It was 1975: the Vietnam war was over, but the people of Berkeley were still marching, now against nuclear arms and nuclear power. It was the prerecombinant era: in our laboratories at the University of California we focused on protein and nucleic acid biochemistry. For a small town, Berkeley was a big place, populated by huge vistas, giant eucalyptus, and biochemists who cast tall shadows. From the roof of the Biochemistry Building, where graduate students often sat and decompressed, the Golden Gate Bridge sparkled through blue skies over San Francisco Bay. To the south we looked down and across campus to the Life Sciences Building, where a determined man was preoccupied with the antibiotic resistance of bacteria, endeavoring to characterize the components of their membranes. The ensuing 30 years witnessed a remarkable advancement in the understanding of biological transport, in large part from his research, that led us from a time of cartoons to the intricate, mechanistic view of the gram-negative bacterial cell envelope that now exists (Fig. 1).
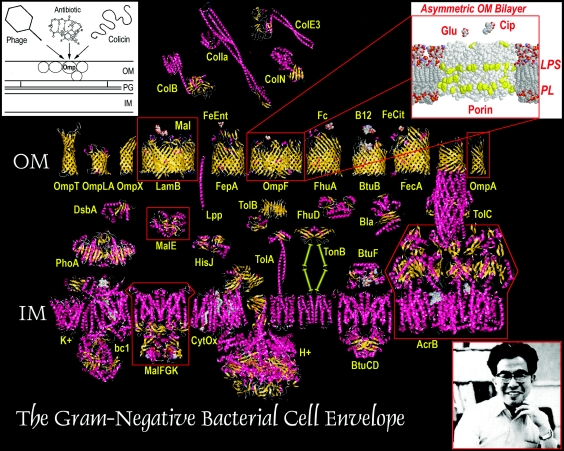
FIG. 1.
Preoccupations of Hiroshi Nikaido. Since the inception of his career, knowledge of the cell envelope has progressed from the cartoon visions of 1975 (left inset) to the crystallographically inspired models of today. Well before they were solved by X-ray data, however, Hiroshi's biochemical data on OmpF, LamB, MalE, MalFGK, and AcrB (boxed in red) gave him the first insights into their mechanistic properties, and the overall organization of the OM (right inset). Protein structures were created using Rasmol (UC Berkeley), with crystallographic coordinates from the Protein Data Bank (http://www.rcsb.org/pdb/). In some cases (MalFGK, TolA, TonB), existing structural information is incomplete, and the figure depicts a postulated conformation based on composite data from other systems. Abbreviations: Col, colicin; Mal, maltose, Glu, glucose; Cip, ciprofloxacin; FeEnt, ferric enterobactin; Fc; ferrichrome; B12, vitamin B12; FeCit, ferric citrate; K+, potassium channel; bc1, cytochrome c reductase complex; cytC, cytochrome c; CytOx, cytochrome oxidase; H+, proton ATP synthase; IM, inner membrane. The remaining proteins are identified by standard nomenclature.
Hiroshi Nikaido is a single-minded person who worked on the bacterial cell envelope from the beginning. His first paper as a graduate student, almost half a century ago (6), dealt with the lysis of what turned out to be galE mutants of Salmonella enterica in the presence of galactose: they could not form lipopolysaccharide (LPS). So began the career of one of the greatest prokaryotic membrane biochemists. His research since then encompasses over 250 publications that explained the functions of bacterial cell envelopes in innovative ways. It addressed four major themes: the barrier properties of LPS, the transport functions of general and specific porins, the structure of the mycobacterial cell envelope, and the mechanism of antibiotic efflux through multidrug resistance pumps. Solute recognition by periplasmic binding proteins, the biochemistry of ABC-type transporters, and outer membrane protein A (OmpA) were three other research interests, and Nikaido's papers in these areas also broke new ground.
