Abstract
Nipple discharge is a common presenting symptom of underlying breast pathology. This study examined the impact of galactography on the evaluation of abnormal nipple discharge. Thirty-five women with spontaneous, unilateral nipple discharge who underwent galactography from 1995 to 1997 were retrospectively studied. Their presenting signs as well as mammographic, galactographic, and pathology findings were evaluated. Nipple discharge was bloody (n = 24), clear (n = 7), or serous (n = 4). A palpable mass was found in 5 patients, and discharge was spontaneous in 29 patients (83%). Mammography was normal in 25 patients (71%). Thirty patients (86%) had an abnormal ductogram that was characterized as a filling defect (n = 20), cutoff sign (n = 5), or ductal dilatation (n = 5). The ductogram demonstrated the location and depth of the lesion in 29 patients (97%). Excision was performed in 27 of 30 patients with an abnormal ductogram: 14 received complete subareolar duct excisions; 12, focused excisions; and 1, excision with a vacuum-assisted biopsy device. Pathology included intraductal papilloma (n = 20) and ductal ectasia (n = 7). Follow-up was completed in 24 patients, including 2 postoperative patients who had persistent discharge on manipulation. In conclusion, galactography is accurate in identifying the location of the ductal abnormality. It allows a focused surgical approach to the pathologic lesion in these patients.
Nipple discharge is a common complaint and is usually associated with a benign etiology. Characteristics of a benign source include a milky, bilateral discharge, which does not require further follow-up. The discharge is due to an underlying malignant lesion, however, in up to 15% of cases (1–5). Discharge from a malignant source is spontaneous, unilateral, persistent, and nonlactational (6). The fluid may be bloody, clear, serous, or milky (2, 7). In most cases of nonlactational discharge, the options for demonstrating and localizing ductal abnormalities and intraductal mass lesions are quite limited. These include the infrequent identification of a mass lesion on examination and the occasional abnormal mammogram. Cytology of the discharge itself is often unrevealing and is not helpful in localizing a tumor (1, 5). Galactography (ductography) is often used to localize and characterize ductal abnormalities. This paper reviews the information provided by ductography at a single institution to determine its impact on the management of these patients.
MATERIALS AND METHODS
A retrospective chart review was performed on patients presenting with abnormal nipple discharge who underwent ductography at Baylor University Medical Center from 1995 to 1997. Information on presenting symptoms, physical signs, mammographic and sonographic findings, and cytology was collected.
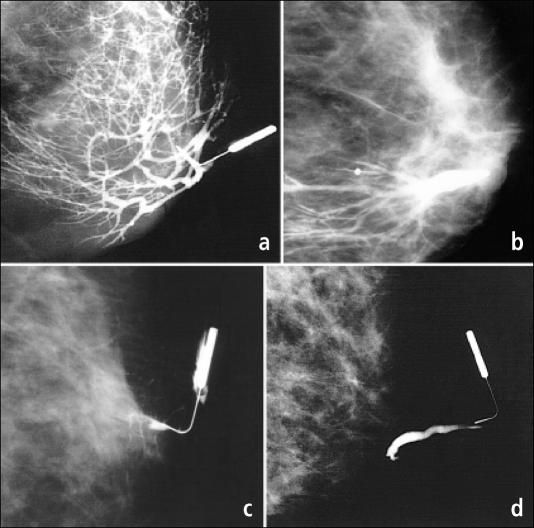
Ductography was performed at the Komen Breast Center. A 30-gauge cannula was inserted into a single duct, and 0.1 to 0.4 cm3 of water-soluble contrast (iothalamate meglumine 60%) was injected. Craniocaudal and 90° lateral views were obtained, as were compression views when appropriate. The results of these ductograms were characterized as 1) normal, 2) ductal dilatation, 3) filling defect, or 4) cutoff sign (Figure 1). Information regarding the radial location and depth of any abnormalities was collected.
Figure 1.
Examples of ductogram findings. (a) Normal ductal appearance. (b) Dilated duct due to ectasia. (c) A filling defect caused by an intraductal lesion. (d) Cutoff of contrast by an intraductal papilloma.
Operative reports were obtained for patients who had surgical excision. The technique of the surgery was recorded with the histology of any abnormal breast tissue. Clinic and telephone follow-up questions focused on persistence of symptoms and postoperative complications.
