Abstract
Use of the standard dual-platform flow cytometric method for determination of CD4+ T-lymphocyte counts, which needs both a flow cytometer (FCM) and hematological analyzer, would inevitably lead to increased variability. The development of new single-platform (SP) FCMs that provide direct CD4+ T-lymphocyte counts for improved assay precision and accuracy have recently attracted attention. This study evaluated one of those systems, CyFlowgreen (Partec), a single-parameter SP volumetric FCM. The performance of CyFlowgreen was compared with those of two reference standard SP microbead-based technologies of the three-color TruCOUNT tube with the FACScan FCM and a two-color FACSCount system (Becton Dickinson Biosciences). Absolute CD4+ and CD8+ T-lymphocyte counts in 200 human immunodeficiency virus type 1-seropositive blood specimens were determined. Statistical analysis for correlation and agreement were performed. A high correlation of absolute CD4 counts was shown when those obtained with CyFlowgreen were compared with those obtained with the bead-based three-color TruCOUNT system (R2 = 0.96; mean bias, −69.1 cells/μl; 95% confidence interval [CI], −225.7 to +87.5 cells/μl) and the FACSCount system (R2 = 0.97; mean bias, −40.0 cells/μl; 95% CI, −165.1 to +85.1 cells/μl). The correlation of the CD4+ T-lymphocyte counts obtained by the two bead-based systems was high (R2 = 0.98). Interestingly, CyFlowgreen yielded CD4+ T-lymphocyte counts that were 21.8 and 7.2 cells/μl lower than those obtained with the TruCOUNT and the FACSCount systems, respectively, when CD4+ T-lymphocyte counts were <250 CD4+ T-lymphocyte counts/μl range or 17.3 and 5.8 cells/μl less, respectively, when CD4+ T-lymphocyte counts were <200 cells/μl. The single-parameter CyFlowgreen volumetric technology performed well in comparison with the performance of the standard SP bead-based FCM system. However, a multicenter comparative study is needed before this FCM machine is implemented in resource-limited settings.
The advent of potent antiretroviral therapy in 1996 led to a revolution in the care of patients with human immunodeficiency virus (HIV) infection or AIDS. In countries such as Thailand, where antiretroviral drug therapy is now available due to the introduction of generic drugs, the provision of affordable and reliable CD4 testing for the initiation and monitoring of antiretroviral therapy has emerged as a vital issue. In Thailand, dual-platform (DP) flow cytometry immunophenotyping is the accepted standard method for the determination of the absolute counts of CD4+ T lymphocytes (6). The DP technique uses a flow cytometer (FCM), along with a hematology analyzer, which provides the absolute lymphocyte count, to provide the percentage of CD4+ T lymphocytes. The Centers for Disease Control and Prevention (CDC)-recommended three-tube, three-color monoclonal antibody panel with the lyse-and-wash or lyse-no-wash whole-blood method (6, 7) is commonly used in most regional hospitals. However, there are two main drawbacks of such a method: (i) many studies have highlighted problems associated with the DP-derived CD4+ T-lymphocyte count, especially in relation to the generation of an absolute lymphocyte count by the hematology analyzer (1, 3, 11, 16, 23); and (ii) the cost of DP FCM CD4 testing in Thailand remains relatively high ($12 to $20). Several single-platform (SP) flow cytometric technologies, including FCM volumetric counting and microfluorometry (8, 9, 13, 14, 19), as well as, most commonly, the bead-based flow cytometric method for measurement of the absolute CD4+ T-lymphocyte counts, have been developed and successfully evaluated (21, 25). Although this bead-based flow cytometric method eliminates the need for multiple technologies, it is still limited by the high cost of the currently available FCMs and fluorescent beads. Although alternative nonflow cytometric technologies are simple and less expensive, they have not been implemented widely due to their complexity, low-volume CD4 counting application (1 to 10 samples/day), and poor quality control (4, 5, 12, 22). The recent introduction of the inexpensive single-parameter CyFlowgreen FCM (Partec GmbH, Münster, Germany), which uses a single phycoerythrin (PE) conjugated-monoclonal antibody to CD4, has increased access to CD4+ count determinations for patients in areas that are not equipped with an FCM, particularly in district hospitals in the rural areas of Thailand.
The purpose of this study was to evaluate this single-parameter SP CyFlowgreen technology as an alternative for determination of absolute CD4+ T-lymphocyte counts by comparing the values obtained with CyFlowgreen with those obtained by the standard SP bead-based systems, consisting of TruCOUNT tubes (Becton Dickinson Biosciences [BDB], San Jose, CA) with the FACScan (BDB) system and the FACSCount FCM (BDB).
This study was done as part of the CDC Global AIDS Program, which supports the evaluation of alternative methodologies for CD4+ T-lymphocyte subset enumeration.
MATERIALS AND METHODS
Patients and blood samples.
Blood samples were obtained from 200 HIV type 1 (HIV-1)-seropositive patients. Two milliliters of venous blood was collected by venipuncture into K3EDTA-containing tubes, kept at room temperature (24°C to 26°C), and processed for immunophenotyping within 6 h. The blood samples used in this study were residual routine clinical specimens that were unlinked from identifiers and that were tested at the Department of Immunology, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand. HIV-1 infections were diagnosed by serologic testing, with confirmation by two other different serologic tests. This study was approved by the Ethical Committee of the Faculty of Medicine, Siriraj Hospital, and CDC as research not involving human subjects.
Equipment used in the study.
