Abstract
Previous studies have indicated that neopterin is synthesized in vitro by human monocyte-derived macrophages and dendritic cells upon stimulation with gamma interferon (IFN-γ). Neopterin production under specific conditions in vitro has also been obtained upon stimulation with IFN-α and/or IFN-β. However, it is unknown if any IFN-γ-independent neopterin synthesis is possible in vivo. In the present study we investigated the serum neopterin concentrations in patients affected by the syndrome of Mendelian susceptibility to mycobacterial disease (MSMD). Indeed, this syndrome is characterized by deeply impaired or absent IFN-γ production or function due to severe mutations in molecules involved in IFN-γ/interleukin-12 (IL-12)/IL-23-dependent pathway. Serum neopterin levels were measured by an enzyme-linked immunosorbent assay in 27 patients with MSMD. We found that serum neopterin levels are elevated in the complete absence of IFN-γ activity due either to a complete deficiency of its receptor or to deleterious mutations of IL-12 or its receptor. These data clearly indicate that, as reported from in vitro studies, other stimuli are able to induce neopterin synthesis in vivo. Consequently, neopterin cannot be used as means of diagnosis of MSMD due to IFN-γ-, IL-12-, and IL-23-dependent pathway defects.
2-Amino-4-oxo-6-erythro-1,2,3 trihydroxy-propyl-pteridine (neopterin) is a molecule derived from GTP by GTP cyclohydroxylase I. Detectable amounts of neopterin can be found in various body fluids, such as serum, urine, and cerebrospinal fluid (15, 30, 32). Neopterin is considered an excellent marker of cellular immune activation. Increased concentrations of serum neopterin were reported in patients with viral (3), bacterial (13), and protozoal (28) infections. Increased neopterin concentrations are observed in the majority of patients before seroconversion becomes detectable. In Austria, blood donations have been screened for elevated neopterin concentrations since 1994, and those with elevated neopterin concentrations are excluded from transfusion (12). Neopterin levels also rise in patients with autoimmune diseases (35), transplant rejection (22), and malignant diseases (23). It was shown previously that human macrophages and dendritic cells release neopterin into culture supernatants and that gamma interferon (IFN-γ) is the major factor inducing this phenomenon (18).
To evaluate the in vivo effect of IFN-γ in the synthesis of neopterin, we evaluated the serum neopterin concentrations of patients with the syndrome of Mendelian susceptibility to mycobacterial disease (MSMD). The latter is a heterogeneous group of rare inherited immune deficiencies characterized by recurrent and disseminated infections caused by weakly virulent mycobacterial species (5, 6). Some patients with MSMD are also susceptible to virulent Mycobacterium tuberculosis. The following mutations have been identified in a score of patients with MSMD: in the IFN-γ receptor ligand-binding chain (IFN-γR1) (19, 20, 21, 26); the IFN-γ signaling chain (IFN-γR2) (7, 8); the interleukin-12 (IL-12) p40 subunit, which is shared by IL-23 (2, 11, 27); the IL-12Rβ1 chain, which is shared by the IL-23 receptor (1); or signal transducer and activator of transcription 1 (STAT1) (9, 10). These mutations offer a very interesting condition characterized by a deeply impaired IFN-γ-dependent activation of macrophages. Complete IFN-γR1 deficiency (cIFN-γR1D) and complete IFN-γR2 deficiency (cIFN-γR2D) are characterized by a completely abolished cell response to IFN-γ. They are associated with early-onset infections caused by bacillus Calmette-Guérin (BCG) vaccines and/or environmental mycobacteria, such as Mycobacterium avium and Mycobacterium smegmatis; poorly delimited and differentiated tissue granulomas; and fatal outcomes in childhood. Partial IFN-γR1 deficiency (pIFN-γR1D) and partial IFN-γR2 deficiency (pIFN-γR2D), like partial STAT1 deficiency, are characterized by an impaired response to IFN-γ. They are associated with late-onset infections, well-delimited and differentiated granulomas, and a much better prognosis. IL-12 is a 75-kDa heterodimeric cytokine produced by dendritic cells and activated macrophages, which play a major role in the IFN-γ production by Τh1 lymphocytes. IL-12 binds to its high-affinity receptor, composed of two chains, IL-12Rβ1 and IL-12Rβ2. Patients with mutations in IL-12p40- and IL-12Rβ1-encoding genes experience severe and disseminated infections caused by poorly pathogenic mycobacteria and, occasionally, Salmonella infections.
