S. meliloti and affect the ability to produce the symbiotically important exopolysaccharide EPS II.
Structural investigation of one of the QSI molecules in alfalfa seed exudates identified it as l-canavanine, a compound produced in large amounts by the seeds of alfalfa and other legumes. l-Canavanine is an arginine analog that inhibits the growth of certain bacteria, including some strains of S. meliloti (11, 51). However, an effect on gene regulation has not been recorded to date. We show in this work that synthetic l-canavanine behaves in a manner similar to that seen with the signal isolated from alfalfa seed exudates. We also show that l-canavanine inhibits the expression and production of EPS II, independent of its effect on the growth of S. meliloti.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All S. meliloti strains were grown in Luria-Bertani (LB) media supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 with 500 μg/ml streptomycin. C. violaceum was grown in LB media with no drugs. Minimal medium (M-1) (0.5 g NaCl, 1 g glutamic acid, 10 g mannitol, 10.45 g morpholinepropanesulfonic acid [MOPS], 2 mg biotin, 10 μg cobalt chloride, 0.25 mM CaCl2, 1 mM MgSO4, and 0.2 mM K2HPO4-KH2PO4 per liter of water) was used to grow S. meliloti strains for β-galactosidase assays and carbohydrate analysis.
Preparation of M. sativa seed and seedling exudates.
Ten grams of M. sativa (alfalfa) seeds (Iroquois) was surface sterilized using 100 ml 70% alcohol for 30 min, followed by 100 ml absolute alcohol for 30 min and 100 ml 50% bleach for 10 min. The seeds were then repeatedly rinsed with 1 liter of sterile water. Seed exudates were prepared by soaking surface-sterilized alfalfa seeds overnight (24 h) in 100 ml of sterile water. The exudates were tested for bacterial contamination by plating 0.1 ml on LB plates and incubating at 30°C for 24 to 48 h. Contaminant-free exudates were further treated as described previously (49) with minor modifications. The seed exudates were filtered through 0.2-μm Millipore membrane filters, lyophilized, and resuspended in 50% methanol (5mg lyophilized material per ml of solvent). After 15 min, the insoluble material was removed by centrifugation and the supernatant was mixed with an equal volume of 100% methanol and one-fourth final volume of isopropanol. After 15 min, the precipitated impurities were removed by filtration through Whatman no. 1 filter paper. The solvents in the filtrate were removed by rotary evaporation, and the final viscous residue was resuspended in sterile water (35 mg of lyophilized material/ml). This suspension was used as the source of alfalfa seed crude exudates.
Seedling exudates were obtained by first germinating surface-sterilized seeds on sterile filter paper for 3 days. The 3-day-old seedlings were rinsed with sterile water to collect the exudates. This rinsate was then tested for bacterial contamination by plating 0.1 ml on LB plates as described above. Contaminant-free rinsates were filtered through 0.2-μm membrane filters and lyophilized. The lyophilized material was extracted with solvents in the same manner as that for seed exudates, and the final residue was resuspended in sterile water. This was used as the source of crude exudates for alfalfa seedlings.
The crude exudates were extracted twice with an equal volume of ethyl acetate and centrifuged. The ethyl acetate and aqueous layers were separated. The top ethyl acetate layer was then evaporated under vacuum, and the residue was resuspended in an equivalent amount of sterile water. This is referred to as the ethyl acetate-soluble seed extract (SE) throughout the manuscript. The lower aqueous layer is referred to as the water-soluble seed extract (SW).
Detection of QSI compounds. (i) Bacterial overlays.
C. violaceum CV026 indicator strain was used to overlay thin-layer chromatography (TLC) plates containing QSI compounds. Uninduced CV026 overlays were prepared by growing CV026 culture to exponential phase and mixing it with an equal volume of LB containing 7.5 g/liter agar (32). Induced CV026 overlays were prepared in the same fashion but included 3.5 nmol/ml C4-HL.
(ii) PMA staining.
Ten percent phosphomolybdic acid (PMA) in ethanol was sprayed evenly over dried TLC plates containing the desired sample. The plate was then slowly heated to 200°C on a hot plate until dark spots were detected on the plate, indicating the presence of organic compounds in the sample (7, 13).
Chromatographic separation of QSI compounds. (i) High-pressure liquid chromatography (HPLC).
SW was separated using a silica column and a linear gradient of 50% to 0% acetone at a flow rate of 1 ml/min over a period of 35 min. Thirty-five 1-ml fractions were collected. The fractions were dried overnight under vacuum and resuspended in a minimal volume of sterile water. The samples were analyzed by spotting on TLC plates and overlaying with the induced CV026 indicator strain. The active fractions are referred to as SWI (for the inhibitory signal in the SW fraction of alfalfa seed exudates).
(ii) Thin-layer chromatography.
Seed extract samples (SE and SW) were spotted on a reverse-phase (RP)-C18 or silica TLC plate, dried under a steady stream of air, and allowed to cool. The samples were then chromatographed with a mixture of 7:3 methanol:water.
SWI and l-canavanine were separated using silica TLC plates and 7:3 methanol:water as the solvent. The plates were air dried and then overlaid with the CV026 indicator strain or stained with PMA as described above.
β-Galactosidase assays.