The FACScan system is a multicolor benchtop FCM equipped with a 15-mW argon ion laser which operates at 488 nm for the excitation of three fluorescent parameters: fluorescein isothiocyanate (FITC), PE, and peridinin chlorophyll protein (PerCP). When this system is combined with three fluorescence (FITC, PE, and PerCP)-conjugated TriTEST monoclonal antibody reagents for CD3/CD4/CD45 and CD3/CD8/CD45 (BDB) and fluorescent-integrated TruCOUNT beads of known density, it is able to generate absolute CD4+ and CD8+ T-lymphocyte counts.
The FACSCount system is an SP benchtop FCM consisting of a green laser with two-color monoclonal antibody reagents in a twin tube containing calibrated beads, additional control beads, and built-in software. The first tube in each pair consists of a mixture of monoclonal antibody reagents for CD4/CD3 conjugated to PE and PE.Cy5 fluorescence and a known density of fluorescent beads. The second tube contains CD8/CD3. The control set consists of fluorescent beads at four different densities: zero (0 beads/μl), low (50 beads/μl), medium (250 beads/μl), and high (1,000 beads/μl).
The CyFlowgreen evaluated in this study is a benchtop flow cytometer equipped with a 532-nm green solid-state laser. It is a single-parameter (PE detection) SP volumetric FCM, one of the most basic models in the CyFlow system family. Absolute counting occurs when the tips of two electrodes dip into the fluid at different levels. The counting is triggered when the higher electrode during the aspiration is no longer surrounded by fluid; when the fluid level falls below the second electrode, counting stops. From the aspirated volume (200 μl) and the dilution factor, the absolute cell count is given.
Immunophenotypic staining of peripheral blood.
All three immunophenotypic staining methods required the use of two tubes, one for CD4 T-lymphocyte enumeration and the other for CD8 T-lymphocyte enumeration.
Exactly 20 μl of TriTEST three-color monoclonal antibodies and 50-μl aliquots of EDTA-anticoagulated whole blood obtained by the reverse pipetting technique were added to a TruCOUNT tube containing a known concentration of beads. The mixture was incubated for 20 min at room temperature in the dark before 450 μl of FACS lysing solution (BDB) was added. After 15 min of incubation, the lyse-no-wash stained samples were analyzed with the FACScan system.
For the FACSCount method, exactly 50 μl of whole blood was added to each of the pair of CD4/CD3 and CD8/CD3 reagent tubes with an electronic pipette (BDB). The tubes were vortexed for 5 s and incubated in the dark at room temperature for 60 min. After incubation, 50 μl of fixative provided in the reagent kit was added to each tube. After the tubes were vortexed, no-lyse stained samples were run on the FACSCount FCM.
Staining with CyFlowgreen reagents was performed by adding 100 μl of EDTA-anticoagulated whole blood and 10 μl of monoclonal antibody reagents for CD4 or CD8 (Partec) to Röhren polystyrene tubes (Sarstedt, Nümbrecht, Germany). The mixtures were incubated for 10 min at room temperature in the dark before the addition of 2,500 μl of No-Lyse Dilution Buffer (Partec), for a total mixture volume of 2,610 μl for the counting of CD4+ T lymphocytes; 850 μl of this well-mixed no-lyse stained sample was transferred to a Röhren tube and analyzed with the CyFlowgreen FCM with FloMax software (Partec).
Flow cytometric analysis.
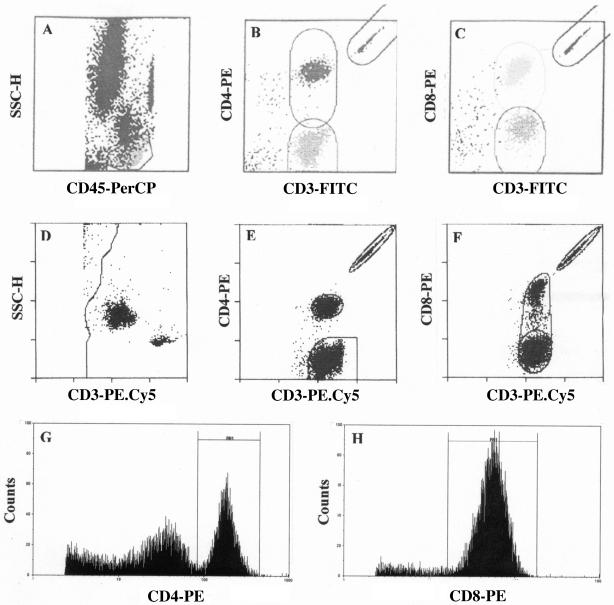
Data were acquired for each sample stained with the TriTEST reagents and analyzed with MultiSET software (BDB), an automated acquisition and analysis interface designed specifically for use with the three-color or four-color monoclonal antibody reagents. Briefly, cells stained with FITC-, PE-, and PerCP-conjugated monoclonal antibodies were detected by the logarithmic amplification of the green, orange, and red fluorescences, respectively. The forward scatter (FSC-H) and the side scatter (SSC-H) of the cells were measured on a linear scale. After the acquisition of data for 15,000 cells, a region was automatically set on SSC-H low and CD45 PerCP high positive cells (Fig. 1A). Cells in this gate were regarded as lymphocytes, while cells outside this gate represented monocytes (SSC-H medium and CD45 PerCP intermediate positive cells) and granulocytes (SSC-H high and CD45 low positive cells). Once this was established, the percentages and absolute counts of CD3+/CD4+ and CD3+/CD8+ T lymphocytes were then automatically enumerated by their CD3-FITC/CD4-PE and CD3-FITC/CD8-PE fluorescences by the software (Fig. 1B and C).