Hiroshi's experiments, especially those with long-time associate Emiko Rosenberg, almost single-handedly demonstrated the selective permeability of the bacterial outer membrane (OM). This happened in two stages that focused first on LPS (about 30 papers; 1961 to 1975), and second on porins (70 papers, 1973 to 1994). Using Bruce Stocker's collection of rfa mutants, Hiroshi determined the effect of LPS structure on outer membrane permeability. The result was a seminal contribution to bacterial physiology: LPS creates a barrier that excludes the possibility of hydrophobic molecules dissolving in the OM. This property prevents the nonspecific penetration of many antibacterial agents, like bile salts, in the digestive tract and hydrophobic antibiotics produced by other organisms, including, for example, rifamycin and erythromycin. His later experiments on the protein composition of deep rough strains (1) showed that rfaD and rfaE mutants were much more susceptible to the permeation of hydrophobic molecules because of decreased protein concentration in their OM bilayers, for which the cell compensates by increasing the amount of phospholipids. This effect especially involves glycerophospholipids, which produce aberrant “bilayer” patches in the normally asymmetric OM and which allow rapid permeation of lipophilic molecules into the periplasm (10). These data ultimately led to his view that the low permeability of the OM derives from the exceptionally low fluidity of the LPS hydrocarbon domain, presumably brought about by the absence of unsaturated fatty acids, by the presence of six or seven fatty acids bound to the single head group, and by the tight interaction between the neighboring LPS molecules (mainly through the bridging effect of divalent cations).
These studies preceded his discovery of proteins that form trans-OM channels, the porins (2, 20). Soon thereafter, Nikaido's articles described the permeability limits of porins with an ingenious liposome swelling assay that required only a few components: lipids, proteins or membranes, solutes of interest, and a spectrophotometer (Fig. 2) (19). With this simple assay, employing sugars and antibiotics as solutes, Hiroshi determined their rates of diffusion through OmpF, OmpC, and PhoE, the major general porins of gram-negative bacteria. From these data he predicted the diameter of the OmpF channel to be about 10 Å. It was a striking discovery that revolutionized the understanding of not only antibiotic susceptibility and resistance but also the basic transport pathways into bacterial cells. This work, in the late 1970s and early 1980s, led to a historical review (21) that postulated the concept of selective permeability through the OM. Its main idea was the asymmetry of the bilayer, with LPS in the outer leaflet (7) and phospholipids in the inner leaflet. Others disputed this departure from traditional membrane physiology on thermodynamic grounds, but it was an insight that rationalized the ability of LPS to block the penetration of many noxious molecules. When combined with Hiroshi's biochemical finding that only molecules smaller than approximately 600 Da may diffuse through the water-filled porin channels, the theory of selective permeability was complete. Hiroshi's liposome swelling test revealed how such “general” porins exclude noxious, larger molecules in the environment, how they create diffusional pathways for nutrients, and how other organisms target these uptake pathways with antibiotics. Thus, the outer membrane is akin to a wall with open portals, through which sugars, amino acids, nucleotides, and many vitamins nonspecifically diffuse. Shortly thereafter, he and his coworkers showed that the bacteriophage λ receptor protein, LamB, also contains a pore, but one that has selectivity for maltose and maltodextrins (16). It was another critical discovery that expanded the repertoire of OM transporters to include “specific” porins. Such proteins contain the same transmembrane β-barrel architecture as general porins, but they facilitate the diffusion of particular solutes into the cell by locating weak binding sites for them near or within their pores.
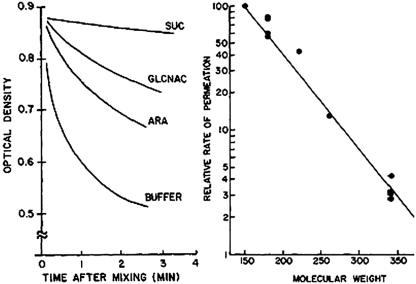
FIG. 2.