Cultures (25 ml) of S. meliloti strain Rm11525 (Rm8530 sinI expE2-lacZ) or Rm8530 sinI ndvB-lacZ were grown to early logarithmic phase in minimal media. Two milliliters of the culture was removed and used as a control for basal-level expression of the gene fusion. To the remaining culture, 2.5 μM C16:1-HL was added and appropriate concentrations of commercially available l-canavanine (Sigma Chemicals), l-arginine (Fisher Scientific), or purified SWI were added to 2-ml aliquots of the culture as indicated in the text. The cultures were then incubated at 30°C. After a 4-h incubation period, the cultures were analyzed for β-galactosidase activity as previously described (36a). All experiments were conducted in triplicate and repeated at least twice.
Quantitation of EPS II production.
Cultures (25 ml) of S. meliloti strain Rm8530 sinI exoY were grown to early logarithmic phase in minimal media. l-Canavanine and C16:1-HL were added as described above for β-galactosidase assays. The cultures were incubated at 30°C for 4 h, after which the cells were centrifuged twice at 20,000 × g for 5 min each time. The carbohydrate content in the supernatant was quantified by the anthrone-sulfuric acid method (29). All experiments were conducted in triplicate and repeated at least twice.
Comparative analysis of S. meliloti AHL production.
Aliquots (2 ml) of early-log-phase cultures of wild-type S. meliloti strain Rm8530 in M-1 media were treated with 56 μM, 0.56 mM, and 5.6 mM l-canavanine or left untreated and incubated for 4 h. AHLs were extracted twice from the culture by adding equal volumes of ethyl acetate. The solvent was evaporated under vacuum, and the AHLs were resuspended in 5 μl 70% MeOH and spotted on an RP C18 TLC plate. The TLC was developed using 70% MeOH, air dried, and overlaid with the NTL4(pZLR4) indicator strain as described in reference 45.
Structural identification of SWI. (i) NMR.
1H nuclear magnetic resonance (NMR) spectra of samples in CDCl3 were recorded at 270 MHz for SWI and l-canavanine. Chemical shifts are reported in δ (parts per million) values relative to a trimethylsilyl internal standard. 1H NMR shows for l-canavanine 2.1 ppm (2H, multiplet), 3.9 ppm (2H, multiplet), and 3.8 ppm (1H, doublet of doublets).
(ii) ESI-MS.
Low-resolution electron-spray ionization-mass spectrophotometric (ESI-MS) analysis of the SWI and l-canavanine was performed by Analytical Services Lab (Evanston, IL). Molecular ions for both were 177 m/z.
(iii) Chemical modification.
The SWI and l-canavanine were both treated with Sanger's reagent and then derivatized with phenylglyoxal to establish the presence of the guanidinium group. l-Canavanine and SWI were both treated with DNFB (dinitrofluorobenzene; Sanger's reagent) as described previously (44). The DNP (dinitrophenyl) derivative thus obtained was further modified by reaction with phenylglyoxal. Approximately 0.8 mg of DNP-l-canavanine was taken up in 200 μl of potassium phosphate buffer, pH 8.0. Phenylglyoxal (500 μg) was also dissolved in 100 μl of potassium phosphate buffer (pH 8.0) and mixed with DNP-l-canavanine solution. The solutions were stirred for 12 h at room temperature. The phenylglyoxal adduct of DNP-l-canavanine and that of identically prepared DNP-SWI were then spotted on precoated TLC plates (silica gel; 60 F254) and developed using butanol-pyridine-acetic acid-water in a 15:10:3:12 ratio by volume (47). Spots were bright yellow due to the presence of DNP in the structure.
Quantitation of l-canavanine present in alfalfa seed exudates.
The concentration of l-canavanine present in alfalfa seed exudate (SWI) purified by HPLC as described earlier was quantified by a colorimetric assay performed with PCAF (trisodium penta cyanoammonio ferrate) (Aldrich Chemical Co., Milwaukee, WI) (41). PCAF solution was photoactivated with 150 W incandescent light for 15 min, filtered, and stored at 4°C for up to 2 weeks. The SWI sample was added to 1 ml of 200 mM potassium phosphate buffer (pH 7.0) and 0.2 ml of 1% (wt/vol) potassium persulfate in a final volume of 2.2 ml. The reaction was initiated by adding 0.1 ml of photoactivated PCAF solution. After 40 min of incubation, the magenta color produced was measured at 530 nm. A standard curve generated using commercially available l-canavanine (Sigma Chemicals) was used to quantitate the amount of l-canavanine present in the SWI sample (41).
RESULTS
Alfalfa seeds and seedlings inhibit violacein production in Chromobacterium violaceum strain CV026.
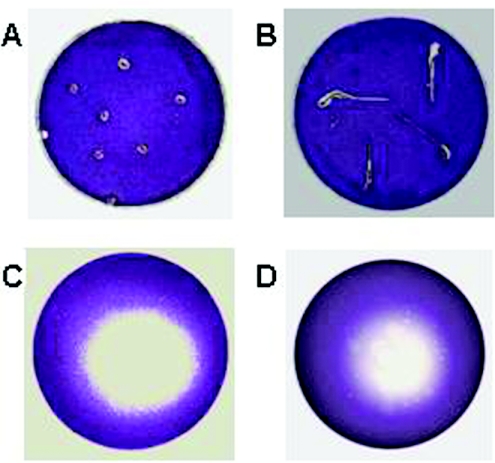
We conducted a preliminary assay to screen for potential QSI compounds produced by alfalfa. For this purpose, we used the C. violaceum CV026 indicator strain. CV026 is an AHL synthase mutant that produces a purple pigment (violacein) in response to short-chain AHLs provided in trans. Long-chain AHLs, on the other hand, competitively inhibit violacein production in a CV026 culture that has been preinduced by a short-chain AHL (35). Surface-sterilized alfalfa seeds and seedlings were placed on either an uninduced or an induced CV026 overlay and incubated overnight (see Materials and Methods). This assay was designed to detect the presence of both short-chain and long-chain AHL-like signal molecules made by alfalfa. The uninduced overlay contains only the reporter strain and therefore will produce violacein in the presence of short-chain AHL-like signal molecules. In contrast, the induced overlay contains a short-chain AHL (C4-HL) along with the reporter strain; therefore, long-chain AHL-like signal molecules can be detected as white zones due to the inhibition of violacein production. No violacein production was observed around the seeds and seedlings on plates overlaid with uninduced CV026 (data not shown). Interestingly, a zone of violacein inhibition was observed around the alfalfa seeds and seedlings against the purple background on plates with the induced overlay (Fig. 1A and 1B).