FIG. 1.
Representative flow cytometric histograms illustrating the software algorithm of the three-color TruCOUNT MultiSET system (A to C), the two-color FACSCount system (D to F), and the single-parameter CyFlowgreen FlowMax system (G and H).
When a stained sample was introduced into the FACSCount system, an elliptical region was automatically set around each cell population and the integrated beads by the built-in software. The ratio of fluorescent cells to beads multiplied by the known concentration of beads in the tube automatically gave the CD3+, CD4+, and CD8+ T-lymphocyte counts (Fig. 1D to F) as the absolute numbers of lymphocytes per μl of blood by the built-in software. The absolute CD4+ and CD8+ T-lymphocyte counts are calculated as the absolute counts of CD3+ T lymphocytes only and not of total lymphocytes since no summation of lymphocyte subsets or absolute lymphocytes are included in the FACSCount software. Moreover, no percentage values of CD4+ and CD8+ T lymphocytes are provided by FACSCount system.
For CyFlowgreen analysis, the single-parameter CyFlowgreen system distinguishes CD4+ T lymphocytes and monocytes as bright and dim cells, respectively, in a histogram plot (Fig. 1G). The bright CD4+ T lymphocytes were manually gated by using the histogram marker set. A dilution factor of 26.1 (obtained from the total stained sample volume of 2,610 μl/100 μl blood sample) was then set in the CyFlowgreen FloMax software to obtain an absolute CD4 count.
The histogram plot for CD8+ T lymphocytes shows only a single population of CD8 bright cells (Fig. 1H). Following the gating of CD8 bright cells by use of the histogram marker set, the absolute CD8 count was determined as described for CD4 T-lymphocyte enumeration. It should be noted that the single-parameter CyFlowgreen system used in this study does not provide the percentage of CD4+ and CD8+ T lymphocytes.
Quality control.
To ensure quality control of the flow cytometric immunophenotyping method with regard to the performance of both the personnel and the instrument, the same lots of reagents were used throughout the study. In addition, all of the immunostaining procedures and the flow cytometric analyses were performed by the same operator for each instrument. Moreover, the FCM photomultiplier tube voltage, sensitivity, and fluorescent compensation settings were optimized prior to sample acquisition and analysis by using Calibrite beads (BDB), a control set of fluorochrome-integrated beads (BDB), and CountCheck and Calibration beads (Partec) for the FACScan, FACSCount, and CyFlowgreen FCMs, respectively. Each instrument operator attended an instrument training workshop to ensure consistency of performance. Adequate training on the use of reverse pipetting technique and electronic pipette was also emphasized during the training.
Assay precision.
To assess the precision of the single-parameter CyFlowgreen system, stabilized whole-blood samples from one CD-Check Plus CD4 Low (Streck, Omaha, NE), a stable whole-blood control with assigned CD4 and CD8 T-lymphocyte values, were assayed by use of replicates of 10 for assessment of the within-run variation of the CyFlowgreen. CountCheck beads were also used periodically to monitor the accuracy of the CyFlowgreen volumetric measurement. For determination of the between-run assay variation, replicate blood samples derived from the same lot of CD-Check Plus CD4 Low were stained and analyzed over the study period. Within-run variation, between-run variation, and, when applicable, the across-instrument pooled coefficient of variation (CV) were calculated for absolute CD4+ and CD8+ T-lymphocyte counts.
Statistical analysis.
Comparison of CD4+ and CD8+ T-lymphocyte counts obtained by different methods was performed by linear regression analysis with StatView (Brainpower, Calabasas, CA). The difference between each pair of measurements was plotted against the average for the pair, as described by the Bland-Altman statistical bias method (2), in order to verify whether the results of the two methods agreed sufficiently well to be used interchangeably. To examine possible differences and the potential clinical impact of the CD4+ T-lymphocyte counts obtained by these methods, significance tests for the absolute CD4+ T-lymphocyte counts with less than 250 cells/μl and 200 cells/μl were also determined.
RESULTS
When the single-parameter SP volumetric CyFlowgreen system was monitored for within-run variation by using CD-Check Plus CD4 Low stabilized whole-blood samples, the mean CVs of CD4+ and CD8+ T-lymphocyte counts derived from 10 replicate samples were less than 4% (Table 1). The accuracies of the CyFlowgreen measurements were also excellent when they were assessed by monitoring the number of beads recovered by using CountCheck. For between-run reproducibility, the means, standard deviations, and CVs of 15 replicate measurements obtained by using the CyFlowgreen system were analyzed over the period of the study. The mean CVs of the absolute CD4+ and CD8+ T-lymphocyte counts were 7.8 and 9.2%, respectively (Table 1). Moreover, the reproducibilities of the three flow cytometric methods were also assessed by analyzing 10 HIV-1-positive blood samples in duplicate by each method. The CVs for the replicates of both CD4+ and CD8+ T-lymphocyte counts did not exceed 10%.
TABLE 1.
Within-run and between-run CVs of CD-Check Plus CD4 Low (Streck) determined by the single-parameter CyFlowgreen system
| Parameter | Within run (na = 10)
|
Between run (n = 15)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | % CV | Mean | SD | % CV | |
| CD4+ T-lymphocyte count (cells/μl) | 515 | 5.8 | 2.8 | 563 | 16.8 | 7.8 |
| CD8+ T-lymphocyte count (cells/μl) | 676 | 23.7 | 3.5 | 694 | 63.8 | 9.2 |
n, number of samples tested.