In this study, we investigated neopterin production in these patients with absent or severely impaired IFN-γ production.
MATERIALS AND METHODS
We evaluated 27 patients with MSMD. Twenty-four patients received the diagnosis at the Laboratory of Human Genetics of Infectious Diseases at René Descartes University in Paris, France, and 3 received the diagnosis at the Laboratory of Immunology of the Institut Pasteur, Tunis, Tunisia. Among these MSMD patients, 14 were males and 13 females, with their ages ranging from 6 months to 33 years (median age, 7 years). We also investigated as controls four non-MSMD patients (one male and three females; median age, 10.5 years) diagnosed with chronic granulomatous disease (CGD), an inherited immunodeficiency characterized by the absence of NADPH oxidase activity and a susceptibility to weakly virulent mycobacteria. Here, they are considered severely infected controls with an intact IL-12 and IFN-γ circuit.
Immunological investigations. (i) IFN-γR1, IFN-γR2, and IL-12Rβ1 cell surface expression.
For IFN-γR1 and IFN-γR2 expression studies, peripheral blood mononuclear cells (PBMCs) were stained with monoclonal anti-human IFN-γR1 (1223-01; Genzyme) or IFN-γR2 (C.11) antibodies or isotype-matched control antibodies. For the IL-12Rβ1 expression study, PBMCs activated with phytohemmaglutinin for 3 days were stained with monoclonal anti-human IL-12Rβ1 antibody (mouse immunoglobulin G1 [IgG1; 204E6; Pharmingen]) or an isotype-matched control antibody. Antibodies bound to IFN-γR1 or IL-12Rβ1 were detected with goat anti-mouse IgG antibody labeled with fluorescein isothiocyanate (Immunotech), and the cells were analyzed with a FACSVantage flow cytometer. Antibodies bound to IFN-γR2 were detected with fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (Dako, Carpinteria, CA).
Cytokine detection by ELISA.
To assess IL-12p40 production, PBMCs from patients were activated for 24 h with Staphylococcus aureus Cowan I (Pansorbin; Calbiochem) diluted 1:10,000 in culture medium. Cell-free supernatants were analyzed by a commercially available quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique (Quantikine ELISA kit; R&D Systems).
To quantify IFN-γ production by PBMCs, 106 cells were activated with phytohemmaglutinin for 72 h, and the culture supernatants were analyzed by use of an ELISA kit (R&D Systems). Each test was performed in duplicate. The absorbance was measured at 450 nm, and the cytokine concentration was determined.
Genetic studies.
Genetic studies were done as described elsewhere (1, 7, 11). The genetic defect was cIFN-γR1D in six patients, pIFN-γR1D in five patients, cIFN-γR2D in four patients, and pIFN-γR2D in one patient. IL-12p40D was detected in four patients, and IL-12Rβ1D was detected in seven other patients.
Serum neopterin measurement.
The serum neopterin concentration was measured by a solid-phase competitive enzyme-linked immunosorbent assay (IBL, Hamburg, Germany). The neopterin present in the sample and a fixed amount of peroxidase-labeled neopterin compete for a rabbit anti-neopterin antibody. The neopterin-rabbit anti-neopterin antibody complexes bind to the wells of the microtiter strips, which are coated with a goat-anti-rabbit antibody. Unbound neopterin is removed by washing. The intensity of the color that develops after substrate incubation is inversely proportional to the amount of neopterin in the sample.