FIG. 1.
Inhibition of violacein production in C. violaceum CV026 by alfalfa seed and seedling exudates. Surface-sterilized alfalfa seeds (A), 3-day-old seedlings (B), seed exudates (C), and seedling exudates (D) were placed on induced CV026 overlays on LB agar plates and incubated overnight.
Exudates from alfalfa seeds and seedlings interfere with violacein production in C. violaceum strain CV026.
In order to determine whether the activity described above was due to signal molecules released by the seeds and seedlings during the germination process, we prepared alfalfa seed and seedling exudates as described in Materials and Methods. A total of 10μl of the seed and seedling exudates was spotted on LB plates containing the induced CV026 overlay. Upon overnight incubation at 30°C, violacein inhibition zones were observed against a purple background (Fig. 1C and 1D), indicating that this activity was indeed due to signal molecules secreted by alfalfa seeds and seedlings. We confirmed through growth studies that the seed and seedling exudates did not inhibit the growth of the C. violaceum and only interfered with the production of violacein pigment (data not shown). Hereafter, we continued using only alfalfa seed exudates for all our experiments.
Alfalfa seed exudates contain only one signal molecule as detected by C. violaceum strain CV026.
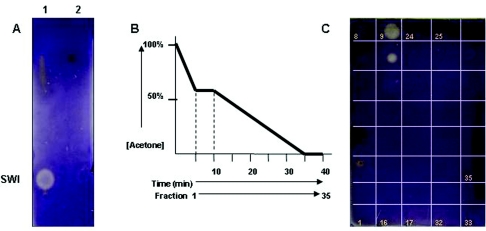
In order to study the biochemical nature of the violacein inhibitory activity and to determine whether this signal molecule has properties similar to those of AHLs, 50 μl of the seed exudates was extracted twice with equal volumes of ethyl acetate. The ethyl acetate (SE) and water (SW) layers were separated and dried under vacuum. The dried pellet was resuspended in 5 μl water, spotted on an RP-C18 TLC plate, dried, and overlaid with induced CV026 overlay. Violacein inhibition was detected in the water layer (SW) but not in the ethyl acetate layer (SE), suggesting that the signal is polar in nature and does not share solubility properties with AHLs (Fig. 2A).
FIG. 2.
Alfalfa seed exudate is detected by CV026. (A) Alfalfa seed exudates SW (lane 1) and SE (lane 2) were separated by TLC and overlaid with induced CV026. (B) Alfalfa seed exudate SW was separated by HPLC using a gradient of acetone-water, and 1-ml/min fractions were collected. (C) The fractions were spotted on a TLC plate and overlaid with induced CV026, and activity was detected in fractions 9 and 10.
A 50-μl volume of the alfalfa seed exudate SW fraction was spotted on RP-C18 or silica TLC plates and chromatographed with 70% MeOH. The plates were allowed to dry and then overlaid with an induced CV026 culture mixed with top agar. After overnight incubation at 30°C, a single violacein inhibition spot (Fig. 2A, lane 1) was observed close to the origin. This suggests that violacein inhibition by alfalfa seed exudates is probably due to a single signal molecule. It should be noted that the spots seen at the top of the TLC plate for lanes 1 and 2 are due to yellow pigments present in the crude exudates anddo not inhibit violacein production in CV026. We observed that this signal molecule bound better to silica than to the RP-C18 plate. This fact, along with the observation that the signal molecule is not extractable with organic solvents, once again suggested that the signal molecule might be polar in nature.
In order to screen for other signal molecules that could potentially interfere with quorum sensing in CV026, the alfalfa SW fraction was separated by TLC as discussed above and overlaid with uninduced CV026 culture. No induction of violacein was observed (data not shown), indicating that there is only one type of signal molecule capable of interfering with the violacein production phenotype in CV026.
Purification of QSI compounds by HPLC.
The SW fraction of the seed extracts was separated using HPLC as described in Materials and Methods. The collected HPLC fractions were dried overnight under vacuum, and the dried pellets were resuspended in 10 μl of water. The fractions were then spotted onto TLC plates, air dried, and overlaid with induced CV026 culture. SW fractions that eluted in a gradient corresponding to 32% to 36% acetone inhibited violacein production in the induced CV026 overlay (Fig. 2B and 2C). It should be noted that the spots seen in fractions 3 and 4 are not due to violacein inhibition but are due to yellow pigments present in the crude exudates.