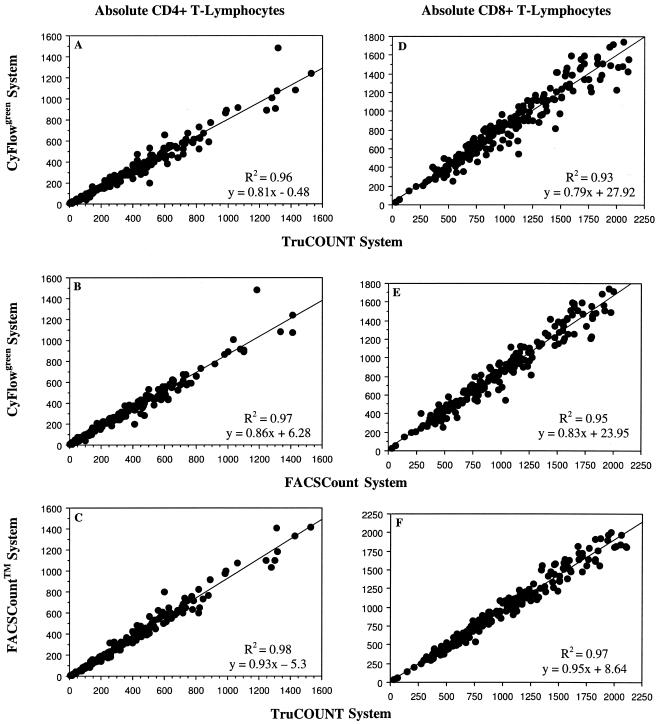
The mean absolute counts of CD4+ and CD8+ T lymphocytes for 200 HIV-1-infected patients obtained by the different flow cytometric methods (i.e., with the three-color TruCOUNT, FACSCount, and CyFlowgreen systems) are shown in Table 2. The CyFlowgreen system gave lower absolute values of both CD4+ and CD8+ T lymphocytes than the two bead-based FCM methods. The absolute CD4+ and CD8+ T-lymphocyte subsets obtained with the CyFlowgreen FCM were compared with those obtained with the two standard bead-based flow cytometric technologies (Fig. 2). Regression analysis of the CD4+ T-lymphocyte counts from 200 HIV-infected blood samples showed that the results were highly correlated: R2 = 0.96; y = −0.48 + 0.81x, and P < 0.0001 (Fig. 2A) and R2 = 0.97; y = 6.3 + 0.86x, and P < 0.0001 (Fig. 2B) when the results obtained with three-color TruCOUNT FCM and FACSCount FCM methods were compared with those obtained with the CyFlowgreen method, respectively. The correlation of CD4+ T-lymphocyte counts by the two bead-based systems was also high (R2 = 0.98; y = −5.3 + 0.93x; P < 0.001) (Fig. 2C). For absolute CD8+ T lymphocytes (Fig. 2D to F), the correlation between the two standard methods and the volumetric method was also excellent (R2 > 0.93; P < 0.001).
TABLE 2.
Absolute CD4+ and CD8+ T-lymphocyte counts in HIV-1-infected patients determined by different flow cytometric methods
| T-lymphocyte subset | Mean ± SD (range) T-lymphocyte count (cells/μl)
|
||
|---|---|---|---|
| FACScan TriTEST, TruCOUNT | FACSCount | CyFlowgreen | |
| CD4 | 360.5 ± 309.1 (0-1526) | 331.4 ± 291.7 (1-1,413) | 291.4 ± 255.4 (7-1,487) |
| CD8 | 1,000.5 ± 476.1 (29-2,110) | 958.2 ± 458.7 (32-2,000) | 814.5 ± 388.1 (26-1,740) |
FIG. 2.
Linear regression analysis of absolute CD4+ and CD8+ T-lymphocyte counts between the volumetric CyFlowgreen flow cytometric method and the two standard bead-based flow cytometric methods. Correlation plots for the CyFlowgreen system versus the three-color TruCOUNT system with FACScan (A and D), the CyFlowgreen system versus the FACSCount system (B and E), and the FACSCount system versus the TruCOUNT system (C and F) are shown.