Liposome swelling assays of OmpF permeability (19). Left. When liposomes harboring pore-forming proteins are diluted into isotonic media containing high concentrations of sugars or other small molecules, the solutes diffuse into the vesicles through the pores (down their concentration gradients). As a result of the increase in solute concentration inside the liposome, water also enters and the vesicles swell, decreasing their optical density. Smaller solutes permeate more rapidly, causing a more rapid drop in absorbance. Right. The relative permeation rate strongly depends on the molecular weight of the solute, showing that the hydrophilic porin channels determine the diffusional limits of the OM. These elegant data are only a snapshot of his work, but they were also some of the most important results in Hiroshi's career, because they demonstrated the fundamental transport pathways of bacteria. An electronic literature search on the keyword “porin” now retrieves over 5,000 scientific articles. (Reproduced from reference 19 with permission of The Rockefeller University Press.)
Hiroshi has an M.D. degree (1955) and a doctorate in medical science, both from Keio University School of Medicine in Tokyo, Japan. He first worked at Harvard Medical School (1963) and then accepted a position as associate professor of bacteriology and immunology at UC Berkeley (1969). I had the good fortune to recruit him to my doctoral committee, and I later sought him as a postdoctoral advisor. Shortly after arriving in his laboratory, I met Steven Martin, whose lab was nearby, and told him of my association with Hiroshi. “Oh, then you must be a ‘porinologist,’” he replied. As I reflected on his comment, it struck me that Hiroshi had discovered and defined a completely novel aspect of bacterial physiology: static, water-filled, transmembrane channels. His accomplishments in other areas are also substantial, but his descriptions of porins were unparalleled in scope and significance.
Hiroshi Nikaido is a man of few words, but also one of tremendous wisdom. He has a clarity of thought that permeates his experiments and his writing. While working with him I was overwhelmed by data from a library of antiporin monoclonal antibodies, about which he offered the advice: “focus on the data that you understand. Write first about the results that are easy to explain.” Another memorable quote from Hiroshi: “Science is measurement!” Many micro/molecular biologists forget this fact, but Nikaido's devotion to quantitation is central to his work. Whether using the Renkin equation, the Donnan equilibrium, or Fick's first law of diffusion, he considers the compatibility of his data with theory and judges it accordingly. This man, who is by nature reserved around other people, has a daring mind that seeks difficulty in science as a place where true rewards are earned. It is a quality that sometimes intimidated those who stood before him in research meetings. Nothing brought more discomfort than the sight of Hiroshi sternly shaking his head from side to side as he listened to the results or, worse, seeing him lean forward to hold his head in his hands. We hoped instead for the simple opposite, a subtle nodding of his head that grew in rhythmic intensity as the data came forth, culminating in a smile and perhaps a compliment or anecdote.
In the wake of the explosion of crystallographic data that followed the solution of the first membrane protein structure (the photosynthetic reaction center in 1984 [5]), the impact of Hiroshi's contributions to porin biochemistry were minimized by some or at least temporarily forgotten. However, when the OmpF crystal structure was finally completed in 1992 by Jurg Rosenbush's group (3), it revealed little new biochemical or overall structural information. Indeed, OmpF contains a hydrophilic channel with a diameter of almost exactly 10 Å at its narrowest point, and its tertiary structure consists of β-strands crossing back and forth through the OM bilayer to form a β-barrel that circumscribes the pore. Hiroshi had reached both conclusions several years beforehand (11, 21). At this time a large group of predominantly crystallographers argued that the L3, or transverse loop of general porins, underwent a conformational change that closed porin channels in response to voltage potential across the membrane bilayer. But Nikaido contended that this conclusion was fallacious, because the observed closure was caused by artificially high voltages that do not exist in vivo. He and colleagues then showed that even changes in voltage as high as 100 mV had no effect on porin permeability in intact cells (22), refuting the idea that porin channels fluctuate in vivo as a result of voltage changes across the OM bilayer. In retrospect, his research in this period established Hiroshi as the ultimate authority on the diffusional transport properties of the gram-negative bacterial OM.