Sera were stored at −80°C until use. All sera were tested in duplicate, and the average results are presented. During all concentration determination procedures, sample exposure to daylight was avoided since it is known that neopterin is susceptible to degradation when it is exposed to direct sunlight. A serum neopterin concentration >10 nmol/liter was considered elevated (30). The assay detection limit was 0.2 nmol/liter.
Statistical analysis was performed by use of the Mann-Whitney test. A P value <0.05 was considered significant.
RESULTS
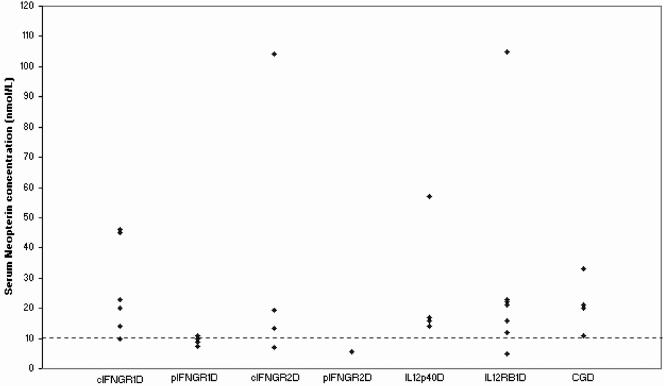
The ages of the MSMD patients at the time of sampling and their clinical manifestations are detailed in Table 1. Compared to the healthy individuals, the serum neopterin concentrations were elevated in all patients (six of six) with cIFN-γR1D, three of four patients with cIFN-γR2D, all patients (four of four) with IL-12p40, and six of seven patients with IL-12Rβ1D (Fig. 1). The neopterin concentrations in these patients varied from 12 to 105 nmol/liter. The highest concentrations were found in a child with IL-12Rβ1D (patient 22) and in a child with cIFN-γR2D (patient 15) and were 105 and 104 nmol/liter, respectively.
TABLE 1.
Clinical, biological, and genetic characteristics of MSMD patients
| MSMD patient no. | Deficiency | Mutation | Age at time of neopterin measurement (yr) | Serum neopterin concn (nmol/liter) | Infection due to: |
|---|---|---|---|---|---|
| MSMD 1 | cIFN-γR1D | 105insT | 8 | 14 | M. avium, M. tuberculosis, Salmonella enterica serovar Enteritidis |
| MSMD 2 | cIFN-γR1D | 295del 12 | 7 | 45 | M. avium |
| MSMD 3 | cIFN-γR1D | 523del T | 9 | 23 | M. peregrinum |
| MSMD 4 | cIFN-γR1D | 230G to A | 11 | 46 | BCG, M. fortuitum |
| MSMD 5 | cIFN-γR1D | 523del T | 5 | 20 | BCG |
| MSMD 6 | cIFN-γR1D | 453del T | 2 | 10 | Nonidentified atypical mycobacteria |
| MSMD 7 | pIFN-γR1D | 818del 4 | 14 | 7.4 | BCG, M. avium, M. kansasii |
| MSMD 8 | pIFN-γR1D | 818del 4 | 20 | 7.4 | BCG |
| MSMD 9 | pIFN-γR1D | 818del 4 | 13 | 8.8 | M. tuberculosis |
| MSMD 10 | pIFN-γR1D | 818del 4 | 8 | 10 | BCG |
| MSMD 11 | pIFN-γR1D | 818del 4 | 33 | 11 | BCG, VZV |
| MSMD 12 | cIFN-γR2D | T168 N | 1 | 19.5 | BCG |
| MSMD 13 | cIFN-γR2D | 663del 27 | 6 | 13.5 | M. avium |
| MSMD 14 | cIFN-γR2D | T168 N | 6 | 7 | BCG, M. fortuitum, VZV |
| MSMD 15 | cIFN-γR2D | 382_387dup | 3 | 104 | M. avium |