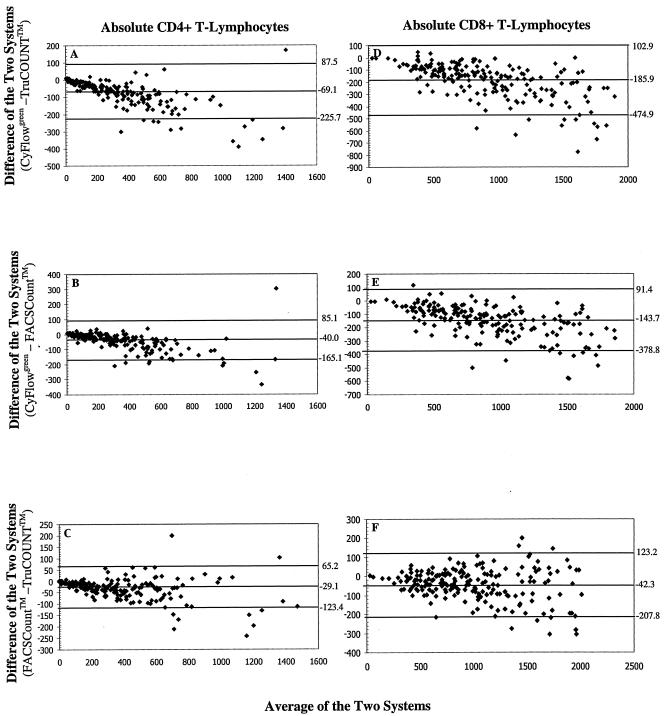
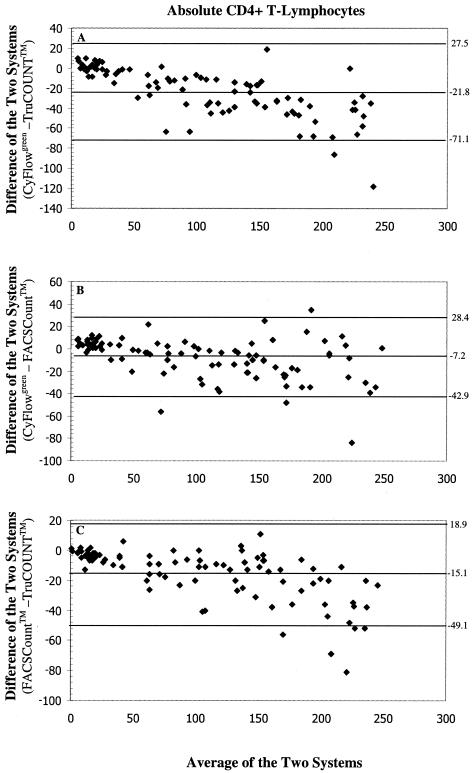
The bias plots obtained by statistical bias method of Bland and Altman (2) were analyzed by plotting the difference in the absolute lymphocyte subset values obtained between the CyFlowgreen system and the standard bead-based flow cytometric method against the mean absolute values of the two methods (Fig. 3). As the mean CD4+ T-lymphocyte counts obtained by the CyFlowgreen FCM and the bead-based methods increased, there tended to be an increase in the bias, with mean biases of −69.1 cells/μl (limits of agreement [LOAs], −225.7 cells/μl to +87.5 cells/μl) and −40.0 cells/μl (LOAs, −165.1 cells/μl to +85.1 cells/μl) when the CyFlowgreen system was compared with the TruCOUNT and FACSCount systems, respectively (Fig. 3A and B). These results indicate that the volumetric CyFlowgreen system yielded lower CD4+ T-lymphocyte counts than the two bead-based TruCOUNT and FACSCount methods. This deviation toward lower CyFlowgreen system results with higher CD4+ T-lymphocyte counts was also reflected in the CD8+ T-lymphocyte counts (Fig. 3D and E). For the two bead-based systems, the FACSCount system yielded lower CD4+ T-lymphocyte counts than the TruCOUNT FCM, in which the mean biases of CD4+ (Fig. 3C) and CD8+ T-lymphocyte counts (Fig. 3F) were −29.1 cells/μl (LOAs, −123.4 cells/μl to +65.2 cells/μl) and +35.7 cells/μl (LOAs, −96.4 cells/μl to 167.8 cells/μl), respectively. With CD4+ T-lymphocyte counts in the range of <250 cells/μl, there was also a bias for lower values from the CyFlowgreen FCM compared to those from the TruCOUNT and FACSCount systems. The CyFlowgreenFCM system yielded biases −21.8 cells/μl (LOAs, −71.1 cells/μl to +27.5 cells/μl; n = 92) and −7.2 cells/μl (LOAs, −42.9 cells/μl to +28.4 cells/μl; n = 96) lower than the CD4+ T-lymphocyte counts obtained with the TruCOUNT and FACSCount systems, respectively (Fig. 4A and B). Again, a lower mean CD4+ T-lymphocyte count of −15.1 cells/μl (LOAs, −49.1 cells/μl to +18.9 cells/μl) was obtained with the FACSCount system compared to the values obtained with the TruCOUNT system (Fig. 4C). When CD4+ T-lymphocyte counts <200 cells/μl were tested, there were 17.3 cells/μl (LOAs, −57.9 cells/μl to +23.3 cells/μl; n = 81) and 5.8 cells/μl (LOAs, −37.6 cells/μl to +25.9 cells/μl; n = 85) less from the CyFlowgreen system than from the two predicate systems, respectively.
FIG. 3.
Bland-Altman (2) bias plots of absolute CD4+ and CD8+ T-lymphocyte counts between the volumetric CyFlowgreen flow cytometric method and the two standard bead-based flow cytometric methods. The differences between the CyFlowgreen system and the three-color TruCOUNT with FACScan system (A and D), the CyFlowgreen system and the FACSCount system (B and E), and the FACSCount system and the TruCOUNT system (C and F) are shown. The horizontal line in the center indicates the mean bias of the two methods. The lower and upper limits of agreement are indicated by the lower and the upper horizontal lines, respectively.
FIG. 4.
Bland-Altman (2) bias plots of absolute CD4+ T-lymphocyte counts of <250 cells/μl between the volumetric CyFlowgreen flow cytometric method and the two standard bead-based flow cytometric methods. The differences between the CyFlowgreen system and the three-color TruCOUNT with FACScan system (A), the CyFlowgreen system and the FACSCount system (B), and the FACSCount system and the TruCOUNT system (C) are shown. The horizontal line in the center indicates mean bias of the two methods. The lower and upper limits of agreement are indicated by lower and upper horizontal lines, respectively.