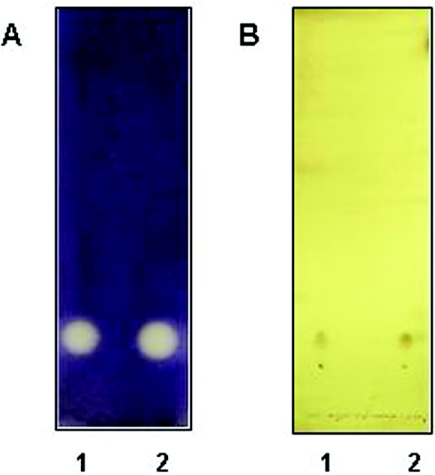
In order to further test the purity of the separated fractions, the active fractions were pooled and separated by TLC as described earlier and then stained with phosphomolybdic acid (PMA) (13). PMA is a generalized stain that interacts with most functional groups and hence is a reliable method to detect the presence of different organic compounds on a TLC plate. A single spot was detected whose migration matched with that of the violacein inhibition activity seen on plates overlaid with induced CV026 (Fig. 3). This also suggested the presence of a single organic compound (hereafter referred to as SWI for “inhibitory signal in SW”) and was probably free of organic impurities. Therefore, we proceeded to use these fractions for structural analysis as described below.
FIG. 3.
l-Canavanine behaves like SWI. HPLC-purified SWI (lane 1) and synthetic l-canavanine (lane 2) were separated by TLC on silica plates and visualized by induced CV026 overlay (A) and PMA staining (B).
Structural identification of the QSI molecule responsible for violacein inhibition in C. violaceum strain CV026.
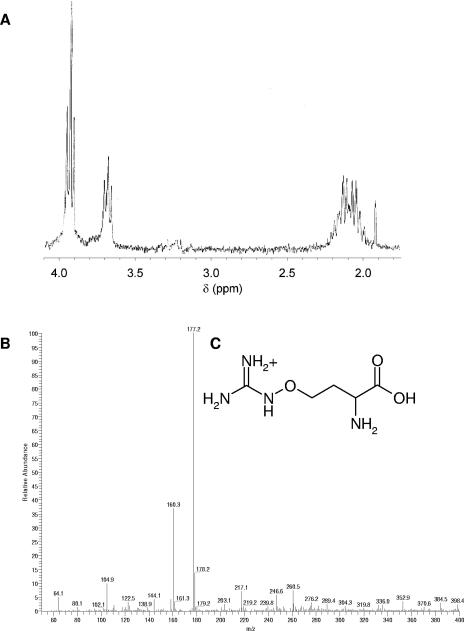
The 1H NMR of SWI exhibited a multiplet at 2.1 ppm and a multiplet at 3.9 ppm (Fig. 4A), which suggested that the compound was a simple derivative of homoserine. A search of the literature for compounds known to be present in biological extracts (especially plant extracts) that would give similar 1H NMR spectra revealed l-canavanine, canaline, phosphohomoserine, and canaline glyoxylate oxime as possible candidates (4, 18, 19, 42). The possibility that the compound was an AHL was easily ruled out, as no acyl protons could be detected at 0.9 ppm and 1.5 ppm. The closest match of 1H NMR appeared to be l-canavanine (Fig. 4C). Synthetic l-canavanine was obtained (Sigma), and the 1H NMR was identical to that for SWI except that the 1H doublet of doublets at 3.65 ppm was shifted by 0.15 ppm to 3.8 ppm. This shift was attributed to a difference in concentration of synthetic sample versus SWI.
FIG. 4.
Structural analysis of SWI. (A) The seed extract fraction recorded at 270 MHz shows multiplets at 2.1 ppm and 3.9 ppm and a doublet of doublets at 3.65 ppm. (B) The mass spectrum indicates a molecular weight of 177 for SWI. (C) Chemical structure of l-canavanine.
The ESI-MS data for SWI showed a molecular ion at 177m/z, which was identical to that obtained for the synthetic l-canavanine (Fig. 4B). This is the expected mass of l-canavanine.
To obtain final confirmation for the structure of SWI, chemical methods were employed. Both SWI and l-canavanine were treated with Sanger's reagent (DNFB), following the protocol as previously described (44). Sanger's reagent reacts with amine groups to give bright yellow dinitrophenyl adducts. The DNP derivatives thus obtained were further derivatized with phenylglyoxal as described in Materials and Methods. Phenylglyoxal selectively reacts with the guanidinium groups in arginine and l-canavanine (47). The phenylglyoxal adduct of l-canavanine and SWI was then separated by normal phase TLC using 15:10:3:12 butanol:pyridine:acetic acid:water. The bright-yellow adducts were easily visualized on the TLC. SWI and synthetic l-canavanine adducts comigrated with an Rf of 0.67, confirming that they were identical compounds (data not shown). Furthermore, synthetic l-canavanine also exhibited violacein inhibition and a PMA spot with migration and shapes identical to that of SWI (Fig. 3). These results further confirm that SWI is indeed the same as l-canavanine.
Quantitation of l-canavanine present in alfalfa seed exudates.
Pooled HPLC fractions 9 and 10 (see Fig. 2C) were used to quantify the amount of l-canavanine present in alfalfa seed exudates by use of a PCAF assay, as described in Materials andMethods (41). Synthetic l-canavanine and SWI were treated with PCAF reagent, and the magenta color developed was measured at 530 nm. A standard curve generated using synthetic l-canavanine was used to quantify SWI. According to our quantitation analysis, 1.04 μmol of l-canavanine is present per ml of seed exudates. This is in agreement with the results shown in Fig. 5 and 6, where 1 ml of SWI added to 2 ml bacterial culture produces about the same amount of inhibition as that seen with 0.56 mM synthetic l-canavanine. This provides further evidence to indicate that l-canavanine is the signal molecule responsible for the quorum-sensing-inhibitory activity.