RESULTS
Ductography was performed at Baylor University Medical Center on 35 patients with abnormal nipple discharge. All patients were women and none were pregnant. The average age was 54 years (range, 26 to 74 years). The mean duration of symptoms was 30 weeks (range, 4 days to 3 years). Although the discharge in most patients was bloody, the majority had no abnormal results after external examination or mammography (Table).
Table.
Patient characteristics
| Characteristic | Number of patients |
| Presenting discharge | |
| Spontaneous | 29 |
| Nonspontaneous | 6 |
| Color of discharge | |
| Clear | 7 |
| Serous | 4 |
| Bloody | 24 |
| Palpable mass | |
| Absent | 30 |
| Present | 5 |
| Mammogram results | |
| Normal | 25 |
| Abnormal | 10 |
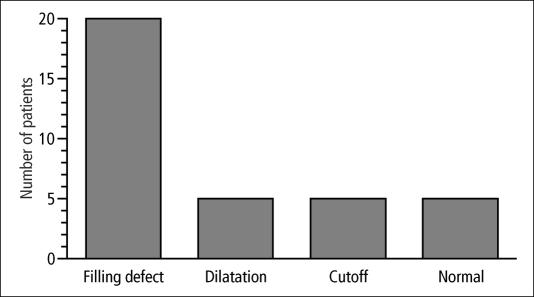
When ductograms were obtained, abnormalities were seen in 30 of the 35 patients. The abnormalities included filling defects (n = 20), dilated ducts (n = 5), and ductal cutoff (n = 5) (Figure 2). In the 10 patients with mammographic abnormalities (5 with suspicious microcalcifications and 5 with evidence of a mass), 9 had galactographic abnormalities as well (filling defect, n = 5; cutoff, n = 5; dilatation, n = 1).
Figure 2.
Results of galactography in 35 patients.
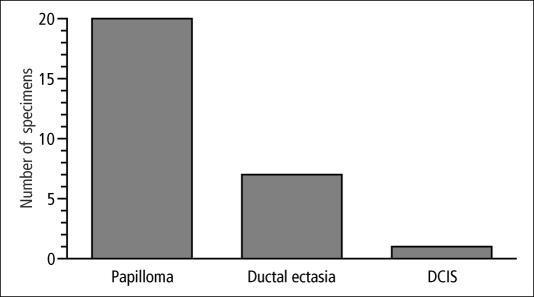
Twenty-seven of the 30 women with abnormal ductograms proceeded to biopsy. The ductographic examination revealed the exact radial location in all patients and the depth of the lesion in 26 of 27 patients (96%). Surgical procedures included complete subareolar ductal excision in 14, focused duct excision in 12, and excision with a directional vacuum-assisted biopsy device (Mammotome system, Ethicon Endo-Surgery, Inc., Cincinnati, Ohio) in 1 patient. All specimens contained a pathologic lesion (Figure 3). Intraductal papilloma was the most common lesion (n = 20), followed by ductal ectasia (n = 7). One patient with an intraductal papilloma also had ductal carcinoma in situ.
Figure 3.
Pathology of 27 surgical specimens. One patient with an intraductal papilloma also had ductal carcinoma in situ (DCIS).
Three patients with an abnormal ductogram (dilated ducts, n = 2; filling defect, n = 1) did not undergo surgical excision. Two did not wish to have a procedure after consultation, and 1 had a significant medical comorbidity that precluded a procedure. The discharge of 2 of these patients has resolved, and they have had normal examinations and mammograms since. The third patient still has discharge but does not wish to have a biopsy.
All 5 patients who had bloody nipple discharge but normal ductograms were offered surgical biopsy. Two chose this option, and a papilloma and ductal ectasia were found. Two patients refused surgery and chose to be followed clinically, both of whom have had a normal physical examination, a normal mammogram, and resolution of spontaneous discharge at 16 and 18 months. One patient with a normal study refused follow-up.
Telephone follow-up (mean, 15 months) was completed in 24 postoperative patients, and 3 patients were lost to follow-up. Two postoperative patients, who presented with spontaneous bloody discharge, had nonspontaneous serous discharge on follow-up. Nine patients, all of whom had subareolar resections, required drainage of a seroma. No patient has required a repeat breast biopsy for nipple discharge or has been found to have invasive breast cancer. Numbness occurred in 3 patients and nipple inversion in 7 after complete subareolar resection. No patients undergoing focused duct excision complained of nipple numbness or inversion.