DISCUSSION
In Thailand, the DP FCM method is expensive in terms of its initial cost and maintenance and requires highly trained personnel. Thus, it is unsuitable for routine use in most laboratories with limited facilities, such as those in district hospitals. An ideal FCM method in a resource-poor setting would be an SP assay that is simple to operate and that uses an inexpensive instrument and the minimum number of monoclonal antibodies that can reliably identify CD4+ T-lymphocytes without sacrificing accuracy and precision. In this study we chose to evaluate the new SP CyFlowgreen FCM, which employs only 1 PE-conjugated monoclonal antibody to CD4, in our search for a cost-effective CD4 testing method that will suit the local situation.
In this CyFlowgreen system, a simple gating strategy with only one green laser and one PE photomultiplier tube is used for CD4+ T-lymphocyte counting. Our aim was to evaluate this new device using one monoclonal antibody reagent against the standard MultiSet TriTEST reagents by using the FACScan and FACSCount systems currently used in Thailand. The identification of CD4+ and CD8+ T lymphocytes on the basis of one-parameter CD4 expression is not novel. Several studies (9, 14, 16, 24) used the concept of primary CD4 gating by reliably identifying CD4+ or CD8+ T lymphocytes by the high level of CD4 or CD8 expression. In one study (16), a series of more than 600 blood samples from both healthy and HIV-infected patients analyzed with the CytoronAbsolute system by primary CD4 gating with a single CD4 monoclonal antibody yielded absolute CD4 counts that were almost identical to those generated by a standard three-tube protocol with nine monoclonal antibodies (r2 = 0.999, with a minimal bias of +4 CD4+ cells/μl).When this primary CD4 gating was further compared with the three-color (CD3/CD4/CD45, CD3/CD8/CD45) and the four-color (CD3/CD8/CD45/CD4) full technologies with different instruments, the mean bias ranged from −2 to +13 CD4 cells/μl. Such a close agreement of the results between the approach with the full antibody set and the approach with the single monoclonal antibody indicated that markers in addition to CD4 failed to influence the CD4 counts (18). Arguably, CD3, the specific pan-T-cell marker, is also not required to identify CD4+ T lymphocytes (17, 20). A quality control measure recommended by CDC, but not included in this CD4 strategy, is the use of isotype controls. However, cursors can be set without the use of isotype controls, since discrimination from non-CD4+ T lymphocytes and of dim CD4 monocytes and dendritic cells can be achieved readily due to the bright expression of CD4 on CD4+ T lymphocytes (14, 17, 20).
In this study, the absolute CD4+ and CD8+ T-lymphocyte values obtained from the CyFlowgreen single-monoclonal-antibody-staining method correlated highly (R2 > 0.95) with those from standard bead-based three-color TruCOUNT with FACScan and two-color FACSCount FCMs. The overall biases for absolute CD4+ values were −69.1 and −40.0 cells/μl when the results obtained with the single-parameter volumetric CyFlowgreen method were compared to those obtained with the standard TruCOUNT and FACSCount systems, respectively. If one considers HIV-infected individuals who need antiretroviral drug therapy because their absolute CD4+ T-lymphocyte counts are less than 250 cells/μl, according to the Thai national antiretroviral therapy program, CD4+ T-lymphocyte counts were −21.8 and −7.2 cells/μl less from the CyFlowgreen system than the two predicate systems, respectively (Fig. 4). These biases will result in CD4+ T-lymphocyte counts that are only 9% and 3% less, respectively, when the CyFlowgreen system is used and 7% and 2% less absolute CD4+ T-lymphocyte counts, respectively, for <200 cells/μl when the CyFlowgreen system is used. Interestingly, the absolute CD4+ and CD8+ T-lymphocyte counts obtained with the CyFlowgreen system were lower than those derived from the two bead-based standard methods. In addition, underestimation of absolute CD8+ T-lymphocyte values was greater than underestimation of CD4 values when the CyFlowgreen data were compared to the data obtained by the other two methods. Previous studies have indicated that two other instruments (DAKO Galaxy and Partec PAS), which are similar in design to the CyFlowgreen system family, regularly underestimated CD4 counts compared to the counts obtained with other FCMs (18). Recent studies also demonstrated that lower CD4+ T-lymphocyte counts were obtained by both the two-parameter and the three-parameter SP CyFlowgreen system compared with those obtained with the FACSCount and TruCOUNT-FACScan systems (10, 26). It is possible that this tendency to provide lower CD4 counts may be a reproducible characteristic of the CyFlow system technology.
The CyFlowgreen method of absolute CD4 or CD8 enumeration performed by a simplified single-color technique is based on primary immunological gating, a heterogeneous gating technique for positivity with a single antibody, and side scatter (SSC) (16). In primary CD4 gating, the CD4+ T lymphocytes (CD4+ and SSC positive) are discriminated from monocytes (strongly CD4+ and SSC positive) and CD4-negative lymphocytes. In CD8 gating for T-lymphocytes, only the strongly CD8+ T lymphocytes are counted. The enumeration of CD8+ T lymphocytes with minimal technology, however, has been less accurate than that of CD4+ T lymphocytes. In a study of 101 adult HIV-positive patients, primary CD8 gating underestimated the absolute CD8 counts by 5.2% compared to those obtained by use of the full technology of CD3+ CD8+ staining (17). In addition, the CyFlowgreen under evaluation in this study uses only one parameter (i.e., FL2 for PE detection) to distinguish the CD4+ or CD8+ T lymphocytes, and it is possible that this less discriminatory approach may have contributed to the greater degree of CD8 underestimation in comparison with the degree of CD4 underestimation when the CyFlowgreen system was compared to the bead-based standard method. The differences in absolute CD4+ T-lymphocyte counts between the two systems may also be due to many different factors. The reference beads used in the two standard FCM systems are preloaded fluorescent microspheres that are strictly prepared and controlled by the manufacturer; this should not allow any technical error except that which may occur in the one pipetting step in the FACSCount system. However, microbeads tend to sediment with time and tend to form aggregates, which in turn may decrease the number of bead events to be used for counting (4, 20) and which could eventually lead to increases in the CD4+ T-lymphocyte counts. The volumetric FCM, on the other hand, gives absolute CD4+ T-lymphocyte counts by defining the absolute CD4+ T-lymphocyte counts in a known final volume. This system requires a high level of dispensing precision in all three pipetting steps, and the final dilution factor must be strictly controlled (3, 4). Moreover, a lack of precision of the volume taken by triggering the two electrodes and the limited sample volume may well result in underestimation of the values of CD4+ T-lymphocyte counts, particularly in HIV-infected patients with lymphopenia and very low CD4+ T-lymphocyte counts.