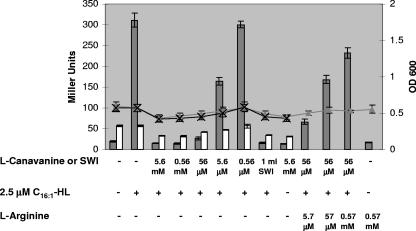
FIG. 5.
l-Canavanine inhibits gene expression in S. meliloti. β-Galactosidase activities (columns) at early log phase (lines) with Rm8530 sinI expE2-lacZ (solid triangles and gray columns) and Rm8530 sinI ndvB-lacZ (cross and white columns) with the addition of AHL, l-arginine, or l-canavanine as indicated are shown. OD, optical density.
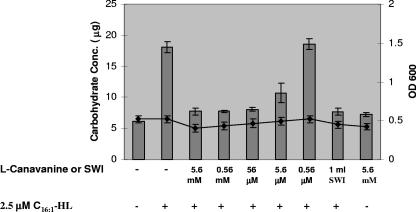
FIG. 6.
l-Canavanine inhibits production of EPS II in S. meliloti. Quantitation of EPS II production (columns) at early log phase (line) in S. meliloti strain Rm8530 sinI exoY with the addition of AHL or l-canavanine as indicated. OD, optical density.
l-Canavanine inhibits expression of an expE-lacZ gene fusion in S. meliloti.
We were specifically interested in determining whether l-canavanine could interfere with S. meliloti quorum sensing, as it appears to do with the CV026 indicator strain. Since we know that synthesis of EPS II is regulated by the Sin quorum-sensing system of S. meliloti, we measured the expression of the exp genes in the presence and absence of l-canavanine. We conducted β-galactosidase assays using strain Rm11525 (Rm8530 sinI expE2-lacZ), which contains a disruption in sinI (the AHL synthase responsible for the production of long-chain AHLs in S. meliloti) and therefore cannot make its own AHLs. In addition, this strain has a lacZ fusion to expE2 (one of the genes involved in the production of EPS II), which is activated in response to externally added long-chain AHLs, specifically the C16:1-HL (31).
Competition assays were set up using C16:1-HL and different concentrations of l-canavanine, and it was found that concentrations of 5.6 mM, 0.56 mM, and 56 μM l-canavanine were capable of competing with 2.5 μM C16:1-HL and inhibiting the expression of the expE2-lacZ fusion up to 20-fold (Fig. 5). It was observed that addition of l-canavanine at the time of subculturing the bacteria resulted in growth retardation (data not shown). Therefore, to look at the effects on gene expression independent of growth effects, the β-galactosidase assay was modified as mentioned in Materials and Methods, where l-canavanine and AHLs were added when the cultures had reached early log phase and β-galactosidase assays conducted 4 h later. This resulted in a negligible difference in the growth of all the cultures used in the assay (Fig. 5).
In order to determine whether l-canavanine has the same inhibitory effect on all genes, irrespective of quorum-sensing control, we studied the expression of ndvB, a gene that is not regulated by the Sin/ExpR quorum-sensing system (Sarah Glenn and Juan E. González, unpublished results). β-Galactosidase assays were conducted as described earlier, using the strain Rm8530 sinI ndvB-lacZ grown in the presence of AHL and different l-canavanine concentrations. We observed that the expression of this gene was inhibited less than 1.5-fold with 5.6 mM l-canavanine (Fig. 5). We also analyzed the effect of l-canavanine on the activity of AHL synthases by growing wild-type S. meliloti (Rm8530) in the presence or absence of up to 5.6 mM l-canavanine and detecting the total amount of AHLs made by the bacteria using indicator strain overlays. The overall amount of AHLs made by the bacteria remained largely unaffected by l-canavanine (data not shown).
The production of EPS II is inhibited in the presence of l-canavanine.
We quantified carbohydrate production in the S. meliloti strain Rm8530 sinI exoY in the presence and absence of l-canavanine. An exoY mutant lacks the ability to make succinoglycan, a second exopolysaccharide that would interfere with our carbohydrate quantification assays. In addition, the presence of a sinI mutation in this strain ensures that no EPS II is made unless external AHLs are provided. As described earlier, 2.5 μM C16:1-HL and different concentrations of l-canavanine or SWI were added to the cultures at early log phase and EPS II was quantified 4 h later using anthrone-sulfuric acid analysis. We found that 5.6 mM, 0.56 mM, and 56 μM l-canavanine inhibited EPS II production when provided along with AHLs (Fig. 6). These results strongly suggest that l-canavanine inhibits the production of EPS II in S. meliloti.
l-Arginine competitively inhibits the QSI activity of l-canavanine.
l-Canavanine is a beta-oxo analog of l-arginine. It has been shown that l-canavanine is incorporated in place of l-arginine during protein synthesis, eventually resulting in misfolded proteins. We were therefore interested to see whether l-arginine would prevent the QSI effect of l-canavanine. We studied the expression of the expE2-lacZ gene in the presence of C16:1-HL, l-canavanine, and increasing amounts of l-arginine. We observed that 0.57 mM l-arginine was required to substantially inhibit the QSI activity of 56 μM l-canavanine (Fig. 5).