| MSMD 16 | pIFN-γR2D | R114C | 17 | 5.3 | BCG, M. abscessus |
| MSMD 17 | IL-12p40D | g.697+5G>A | 6 | 14 | BCG, M. tuberculosis |
| MSMD 18 | IL-12p40D | g.482+82_856-854del | 5 | 17 | BCG, S. enterica serovar Enteritidis |
| MSMD 19 | IL-12p40D | G315_316insA | 5 | 16 | M. tuberculosis, S. enterica serovar Enteritidis |
| MSMD 20 | IL-12p40D | 295del 8 | 0.5 | 57 | BCG |
| MSMD 21 | IL-12Rβ1D | 557_563delins8 | 11 | 22 | BCG, S. enterica serovar Enteritidis |
| MSMD 22 | IL-12Rβ1D | R173P | 7 | 105 | M. chelonae |
| MSMD 23 | IL-12Rβ1D | 783+IG>A | 10 | 5 | BCG |
| MSMD 24 | IL-12Rβ1D | 1745_1746ins CA+1483+ 182-1619-1073del | 25 | 16 | BCG, S. enterica serovar Dublin |
| MSMD 25 | IL-12Rβ1D | G467_484del | 4 | 12 | BCG |
| MSMD 26 | IL-12Rβ1D | 550-2A to G | 6 | 21 | BCG |
| MSMD 27 | IL-12Rβ1D | 64+5G to A | 1 | 23 | BCG |
FIG. 1.
Serum neopterin concentrations in different subgroups of MSMD patients. cIFNGR1D, complete IFN-γR1 deficiency; pIFNGR1D, partial IFN-γR1 deficiency; cIFNGR2, complete IFN-γR2 deficiency; pIFNGR2D, partial IFN-γR2 deficiency; IL12p40D, IL-12p40 deficiency; IL12Rβ1D, IL-12Rβ1 deficiency. Each dot represents an individual serum neopterin concentration quantified in duplicate by ELISA. The threshold of 10 nmol/liter (the discontinuous line) corresponds to the highest value within the normal range in healthy individuals. The serum neopterin concentration was also measured in patients with CGD, who were considered infected controls with an intact IL-12-IFN-γ circuit.
In patients affected by partial deficiencies, either pIFN-γR1D or pIFN-γR2D (pIFN-γRD), the neopterin concentration was normal in all patients but one, who had a very slightly increased level.
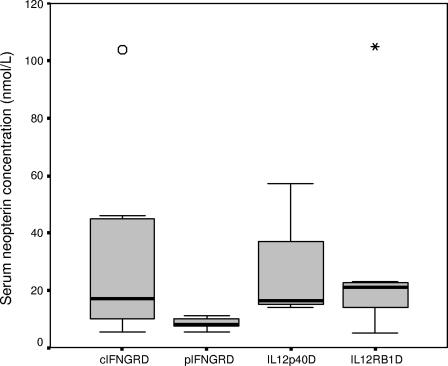
The neopterin level was increased in patients with cIFN-γR1D and cIFN-γR2D (cIFN-γRD), with a mean value of 30.2 nmol/liter, and healthy patients with pIFN-γR1D and pIFN-γR2D, with a mean value of 8.32 nmol/liter (P = 0.011) (Fig. 2). The mean neopterin value was high both in patients with IL-12p40D and patients with IL-12Rβ1D (26 and 29 nmol/liter, respectively).
FIG. 2.
Mean serum neopterin concentrations in patients with MSMD. The mean serum neopterin concentrations in patients with complete IFN-γ receptors deficiencies (cIFNGRD), IL-12p40D, and IL-12Rβ1D were elevated, while they were normal in patients with partial IFN-γ receptors deficiencies (pIFNGRD) (Mann-Whitney test, P = 0.011).