In this study the bead-based TruCOUNT method showed higher CD4+ T-lymphocyte values compared with those obtained by the CyFlowgreen and FACSCount systems. A similar bias was also observed when the TruCount bead-based system was compared with the DP system (21) and the volumetric CytoronAbsolute system (15), indicating that this tendency of the TruCOUNT system to provide higher CD4 counts than other methods may be a reproducible characteristic of the TruCOUNT bead-based technology.
This study demonstrates that the volumetric CyFlowgreen FCM system is a valid method of CD4 enumeration. Although the evaluation suggests that the method seems to be promising compared with the predicate bead-based FCM methods for determination of absolute CD4+ T lymphocyte counts, the operation of CyFlowgreen still requires substantial technical expertise, i.e., differentiation of monocytes expressing CD4 antigen and CD4+ T lymphocytes. However, this device is compact and can easily be moved from a central laboratory to a remote laboratory. Most importantly, the use of this CyFlowgreen device together with the use of one monoclonal antibody can reduce the cost of CD4 testing from $10 to 12 per test to $4 to 5 per test. The price quoted refers to the cost per reportable result and includes consumables, transport, staff, and overhead, such as electricity.
The use of antiretroviral drugs has dramatically reduced the rates of mortality and morbidity and has thus increased the life expectancies of AIDS patients. Since Thailand has already declared that 50,000 HIV-infected patients will have access to antiretroviral drug therapy program by the year 2005 (27), it is foreseeable that more and more CD4 testing will be required to cope with such treatment demand, i.e., between 150,000 and 200,000 CD4 tests per year. Nevertheless, since this evaluation study was performed in a reference laboratory that fulfills all required flow cytometry standards, a multicenter validation study should be initiated if this system is to be widely implemented in resource-limited settings.
Acknowledgments
This study was supported by the Global AIDS Program, Centers for Disease Control and Prevention, contract grants DOA02 and NIH02.
We thank Hla Shain for valuable comments.
REFERENCES
- 1.Barnett, D., V. Granger, L. Whitby, I. Storie, and J. T. Reilly. 1999. Absolute CD4+ T lymphocyte and CD34+ stem cell counts by single platform flow cytometry: the way forward. Br. J. Haematol. 106:1059-1062. [DOI] [PubMed] [Google Scholar]
- 2.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 3.Brando, B., D. Barnett, G. Janossy, F. Mandy, B. Autran, G. Rothe, B. Scarpati, G. D'Avanzo, J. L. D'Hautcourt, R. Lenkei, G. Schmitz, A. Kunkl, R. Chianese, S. Papa, and J. W. Gratama for the European Working Party on Clinical Cell Analysis (EWGCCA). 2000. Cytofluorometric methods for assessing absolute numbers of cells in blood. Cytometry 42:327-346. [DOI] [PubMed] [Google Scholar]
- 4.Brando, B., W. Göhde, Jr., B. Scarpati, and G. D'Avanzo. 2001. The “vanishing counting bead” phenomenon: effect on absolute CD34+ cell counting in phosphate-buffered saline-diluted leukapheresis samples. Cytometry 43:154-160. [DOI] [PubMed] [Google Scholar]
- 5.Carella, A. V., M. W. Moss, V. Provost, and T. C. Quinn. 1995. A manual bead assay for the determination of absolute CD4+ and CD8+ lymphocyte counts in human immunodeficiency virus-infected individuals. Clin. Diagn. Lab. Immunol. 2:623-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV). Morbid. Mortal. Wkly. Rep. 46(No. RR-2):1-29. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2003. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. Morbid. Mortal. Wkly. Rep. 52(No. RR02):1-13. [PubMed] [Google Scholar]
- 8.Connelly, M. C., M. Knight, J. V. Giorgi, J. Kagan, A. L. Landay, J. W. Parker, E. Page, C. Spino, C. Wilkening, and T. J. Mercolino. 1995. Standardisation of absolute CD4+ lymphocyte count across laboratories: an evaluation of the Ortho CytoronAbsolute flow cytometry system on normal donors. Cytometry 22:200-210. [DOI] [PubMed] [Google Scholar]
- 9.Dietz, L. J., R. S. Dubrow, B. S. Manian, and N. L. Sizto. 1996. Volumetric capillary cytometry: a new method for absolute cell enumeration. Cytometry 37:29-37. [DOI] [PubMed] [Google Scholar]
- 10.Dieye, T. N., C. Verrecken, A. A. Diallo, P. Ordoa, P. A. Diaw, Camara, M., F. Karam, S. Mboup, and L. Kestens. 2005. Absolute CD4 T-cell counting in resource-poor settings. Direct volumetric measurements versus bead-based clinical flow cytometry instruments. J. Acquir. Immune Defic. Syndr. 39:32-37. [DOI] [PubMed] [Google Scholar]