In the course of these studies Hiroshi engaged in many collaborations, including experiments with the great bacterial geneticist Maurice Hofnung at the Institut Pasteur, who was studying the transport of maltose and maltodextrins. Nikaido spent a sabbatical year with Maurice in the Unit é de Programmation Moléculaire et Toxicologie Génétique at the Institut Pasteur in Paris, France, and visited many times for science and pleasure. Maurice once related that during his first stay, Hiroshi was extremely prolific from his dedication to lab work; in subsequent visits he further expanded his horizons to the fine restaurants and culture of Paris. Indeed, Hiroshi is a gourmet, of science as well as food, which was well illustrated by his work on the E. coli maltose/maltodextrin uptake system. He and colleagues characterized its periplasmic binding protein, MalE, and its inner membrane ABC-type transporter, the maltose permease. They purified and functionally reconstituted, for the first time, the MalFGK permease complex and showed its dependence on interaction with MalE for ATP hydrolysis and solute internalization (4). Later, in a particularly incisive set of experiments, they showed by three spectroscopic methods (UV, electron paramagnetic resonance, and fluorescence) that the two lobes of MalE undergo large conformational changes when sugars bind, effectively closing the protein structure and preparing it for interaction with MalFGK (8, 9).
In the past 15 years Nikaido's lab diverged into two new areas, the mycobacterial cell wall and antibiotic efflux pumps. With his coworkers, Hiroshi's experiments forced a reevaluation of the architecture of the mycobacterial cell wall, resulting in the current view of its composition and structure (15). Using X-ray diffraction and electron paramagnetic resonance (15, 18), they showed that mycobacteria do contain an “outer membrane”—an asymmetric lipid bilayer composed of mycolic acids and other glycolipids. This was against the long-standing view that (gram-positive) mycobacterial cells only have one (cytoplasmic) membrane. But, their new structural model (14) and subsequent work explained the intrinsic resistance of mycobacteria to most antibiotics. It later received support from many other groups, including the discovery of a porin in the outer layer of the cell wall (23), and now stands as the working hypothesis that guides the design of new drugs against tuberculosis.
Similarly, in the 1990s Nikaido and coworkers began to explain the mysteries of antibiotic efflux pumps. At that time, Hiroshi made tremendous efforts to change the outdated thinking of others, ironically because the field was dogmatized by his own theories of low OM permeability. He emphasized that OM permeability alone did not explain intrinsic bacterial resistance to antibiotics, but many experts could not accept these new ideas on efflux pumps, leading to the initial rejection of two important papers on antibiotic export by Pseudomonas spp. Hiroshi once said that the two manuscripts were the most difficult he had ever written. The responses of referees to this work were so negative (one of them contained 38 points of criticism!) that it prompted a comment to his coauthors: “Life is not easy.” Ultimately, these papers were held from publication for months because the reviewers did not believe that a single pump of such a wide specificity could exist in this world. But Nikaido remained confident of the data, which were solid, novel, and later published (12, 13). In the ensuing years his experiments elucidated the nature of the pumps and addressed the mechanism of the AcrA-AcrB-TolC multidrug efflux complex. Hiroshi and coworkers recently published a crystal structure of the AcrB inner membrane component in association with drugs that defined the location of its broad-specificity substrate collection domain (24). Perhaps the biggest contribution of his lab to this subject was the demonstration that such export pumps play a major role in defining the net influx rate of most antibiotics, especially lipophilic ones.
As one considers the series of contributions made by Nikaido, their extent and magnitude are impressive. Instead of unraveling one or two perplexing problems, which most scientists are happy to accomplish in the course of a career, he well explained the fundamental biochemistry and physiology of six or seven systems. Some of these findings revolutionized clinical approaches to bacterial disease. With the original goal of understanding the interaction of antibiotics with bacteria, he elucidated cell envelope processes underlying both their susceptibility and their resistance. Especially important in light of expanding resistance to antibiotics, his descriptions of cell envelope uptake and export processes paved the way for rational designs of antibacterial agents. Such experiments could not proceed without the information that he provided on the relationships of solute size, charge, and hydrophobicity to permeation into, and out of, the cell envelope. In this light, a statement from one of his papers (17) rings true: “The greatest attraction of the field of microbiology is our ability to analyze at the molecular level the physiological and ecological responses of microbes.” Regarding the cell envelopes of bacteria, no one has done so more thoroughly than Hiroshi Nikaido.