We also investigated four non-MSMD patients with CGD. They were considered severely infected “wild-type” controls. Neopterin levels in this control group were elevated compared to those in healthy individuals (mean value, 21.25 nmol/liter). These levels were lower than those obtained in cIFN-γRD patients (mean value, 30.2 nmol/liter), but this difference was not statistically significant (P = 0.514).
DISCUSSION
Monitoring of neopterin in body fluids is considered a sensitive way to detect Th1-type immune responses (14). Moreover, neopterin enhances the oxidative potential of the reactive oxygen species (ROS) produced by immunocompetent cells. It was demonstrated that the amounts of neopterin produced by activated macrophages correlate with their capacity to produce ROS (17). Schobersberger et al. demonstrated that incubation of vascular smooth muscle cells (VSMCs) with neopterin promotes nitric oxide synthase gene expression and nitric oxide release (29). In addition, neopterin was found to increase intracellular Ca2+, indicating a direct role in the production of ROS and NO (37). Furthermore, neopterin was found to amplify the secretion of tumor necrosis factor alpha (TNF-α) by peripheral blood mononuclear cells induced by lipopolysaccharide (LPS), IFN-γ, and interleukin-2 (4) and to stimulate TNF-α gene expression as well as TNF-α release and nuclear uptake of NF-κB in VSMCs (16).
Previous investigations demonstrated that antigenic stimulation of human peripheral blood mononuclear cells leads to neopterin release into cell culture medium. Macrophages were identified as the major type of human cell that produces neopterin when they are stimulated in vitro with IFN-γ (18). Other cells like dendritic cells and other cell lines, including endothelial and kidney epithelial cells, fibroblasts, and VSMCs, secrete neopterin upon stimulation with IFN-γ (24, 31, 34, 36).
To evaluate the in vivo role of IFN-γ in the production of neopterin, we measured the serum neopterin concentrations in patients with MSMD, who offer a unique human model in whom the IFN-γ and IL-12 axis is deeply impaired and in whom the production or function of IFN-γ is absent. It represents a rare condition in which macrophage infection by mycobacteria occurs in the absence of IFN-γ.
We found that neopterin levels are increased in patients with cIFN-γR1D, cIFN-γR2D, IL-12p40D, and IL-12Rβ1D. All these patients experienced early and severe mycobacterial infections, Salmonella infections, and, in some cases, viral infections (varicella-zoster virus [VZV] infection). These data clearly demonstrate that the levels of serum neopterin are increased in vivo, including in patients in whom no effect of IFN-γ is possible due to the complete absence of expression of its receptor. Huber et al. (18) added various interferons to macrophages in culture and demonstrated that IFN-γ is the most potent in vitro inducer of neopterin release, whereas an approximately 1,000 times higher concentration of IFN-α was required for the same effect. In contrast, IFN-β was unable to induce neopterin secretion (18). Furthermore monoclonal antibodies against IFN-γ but not against IFN-α were able to completely neutralize the induction of neopterin release, which could be overcome by the readdition of IFN-γ. Zymosan, phorbol myristate acetate, or granulocyte-monocyte colony-stimulating factor could not induce neopterin release (25).
IFN-γ was shown to exhibit the strongest effect on neopterin release in the human monocytic cell line THP-1. At very much higher concentrations, IFN-α and IFN-β were shown to exert the same effect. In contrast, IFN-α and IFN-β show the same capacity to induce neopterin in human monocyte-derived dendritic cells (36). TNF-α is unable to induce neopterin when it was added alone, but it amplifies the action of IFN-γ on cultured monocyte cell lines. Furthermore, Werner-Felmayer et al. (33) tested the effects of numerous bacterial pyrogens, like LPS, lipid A, lipoteichoic acid, toxic shock syndrome toxin 1, and cell wall compounds from Mycobacterium tuberculosis, on the formation of neopterin in THP-1 cell lines. The whole LPS molecule, when added at a high dose only, potently stimulated neopterin when it was used as a single stimulus (33).