DISCUSSION
Abnormal nipple discharge is associated with an underlying malignancy in 1.2% to 15% of patients (1–5). Abnormal discharge is defined as nonlactational, persistent, spontaneous, and unilateral (6). Bloody or Hemoccult-positive discharge is more likely to be associated with cancer (5% to 28%) and should prompt further evaluation (1, 8). However, clear or watery discharge has been associated with breast cancer in up to 7% of cases (1, 9, 10). Although cytology has been used to further evaluate the discharge, it has been associated with a false-negative rate of up to 18% (1, 5). The examination of this group of patients infrequently reveals a mass lesion.
Mammography is advocated as part of the routine evaluation of women with breast complaints. Mammography is associated with a 9.5% false-negative rate and a 1.6% false-positive rate in detecting breast cancer in patients with nipple discharge (2). Ductal ectasia as well as carcinoma may be suggested by microcalcification or mass (7). Tabar noted that only half of the patients presenting with nipple discharge who were found to have cancer had an abnormal mammogram (1). Magnetic resonance imaging ductography and fiber-ductoscopy remain investigational (11, 12).
Ductography is an increasingly available method of examination and is relatively easy to perform with few complications (1, 13). Ductography has been shown to be accurate in providing the location and depth of ductal abnormalities when a single duct is identified as the source (1, 14, 15). Data regarding the location of the lesion greatly facilitate biopsy, especially with deep lesions. Ductography has also been shown to improve the diagnostic yield of surgical biopsy from 67% in nonstudied patients to 100% in patients receiving a ductogram (3).
The operative management of nipple discharge may be accomplished by a number of techniques that generally involve a periareolar incision located in the direction of the discharging duct. A blind complete subareolar dissection can be performed but may be associated with a high incidence of normal specimens. Van Zee reported that 33% of surgical biopsies without galactographic guidance contained only normal breast tissue (3). A focused excision of affected ducts can also be accomplished following radiographic visualization of the depth of the lesion (1, 7). The duct can be cannulated with a fine probe, injected with methylene blue dye, or radiographically localized with a wire (3, 16, 17). Focused excisions may lessen the possibility of postoperative seroma formation and nipple numbness and may also allow lactation in the ipsilateral breast (7). This is an important consideration in the patient of childbearing age.
The histopathologic causes of abnormal bloody or serous discharge are usually benign. Solitary or multiple papillomas are the most common cause of nipple discharge reported (35%–62%) (1, 4, 5, 9, 16–19); in our study, they accounted for 74% of the cases. Papillomas, although benign, are associated with a 5% risk of developing into invasive carcinoma (20). The depth of papilloma is typically 1 to 2 cm but may be 4 to 5 cm from the nipple. There may also be multiple papillomas in the same ductal system or sporadic epitheliosis (papillomatosis) in several duct systems.
Ductal ectasia is the cause of nipple discharge in 11% of patients (1). The etiology of ductal ectasia is related to occlusion of the ductal system with subsequent dilatation, pressure buildup, and leakage of secretions (21). Complete subareolar excision is usually performed since multiple ducts are usually involved. However, when a ductogram confirms this finding, no abnormal filling defect is noted, and the discharge is not suspicious (i.e., persistent or bloody), simple observation of the patient is an option.
The management of these patients was potentially influenced by the ductograms since 12 of 27 patients underwent a focused duct excision rather than a complete subareolar excision. Patients who underwent a complete subareolar excision had complications, including seroma formation (n = 9), nipple inversion (n = 7), and nipple numbness (n = 3). These complications did not occur in any of the 12 patients undergoing a focused excision. Furthermore, a probable explanation for the discharge was achieved in nearly all patients who underwent ductography. All biopsy specimens contained a pathologic lesion that explained the discharge.
In summary, the nipple ductogram is a useful diagnostic tool to identify and localize the cause of nipple discharge. The localization ensures a high probability of removing the etiology of the discharge. Localization also offers the possibility for a focused ductal excision that preserves greater sensation and function, avoids nipple inversion, and decreases the likelihood of seroma formation.