Acknowledgments
I thank the many associates, colleagues, students, and friends of Hiroshi Nikaido, who kindly offered important information, comments, and insight on this document. Thanks most of all to Hiroshi himself for all that he has shown us and taught us.
REFERENCES
- 1.Ames, G. F., E. N. Spudich, and H. Nikaido. 1974. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations J. Bacteriol. 117:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavoil, P., H. Nikaido, and K. von Meyenburg. 1977. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol. Gen. Genet. 158:23-33. [DOI] [PubMed] [Google Scholar]
- 3.Cowan, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 4.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deisenhofer, J., O. Epp, K. Miki, R. Huber, and H. Michel. 1984. X-ray structure analysis of a membrane protein complex. Electron density map at 3 Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J. Mol. Biol. 180:385-398. [DOI] [PubMed] [Google Scholar]
- 6.Fukasawa, T., and H. Nikaido. 1959. Formation of protoplasts in mutant strains of Salmonella induced by galactose. Nature 183:1131-1132. [DOI] [PubMed] [Google Scholar]
- 7.Funahara, Y., and H. Nikaido. 1980. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J. Bacteriol. 141:1463-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, J. A., K. Gehring, and H. Nikaido. 1997. Two modes of ligand binding in maltose-binding protein of Escherichia coli. Correlation with the structure of ligands and the structure of binding protein. J. Biol. Chem. 272:17605-17609. [DOI] [PubMed] [Google Scholar]
- 9.Hall, J. A., T. E. Thorgeirsson, J. Liu, Y. K. Shin, and H. Nikaido. 1997. Two modes of ligand binding in maltose-binding protein of Escherichia coli. Electron paramagnetic resonance study of ligand-induced global conformational changes by site-directed spin labeling. J. Biol. Chem. 272:17610-17614. [DOI] [PubMed] [Google Scholar]
- 10.Kamio, Y., and H. Nikaido. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 15:2561-2570. [DOI] [PubMed] [Google Scholar]
- 11.Klebba, P. E., S. A. Benson, S. Bala, T. Abdullah, J. Reid, S. P. Singh, and H. Nikaido. 1990. Determinants of OmpF porin antigenicity and structure. J. Biol. Chem. 265:6800-6810. [PubMed] [Google Scholar]
- 12.Li, X. Z., D. M. Livermore, and H. Nikaido. 1994. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob. Agents Chemother. 38:1732-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, X. Z., D. Ma, D. M. Livermore, and H. Nikaido. 1994. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob. Agents Chemother. 38:1742-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., C. E. Barry III, G. S. Besra, and H. Nikaido. 1996. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 271:29545-29551. [DOI] [PubMed] [Google Scholar]
- 15.Liu, J., E. Y. Rosenberg, and H. Nikaido. 1995. Fluidity of the lipid domain of cell wall from Mycobacterium chelonae. Proc. Natl. Acad. Sci. USA 92:11254-11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckey, M., and H. Nikaido. 1980. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 77:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido, H. 1999. Microdermatology: cell surface in the interaction of microbes with the external world. J. Bacteriol. 181:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido, H., S. H. Kim, and E. Y. Rosenberg. 1993. Physical organization of lipids in the cell wall of Mycobacterium chelonae. Mol. Microbiol. 8:1025-1030. [DOI] [PubMed] [Google Scholar]
- 19.Nikaido, H., and E. Y. Rosenberg. 1981. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J. Gen. Physiol. 77:121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H., S. A. Song, L. Shaltiel, and M. Nurminen. 1976. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem. Biophys. Res. Commun. 76:324-330. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen, K., J. Hellman, and H. Nikaido. 1988. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 263:1182-1187. [PubMed] [Google Scholar]
- 23.Trias, J., V. Jarlier, and R. Benz. 1992. Porins in the cell wall of mycobacteria. Science 258:1479-1481. [DOI] [PubMed] [Google Scholar]
- 24.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]