The presence of high levels of neopterin in patients with impairment of the IFN-γ pathway demonstrates that IFN-γ is not the only stimulus needed for the synthesis of neopterin in vivo. Because the high levels of IFN-α and/or IFN-β needed in vitro to obtain neopterin secretion by monocytes/macrophages could not be reached under in vivo conditions and because the other cells have been shown to secrete only very limited amounts of neopterin in vitro (17), we might speculate that the increase in neopterin levels observed in MSMD patients is due to neopterin secretion by dendritic cells stimulated by IFN-α and/or IFN-β, as already observed in vitro.
The investigation of MSMD patients with STAT1 deficiency could be relevant to obtaining a better understanding of the roles of IFN-α and IFN-β, since STAT1 is a critical molecule known to be shared by the IFN-γ and the IFN-α-IFN-β signaling pathways. Unfortunately, we were unable to investigate serum neopterin levels in STAT1-deficient patients because such sera from unique patients were not available.
Surprisingly, we have observed higher levels of neopterin in the sera of patients with cIFN-γ RD than in those with pIFN-γRD. Although no definitive explanation could be given, the fact that the latter patients have milder infections might play a role in this regard, since they will secrete neopterin at levels close to those of healthy individuals.
To investigate the role of infection per se in the production of neopterin in vivo, we did measure the serum neopterin levels in a limited number of CGD patients presenting with severe infections comparable to those occurring in MSMD patients but that were due to an immune defect unrelated to IFN-γ. Interestingly, although serum neopterin levels were lower in CGD patients, the difference compared to the levels in cIFN-γRD patients was not statistically significant. These are preliminary results, and more patients from this control group must be included in order to get a clear-cut interpretation.
Our data definitely demonstrate that IFN-γ is dispensable to neopterin production in vivo. Because of its involvement mainly in oxidative stress, neopterin might play an important role in the course of host defense reactions. Thus, its synthesis in vivo in the absence of IFN-γ seems to be regulated by other cytokines and/or factors. Further investigations are needed to better identify the molecules involved and their respective roles.
REFERENCES
- 1.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Döffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. S. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 2.Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Döffinger, S. Lamhamedi, P. Drysdale, D. Toellner, J. Girdelstone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102:2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baier-Bitterlich, G., D. Fuchs, and H. Wachter. 1997. Chronic immune stimulation, oxidative stress, and apoptosis in HIV infection. Biochem. Pharmacol. 53:755-763. [DOI] [PubMed] [Google Scholar]
- 4.Barak, M., and N. Gruener. 1991. Neopterin augmentation of tumor necrosis factor production. Immunol. Lett. 30:101-106. [DOI] [PubMed] [Google Scholar]
- 5.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 6.Casanova, J. L., and L. Abel. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 4:55-66. [DOI] [PubMed] [Google Scholar]
- 7.Doffinger, R., E. Jouangy, S. Dupuis, M. C. Fondaneche, J. Stephan, J. F. Emile, S. Lamhamedi-Cherradi, F. Altare, A. Pallier, G. Barcenas-Morales, E. Meinl, C. Krause, S. Pestka, R. D. Schreiber, F. Novelli, and J. L. Casanova. 2000. Partial interferon-γ receptor signalling chain deficiency in a patient with bacille Calmette-Guérin and Mycobacterium abscessus infection. J. Infect. Dis. 181:379-384. [DOI] [PubMed] [Google Scholar]