DISCUSSION
Our initial studies of alfalfa seed exudates suggested the presence of multiple signal molecules capable of interfering with quorum-sensing-regulated gene expression in different bacterial strains. The QSI compounds differed from each other in their biochemical properties as well as their specificity to particular strains (unpublished observations). We decided to choose one of these QSI molecules (SWI) for further characterization in this study. This particular compound was polar in nature, as indicated by its preferential partition to the aqueous phase during organic extraction and its strong binding to silica matrix as well as its mobility on TLC plates. SWI inhibited violacein production, a quorum-sensing-regulated phenotype in C. violaceum (CV026). We further confirmed that this signal molecule did not inhibit the growth of CV026 and noticed that addition of SWI to an overlay that had already developed purple color did not result in alteration or degradation of the pigment. Structural characterization coupled with induced CV026 overlay confirmed that l-canavanine was structurally identical to SWI and had QSI properties similar to SWI.
l-Canavanine is an arginine analog found in nature only in the seeds of legumes, where it occurs in concentrations of up to 5% of the dry weight of the seeds (51). l-Canavanine serves as the principal nitrogen storage metabolite for developing legumes. However, the synthesis of l-canavanine commences with the emergence of the very first leaf, indicating that it may not function solely as a storage metabolite. It has been suggested that l-canavanine may act as an allelopathic substance, since it is known to be a potent inhibitor of growth of several microorganisms and phytophagous insects (40, 51).
l-Canavanine is a beta-oxo analog of l-arginine and therefore is a substrate for arginyl tRNA synthetase. It is incorporated into nascent proteins in place of l-arginine. The oxyguanidino group of l-canavanine is significantly less basic than the guanidino group of arginine; as a result, l-canavanine-containing proteins are unable to form crucial ionic interactions, resulting in altered protein structure and function that could lead to cell death (2). Interestingly, l-canavanine inhibits violacein production in C. violaceum without interfering with growth. In addition, we noticed that adding excess amounts of l-arginine prevents l-canavanine from inhibiting violacein production in CV026 (data not shown). To our knowledge, this is the first evidence recorded to date of l-canavanine interfering with bacterial quorum sensing.
Since l-canavanine appeared to inhibit quorum sensing in the indicator bacterium CV026, we explored the possibility that it might have a similar effect on S. meliloti quorum sensing. S. meliloti has three quorum-sensing systems, namely, the Sin, the Tra, and the Mel systems (32). The Sin system comprises the AHL synthase sinI and the transcriptional regulator sinR and is responsible for the production of long-chain AHLs (33). It was recently shown that the SinRI quorum-sensing system is responsible for the regulation of EPS II biosynthesis, one of the two symbiotically important exopolysaccharides made by S. meliloti. In the absence of the Sin system, no EPS II is made, but when the Sin AHLs are supplied in trans, EPS II production is restored (31). This requires the presence of an intact gene encoding the regulator ExpR, which is homologous to the model quorum-sensing regulator protein LuxR. The presence of both Sin AHLs and ExpR is required to induce expression of the exp genes resulting in the production of EPS II (31). The expression of expE2 (one of thegenes involved in EPS II production) was analyzed in the presence and absence of l-canavanine, in a sinI strain supplemented with synthetic AHLs. l-Canavanine inhibited the expression of expE2 in the presence of synthetic AHLs and reduced its expression to basal levels.
We observed that l-canavanine delays the growth of S. meliloti in a concentration-dependent manner. This is in agreement with previously recorded observations (51). In order to study the effect on expE2 gene expression independent of the delayed growth rate, we grew all cultures to early logarithmic phase, before addition of AHLs and l-canavanine. The expression of expE2 was quantified 4 h after addition of AHLs and l-canavanine. A negligible growth difference was observed among the different cultures during this 4-h period. Our results indicated that l-canavanine inhibited the expression of the exp gene. A concentration range of 5.6 mM to 56 μM was capable of competing with 2.5 μM C16:1-HL and inhibiting expression up to 20-fold.
Further quantification of the amount of EPS II made by S. meliloti (sinI exoY) in the presence or absence of l-canavanine indicated that 56 mM was sufficient to inhibit EPS II production. This observation supports our results with gene expression studies and suggests that l-canavanine inhibits the production of EPS II in S. meliloti in a concentration-dependent manner. We conducted quantitation analysis of alfalfa seed exudates; on the basis of our quantitation results, we estimate that about 0.2 mg of l-canavanine is recovered per gram of alfalfa seeds. This amount is 10-fold lower than that reported by Emmert et al. (11). The difference in concentration could be attributed to variation in the extraction procedures.
The presence of l-canavanine in different parts of the plant has been recorded and is said to be maximal in growing tissues of the plant (52a). The concentration of l-canavanine would therefore be higher in the fast-growing roots than in the surrounding soil environment. The relatively higher concentrations of l-canavanine along with other factors in the root nodule (e.g., high phosphate concentration) may help to shut down EPS II production, leading to the onset of symbiosis. This prediction is in agreement with a previous hypothesis that EPS II production is inhibited in the root nodule environment (36).
Additional competition experiments were conducted with increasing amounts of AHLs (up to 15 μM) added to S. meliloti cultures along with 56 μM l-canavanine, and it was found that further addition of AHLs did not restore EPS II production (data not shown). This data suggests that l-canavanine is not directly competing with AHLs. This was not surprising to us, as the structure and biochemical behavior of l-canavanine were completely different from those of AHLs. It is possible that l-canavanine is incorporated as an arginine analog in the early biosynthesis of proteins. The predicted protein sequence of ExpR contains about 9% arginine residues. Incorporation of l-canavanine in place of l-arginine in ExpR may hinder the protein folding, thereby having a negative effect on the overall protein structure and function. To test this hypothesis, we studied the expression of the expE2 gene in the presence of AHL, l-canavanine, and increasing amounts of l-arginine. We found that addition of 10-fold more l-arginine was required to inhibit the QSI effect of 56 μM l-canavanine and restore the quorum-sensing-mediated induction of the expE2 gene. This suggests that incorporation of l-canavanine in place of l-arginine might account for the QSI activity. Further confirmation of this hypothesis requires additional experimental support.