- 11.Gelman, R., S. U. Cheng, P. Kidd, M. Waxdal, and J. Kagan. 1993. Assessment of the effects of instrumentation, monoclonal antibody and fluorochrome on flow cytometric immunophenotyping: a report based on 2 years of the NIAID DAIDS flow cytometry quality assessment program. Clin. Immunol. Immunopathol. 66:150-162. [DOI] [PubMed] [Google Scholar]
- 12.Gernow, A., I. M. Lisse, B. Bottiger, L. Christensen, and K. Brattegaard. 1995. Determination of CD4+ and CD8+ lymphocytes with the cytosphere assay: a comparative study with flow cytometry and the immunoalkaline phosphatase method. Clin. Immunol. Immunopathol. 76:135-141. [DOI] [PubMed] [Google Scholar]
- 13.Glencross, D., L. Scott, S. Sonday, H. Aggett, and S. Scott. 1999. Microvolume fluorimetry for the determination of absolute CD4+ and CD8+ lymphocyte counts in patients with HIV: a comparative evaluation. Clin. Lab. Haematol. 21:391-395. [DOI] [PubMed] [Google Scholar]
- 14.Greve, B., U. Cassens, C. Westerberg, W. Göhde, Jr., W. Sibrowski, D. Reichelt, and W. Göhde. 2003. A new no-lyse, no-wash flow-cytometric method for the determination of CD4 T cells in blood samples. Transfus. Med. Hemother. 30:8-13. [Google Scholar]
- 15.Jani, I. V., G. Janossy, A. Iqbal, F. S. Mhalu, E. F. Lyamuya, G. Biberfeld, D. K. Glencross, L. Scott, J. T. Reilly, V. Granger, and D. Barnett. 2001. Affordable CD4+ T cell counts by flow cytometry. II. The use of fixed whole blood in resource-poor settings. J. Immunol. Methods 257:145-154. [DOI] [PubMed] [Google Scholar]
- 16.Janossy, G., I. Jani, and W. Göhde. 2000. Affordable CD4+ T-cell counts on ‘single-platform’ flow cytometers I. Primary CD4 gating. Br. J. Haematol. 111:1198-1208. [DOI] [PubMed] [Google Scholar]
- 17.Janossy, G., I. V. Jani, N. Bradley, A. S. Bikoue, T. Pittfield, and D. K. Glencross. 2002. Affordable CD4+ T cell counts by flow cytometry: CD45 gating for volumetric analysis. Clin. Diagn. Lab. Immunol. 9:1085-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janossy, G., I. V. Jani, and B. Brando. 2003. New trends in affordable CD4+ T-cell enumeration by flow cytometry in HIV/AIDS. Clin. Appl. Immunol. Rev. 4:91-107. [Google Scholar]
- 19.Malone, J. L., T. E. Simms, G. C. Gray, K. F. Wagner, J. R. Burge, and D. S. Burke. 1990. Sources of variability in repeated T-helper lymphocyte counts from human immunodeficiency virus type 1 infected patients: total lymphocyte count fluctuation and diurnal cycle are important. J. Acquir. Immune Defic. Syndr. 3:144-151. [PubMed] [Google Scholar]
- 20.Mandy, F., and B. Brando. 2000. Enumeration of absolute counts using immunophenotypic techniques, p. 6.8.1-6.8. 26. In J. P. Robinson, Z. Darzynkiewicz, P. N. Dean, et al. (ed.), Current protocols in cytometry. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 21.Nicholson, J. A., D. Stein, T. Mui, R. Mack, M. Hubbard, and T. Denny. 1997. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin. Diagn. Lab. Immunol. 4:309-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson, J. K., W. M. Velleca, S. Jubert, T. A. Green, and L. Bryan. 1994. Evaluation of alternative CD4 technologies for the enumeration of CD4 lymphocytes. J. Immunol. Methods 177:43-54. [DOI] [PubMed] [Google Scholar]
- 23.Robinson, G., L. Morgan, M. Evans, S. McDermott, S. Pereira, M. Wansborough-Jones, and G. Griffen. 1992. The effect of type of hematology analyzer on CD4 count. Lancet 340:485. [DOI] [PubMed] [Google Scholar]
- 24.Sherman, G. G., J. S. Galpin, J. M. Patel, B. V. Mendelow, and D. K. Glencross. 1999. CD4+ T cell enumeration in HIV infection with limited resources. J. Immunol. Methods 222:209-217. [DOI] [PubMed] [Google Scholar]
- 25.Strauss, K., I. Hannet, S. Engels, A. Shiba, D. M. Ward, S. Ulley, G. Jinguji, J. Valinsky, D. Barnett, A. Orfao, and L. Kestens. 1996. Performance evaluation of the FACSCount system: a dedicated system for clinical cellular analysis. Cytometry 26:52-59. [DOI] [PubMed] [Google Scholar]
- 26.Teav, S., L. Lynen, C. Vereecken, P. De Munter, C. Srey, and L. Kestens. 2004. Alternative CD4 counting using Cyflow in Cambodia: precision and comparison with Facscount. Proc. XV Int. AIDS Conf. http://www.iasociety.org/abstract/show.asp?abstract id=2170843. [Online.]
- 27.World Health Organization. 2005. http://www.who.int/3by5/publication/documents/en/3by5StategyMakingItHappen.pdf. [Online.] Accessed March 18, 2005.