- 8.Dorman, S. E., and S. M. Holland. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Investig. 101:2364-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuis, S., C. Dargemont, C. Fieschi, N. Thomassin, S. Rosenzweig, J. Harris, S. M. Holland, R. D. Schreiber, and J. L. Casanova. 2001. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293:300-303. [DOI] [PubMed] [Google Scholar]
- 10.Dupuis, S., E. Jouanguy, S. Al-Hajjar, C. Fieschi, I. Z. Al-Mohsen, S. Al-Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al-Gazalan, H. Al-Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 11.Elloumi-Zghal, H., M. R. Barbouche, J. Chemli, M. Bejaoui, A. Harbi, N. Snoussi, S. Abdelhak, and K. Dellagi. 2002. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille Calmette-Guérin infection. J. Infect. Dis. 185:1468-1475. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs, D., M. P. Dierich, and H. Wachter. 1994. Neopterinbestimmung als zusätzliche Screeningmethode zur Erhöhung der Sicherheit der Bluttransfusion. Österr. Krankenhaus-Zeitung 35:75-79. [Google Scholar]
- 13.Fuchs, D., A. Hausen, M. Kofler, H. Kosanowski, G. Reibnegger, and H. Wachter. 1984. Neopterin as an index of immune response in patients with tuberculosis. Lung 162:337-346. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, D., A. Hausen, G. Reibnegger, E. R. Werner, M. Dierich, and H. Wachter. 1988. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol. Today 9:150-154. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, D. E., J. C. McArthur, and D. R. Cornblath. 1991. Neopterin and interferon-gamma in serum and cerobrospinal fluid of patients with HIV-associated neurologic disease. Neurology 41:69-74. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, G., S. Frede, S. Kenn, M. Smolny, H. Wachter, D. Fuchs, J. Grote, J. Rieder, and W. Schobersberger. 1998. Neopterin-induced tumor necrosis factor-alpha synthesis in vascular smooth muscle cells in vitro. Int. Arch. Allergy Immunol. 116:240-245. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, G., B. Wirleitner, and D. Fuchs. 2003. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm. Res. 52:313-321. [DOI] [PubMed] [Google Scholar]
- 18.Huber, C., J. R. Batchelor, D. Fuchs, A. Hausen, A. Lang, D. Niederwieser, G. Reibnegger, P. Swelty, P., J. Troppmair, and H. Wachter. 1984. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon gamma. J. Exp. Med. 160:310-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 20.Jouanguy, E., S. Lamhamedi-Cherradi, F. Altare, M. C. Fondaneche, D. Tuerlinckx, S. Blanche, J. F. Emile, J. L. Gailard, R. Schreiber, M. Levin, A. Fischer, C. Hivroz, and J. L. Casanova. 1997. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Investig. 100:2658-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouanguy, E., S. Lamhamedi-Cherradi, D. Lammas, S. E. Dorman, M. C. Fondaneche, S. Dupuis, R. Doffinger, F. Altare, J. Girdlestone, J. F. Emile, H. Ducoulombier, D. Edgar, J. Clarke, V. A. Oxelius, M. Brai, V. Novelli, K. Heyne, A. Fischer, S. M. Holland, D. S. Kumararatne, R. D. Schreiber, and J. L. Casanova. 1999. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet. 21:370-378. [DOI] [PubMed] [Google Scholar]
- 22.Kotanko, P., R. Margreiter, and W. Pfaller. 2000. Urinary N-acetyl-beta-d-glucosaminidase and neopterin aid in the diagnosis of rejection and acute tubular necrosis in initially nonfunctioning kidney grafts. Nephron 84:228-235. [DOI] [PubMed] [Google Scholar]
- 23.Manes, G., O. A. Spada, P. G. Rabitti, B. Feola, S. Misso, A. Minerva, and G. Uomo. 1999. Neopterin serum levels in pancreatic adenocarcinoma. Int. J. Pancreatol. 25:31-37. [DOI] [PubMed] [Google Scholar]
- 24.Moutabarrik, A., S. Takahara, I. Nakanishi, Y. Kokado, Y. Takano, H. Kameoka, M. Ishibashi, and D. Zaid. 1994. Interferon-gamma stimulates neopterin release from cultured kidney epithelial cells. Scand. J. Immunol. 39:27-30. [DOI] [PubMed] [Google Scholar]
- 25.Nathan, C. F. 1986. Interferon 7, p. 125-143. In I. Gresser and J. Vilcek (ed.). Academic Press, Inc., New York, N.Y.