The crystal structure of TraR (the transcriptional activator in the quorum sensing system of Agrobacterium tumefaciens) shows the presence of five arginine residues in positions required for the DNA binding and dimerization functions (50, 56). Although the crystal structure of ExpR remains to be obtained, it is possible that arginine may play a similar role in maintaining the structure and function of the ExpR protein. Incorporation of l-canavanine into crucial positions within the ExpR protein could potentially disrupt its function. The overall growth rate of the different cultures remained the same during the 4-h incubation period with l-canavanine, although we did see a drastic inhibition of expE2-lacZ expression, which is dependent on ExpR function. This suggests that l-canavanine might have a greater effect on ExpR-regulated genes than other cellular proteins, possibly due to the high arginine content and the specific role that this particular amino acid may play in the overall function of the protein. To further test this hypothesis, we studied the expression of ndvB, a gene that is not regulated by the Sin/ExpR quorum-sensing system (S. Glenn and J. E. González, unpublished observations). β-Galactosidase assays performed using Rm8530 sinI ndvB-lacZ indicated that the expression of this gene is barely affected by l-canavanine (1.5-fold inhibition) compared to the expression of expE2-lacZ (20-fold inhibition) (Fig. 5).
We also analyzed the effect of l-canavanine on the activity of the S. meliloti AHL synthases. S. meliloti AHL synthases do not appear to be regulated by ExpR (23). Early-log-phase cultures of wild-type S. meliloti (Rm8530) were incubated with up to 5.6 mM l-canavanine, and AHLs were extracted, separated by TLC, and analyzed using indicator strain overlays. The overall amount of the different AHLs made by Rm8530 remained largely unaffected by the presence of l-canavanine under our experimental conditions (data not shown).
It is, however, surprising that l-canavanine inhibits violacein production in CV026 without interfering with its growth, suggesting that l-canavanine, in this case, may not interfere with protein synthesis. According to previous reports, bacteria resistant to l-canavanine break it down to homoserine (25, 40). It is possible that this homoserine could then be converted by the cell to an AHL mimic that interferes with the quorum-sensing behavior of CV026. Alternatively, l-canavanine could be destabilizing the response regulators by allosteric binding or other interactions with the proteins. Nonetheless, we think it is interesting that l-canavanine can act as a quorum-sensing inhibitor irrespective of its effect on the growth of a given bacterium and thus holds promise for the manipulation of the quorum-sensing systems in other bacteria.
Acknowledgments
We thank the members of our laboratory for helpful discussions and critical reading of the manuscript. We also thank Anatol Eberhard for providing us with the synthetic AHLs and Jo Handelsman for her generous gift of the PCAF reagent used in this study.
This work was supported by the Texas Advanced Research Program under grant 0009741-0022-2001 to J.E.G. and by National Science Foundation grant MCB-9733532 to J.E.G.
REFERENCES
- 1.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421-424. [DOI] [PubMed] [Google Scholar]
- 2.Bence, A. K., and P. A. Crooks. 2003. The mechanism of l-canavanine cytotoxicity: arginyl tRNA synthetase as a novel target for anticancer drug discovery. J. Enzyme Inhib. Med. Chem. 18:383-394. [DOI] [PubMed] [Google Scholar]
- 3.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, A. J. 1984. Oxime formation between alpha-keto acids and l-canaline. Arch. Biochem. Biophys. 233:603-610. [DOI] [PubMed] [Google Scholar]
- 5.Cubo, M. T., A. Economou, G. Murphy, A. W. Johnston, and J. A. Downie. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels, R., D. E. De Vos, J. Desair, G. Raedschelders, E. Luyten, V. Rosemeyer, C. Verreth, E. Schoeters, J. Vanderleyden, and J. Michiels. 2002. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 277:462-468. [DOI] [PubMed] [Google Scholar]
- 7.Davies, J. R. 1967. A thin-layer chromatographic method for distinguishing between natural rubber and synthetic polyisoprene. J. Chromatogr. 28:451-455. [DOI] [PubMed] [Google Scholar]
- 8.Delalande, L., D. Faure, A. Raffoux, S. Uroz, C. D'Angelo-Picard, M. Elasri, A. Carlier, R. Berruyer, A. Petit, P. Williams, and Y. Dessaux. 2005. N-hexanoyl-l-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 52:13-20. [DOI] [PubMed] [Google Scholar]
- 9.Dong, Y. H., and L. H. Zhang. 2005. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 43:101-109. [PubMed] [Google Scholar]
- 10.Elasri, M., S. Delorme, P. Lemanceau, G. Stewart, B. Laue, E. Glickmann, P. M. Oger, and Y. Dessaux. 2001. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl. Environ. Microbiol. 67:1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmert, E. A. B., J. L. Milner, J. C. Lee, K. L. Pulvermacher, H. A. Olivares, J. Clardy, and J. Handelsman. 1998. Effect of canavanine from alfalfa seeds on the population biology of Bacillus cereus. Appl. Environ. Microbiol. 64:4683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan, T. M., A. M. Hirsch, J. A. Leigh, E. Johansen, G. A. Kuldau, S. Deegan, G. C. Walker, and E. R. Signer. 1985. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell 40:869-877. [DOI] [PubMed] [Google Scholar]
- 13.Fried, B., and J. Sherma. 1999. Thin-layer chromatography, 4th ed. Marcel Dekker, New York, N.Y.