References
- 1.Tabar L, Dean PB, Pentek Z. Galactography: the diagnostic procedure of choice for nipple discharge. Radiology. 1983;149:31–38. doi: 10.1148/radiology.149.1.6611939. [DOI] [PubMed] [Google Scholar]
- 2.Fiorica JV. Nipple discharge. Obstet Gynecol Clin North Am. 1994;21:453–460. [PubMed] [Google Scholar]
- 3.Van Zee KJ, Ortega Perez G, Minnard E, Cohen MA. Preoperative galactography increases the diagnostic yield of major duct excision for nipple discharge. Cancer. 1998;82:1874–1880. doi: 10.1002/(sici)1097-0142(19980515)82:10<1874::aid-cncr9>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Paterok EM, Rosenthal H, Sabel M. Nipple discharge and abnormal galactogram. Results of a long-term study (1964–1990) Eur J Obstet Gynecol Reprod Biol. 1993;50:227–234. doi: 10.1016/0028-2243(93)90205-q. [DOI] [PubMed] [Google Scholar]
- 5.Leis HP., Jr Management of nipple discharge. World J Surg. 1989;13:736–742. [PubMed] [Google Scholar]
- 6.Fiorica JV. Breast disease. Curr Opin Obstet Gynecol. 1992;4:897–903. [PubMed] [Google Scholar]
- 7.Jardines L. Management of nipple discharge. Am Surg. 1996;62:119–122. [PubMed] [Google Scholar]
- 8.Chaudary MA, Millis RR, Davies GC, Hayward JL. The diagnostic value of testing for occult blood. Ann Surg. 1982;196:651–655. doi: 10.1097/00000658-198212001-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawes LG, Bowen C, Venta LA, Morrow M. Ductography for nipple discharge: no replacement for ductal excision. Surgery. 1998;124:685–691. doi: 10.1067/msy.1998.91362. [DOI] [PubMed] [Google Scholar]
- 10.Leis HP, Jr, Greene FL, Cammarata A, Hilfer SE. Nipple discharge: surgical significance. South Med J. 1988;81:20–26. doi: 10.1097/00007611-198801000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimoto M, Kasumi F, Iwase T, Takahashi K, Tada T, Uchida Y. Magnetic resonance galactography for a patient with nipple discharge. Breast Cancer Res Treat. 1997;42:87–90. doi: 10.1023/a:1005703927922. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki A, Hirata K, Okazaki M, Svane G, Azavedo E. Nipple discharge disorders: current diagnostic management and the role of fiber-ductoscopy. Eur Radiol. 1999;9:583–590. doi: 10.1007/s003300050715. [DOI] [PubMed] [Google Scholar]
- 13.Cardenosa G, Doudna C, Eklund GW. Ductography of the breast: technique and findings. AJR Am J Roentgenol. 1994;162:1081–1087. doi: 10.2214/ajr.162.5.8165986. [DOI] [PubMed] [Google Scholar]
- 14.Woods ER, Helvie MA, Ikeda DM, Mandell SH, Chapel KL, Adler DD. Solitary breast papilloma: comparison of mammographic, galactographic, and pathologic findings. AJR Am J Roentgenol. 1992;159:487–491. doi: 10.2214/ajr.159.3.1503011. [DOI] [PubMed] [Google Scholar]
- 15.Ciatto S, Bravetti P, Berni D, Catarzi S, Bianchi S. The role of galactography in the detection of breast cancer. Tumori. 1988;74:177–181. doi: 10.1177/030089168807400210. [DOI] [PubMed] [Google Scholar]
- 16.Rongione AJ, Evans BD, Kling KM, McFadden DW. Ductography is a useful technique in evaluation of abnormal nipple discharge. Am Surg. 1996;62:785–788. [PubMed] [Google Scholar]
- 17.Hou MF, Huang CJ, Huang YS, Huang TJ, Chan HM, Wang JY, Liu GC, Wu DK. Evaluation of galactography for nipple discharge. Clin Imaging. 1998;22:89–94. doi: 10.1016/s0899-7071(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 18.Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg. 1982;195:259–264. doi: 10.1097/00000658-198203000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulay H, Bora S, Kilicturgay S, Hamaloglu E, Goksel HA. Management of nipple discharge. J Am Coll Surg. 1994;178:471–474. [PubMed] [Google Scholar]
- 20.Gadd MA. Papillary lesions. In: Harris JR, Lippman ME, Morrow M, editors. Diseases of the Breast. Philadelphia: Lippincott-Raven; 1996. pp. 42–45. [Google Scholar]
- 21.Tanabe KK. Duct ectasia, periductal mastitis, and infections. In: Harris JR, Lippman ME, Morrow M, editors. Diseases of the Breast. Philadelphia: Lippincott-Raven; 1996. pp. 49–54. [Google Scholar]