- 26.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 27.Picard, C., C. Fieschi, F. Altare, S. Al-Jumaah, S. Al-Hajjar, J. Feinberg, S. Dupuis, C. Soudais, I. Z. Al-Mohsen, E. Genin, D. Lammas, D. S. Kumararatne, T. Leclerc, A. Rafii, H. Frayha, B. Murugasu, L. B. Wah, R. Sinniah, M. Loubser, E. Okamoto, A. Al-Ghonaium, H. Tufenkeji, L. Abel, and J. L. Casanova. 2002. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am. J. Hum. Genet. 70:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reibnegger, G., V. Boonpucknavig, D. Fuchs, A. Hausen, E. Schmutzhard, and H. Wachter. 1984. Urinary neopterin is elevated in patients with malaria. Trans. R. Soc. Trop. Med. Hyg. 78:545-546. [DOI] [PubMed] [Google Scholar]
- 29.Schobersberger, W., G. Hoffman, J. Grote, H. Wachter, and D. Fuchs. 1995. Induction of inducible nitric oxide synthase expression by neopterin in vascular smooth muscle cells. FEBS Lett. 377:461-464. [DOI] [PubMed] [Google Scholar]
- 30.Turgan, N., S. Habif, Z. Parildar, D. Ozmen, I. Mutaf, D. Erdener, and O. Bayindir. 2001. Association between homocysteine and neopterin in healthy subjects measured by a simple HPLC-fluorometric method. Clin. Biochem. 34:271-275. [DOI] [PubMed] [Google Scholar]
- 31.Walter, R., P. Linscheid, N. Blau, L. Kierat, A. Schaffner, and G. Schoedon. 1998. Induction of tetrahydrobiopterin synthesis in human umbilical vein smooth muscle cells by inflammatory stimuli. Immunol. Lett. 60:13-17. [DOI] [PubMed] [Google Scholar]
- 32.Werner, E. R., A. Bichler, G. Daxenbichler, D. Fuchs, L. C. Fuith, A. Hausen, H. Hetzel, G. Reibnegger, and H. Wachter. 1987. Determination of neopterin in serum and urine. Clin. Chem. 33:62-66. [PubMed] [Google Scholar]
- 33.Werner-Felmayer, G., G. Baier-Bitterlich, D. Fuchs, A. Hausen, C. Murr, G. Reibnegger, E. R. Werner, and H. Wachter. 1995. Detection of bacterial pyrogens on the basis of their effects on gamma interferon-mediated formation of neopterin or nitrite in cultured monocyte cell lines. Clin. Diagn. Lab. Immunol. 2:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner-Felmayer, G., E. R. Werner, D. Fuchs, A. Hausen, G. Reibnegger, K. Schmidt, G. Weiss, and H. Wachter. 1993. Pteridine biosynthesis in human endothelial cells. Impact of nitric oxide-mediated formation of cyclic GMP. J. Biol. Chem. 268:1842-1846. [PubMed] [Google Scholar]
- 35.Widner, B., N. Sepp, E. Kowald, S. Kind, M. Schmuth, and D. Fuchs. 1999. Degradation of tryptophan in patients with systemic lupus erythematosus. Adv. Exp. Med. Biol. 467:571-577. [DOI] [PubMed] [Google Scholar]
- 36.Wirleitner, B., D. Reider, S. Ebner, G. Bock, B. Widner, M. Jaeger, H. Schennach, N. Romani, and D. Fuchs. 2002. Monocyte-derived dendritic cells release neopterin. J. Leukoc. Biol. 72:1148-1153. [PubMed] [Google Scholar]
- 37.Woll, E., G. Weiss, D. Fuchs, F. Lang, and H. Wachter. 1993. Effect of pteridine derivatives on intracellular calcium concentration in human monocytic cells. FEBS Lett. 318:249-252. [DOI] [PubMed] [Google Scholar]