- 14.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, M., M. Teplitski, J. B. Robinson, and W. D. Bauer. 2003. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe Interact. 16:827-834. [DOI] [PubMed] [Google Scholar]
- 18.Giovanelli, J., S. H. Mudd, and A. H. Datko. 1974. Homoserine esterification in green plants. Plant Physiol. 54:725-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovanelli, J., S. H. Mudd, and A. H. Datko. 1978. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J. Biol. Chem. 253:5665-5677. [PubMed] [Google Scholar]
- 20.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González, J. E., B. L. Reuhs, and G. C. Walker. 1996. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc. Natl. Acad. Sci. USA 93:8636-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray, K. M. 1997. Intercellular communication and group behavior in bacteria. Trends Microbiol. 5:184-188. [DOI] [PubMed] [Google Scholar]
- 23.Hoang, H. H., A. Becker, and J. E. Gonzalez. 2004. The LuxR homolog ExpR, in combination with the Sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J. Bacteriol. 186:5460-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser, D. 2004. Signaling in myxobacteria. Annu. Rev. Microbiol. 58:75-98. [DOI] [PubMed] [Google Scholar]
- 25.Kalyankar, G. D., M. Ikawa, and E. E. Snell. 1958. The enzymatic cleavage of canavanine to homoserine and hydroxyguanidine. J. Biol. Chem. 233:1175-1178. [PubMed] [Google Scholar]
- 26.Leigh, J. A., J. W. Reed, J. F. Hanks, A. M. Hirsch, and G. C. Walker. 1987. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51:579-587. [DOI] [PubMed] [Google Scholar]
- 27.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loh, J., and G. Stacey. 2003. Nodulation gene regulation in Bradyrhizobium japonicum: a unique integration of global regulatory circuits. Appl. Environ. Microbiol. 69:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loewus, F. A. 1952. Improvement in the anthrone method for determination of carbohydrates. Anal. Chem. 24:219. [Google Scholar]
- 30.Manefield, M., T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119-1127. [DOI] [PubMed] [Google Scholar]
- 31.Marketon, M. M., S. A. Glenn, A. Eberhard, and J. E. González. 2003. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 185:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marketon, M. M., and J. E. González. 2002. Identification of two quorum-sensing systems in Sinorhizobium meliloti. J. Bacteriol. 184:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marketon, M. M., M. R. Gronquist, A. Eberhard, and J. E. González. 2002. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J. Bacteriol. 184:5686-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathesius, U., S. Mulders, M. Gao, M. Teplitski, G. Caetano-Anolles, B. G. Rolfe, and W. D. Bauer. 2003. 2 January 2003, posting date. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 100:1444-1449. (Online.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 36.Mendrygal, K. E., and J. E. González. 2000. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 182:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Pappas, K. M., C. L. Weingart, and S. C. Winans. 2004. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling. Mol. Microbiol. 53:755-769. [DOI] [PubMed] [Google Scholar]
- 38.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen, T. B., T. Bjarnsholt, M. E. Skindersoe, M. Hentzer, P. Kristoffersen, M. Köte, J. Nielsen, L. Eberl, and M. Givskov. 2005. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187:1799-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal, G. A. 2001. l-Canavanine: a higher plant insecticidal allelochemical. Amino Acids 21:319-330. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal, G. A. 1977. Preparation and colorimetric analysis of l-canavanine. Anal. Biochem. 77:147-151. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal, G. A., M. A. Berge, and J. A. Bleiler. 1989. Novel mechanism for detoxification of l-canaline. Biochem. Syst. Ecol. 17:203-206. [Google Scholar]
- 43.Salmond, G. P., B. W. Bycroft, G. S. Stewart, and P. Williams. 1995. The bacterial “enigma”: cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 44.Sanger, F. 1945. The free amino groups of insulin. Biochem. J. 39:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swift, S., J. P. Throup, P. Williams, G. P. Salmond, and G. S. Stewart. 1996. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem. Sci. 21:214-219. [PubMed] [Google Scholar]
- 47.Takahashi, K. J. 1968. The reaction of phenylglyoxal with arginine residues in proteins. Biol. Chem. 243:6171-6179. [PubMed] [Google Scholar]
- 48.Teplitski, M., H. Chen, S. Rajamani, M. Gao, M. Merighi, R. T. Sayre, J. B. Robinson, B. G. Rolfe, and W. D. Bauer. 2004. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 134:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teplitski, M., J. B. Robinson, and W. D. Bauer. 2000. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 13:637-648. [DOI] [PubMed] [Google Scholar]
- 50.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. D. Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaks, T. E. 1977. Differences between strains of rhizobium in sensitivity to canavanine. Plant Soil 48:387-395. [Google Scholar]
- 52.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 52a.Williams, S. E., and G. E. Hunt. 1967. Canavanine distribution in jackbean fruit during fruit growth. Planta 77:192-202. [DOI] [PubMed] [Google Scholar]
- 53.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 54.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 55.Yang, C., E. R. Signer, and A. M. Hirsch. 1992. Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol. 98:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, R. G., T. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans, and A. Joachimiak. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417:971-974. [DOI] [PubMed] [Google Scholar]