Abstract
The aims of the present study were to determine (i) the long-term immunogenicity and the decay rate of hepatitis B virus (HBV) surface antibody (anti-HBs) from universal hepatitis B vaccination at infancy for a healthy population in an area of hyperendemicity and (ii) whether the anti-HBs levels measured by enzyme immunoassay (EIA) were closely correlated with those assayed by radioimmunoassay (RIA) methods during long-term monitoring. A total of 1,337 apparently healthy children (696 boys and 641 girls) who were vaccinated against HBV at infancy and monitored for anti-HBs annually from 7 to 16 years of age entered the study. Serum samples were analyzed for anti-HBs by RIA at 7 to 15 years of age and were also analyzed by EIA at 13 to 16 years of age. Antibody titers were quantified in mIU/ml by EIA as well as by the ratio of the count in the sample to the count for a negative control (S/N) by RIA. In nonboosted children, the average decay of anti-HBs from 7 to 16 years of ages indicated that approximately 20% of the geometric mean titer decays per year. There was a good correlation between serum anti-HBs levels measured by the RIA and the EIA methods (r = 0.91; P < 0.0001). An equation for RIA to EIA level conversion was established: log EIA titer = −0.12 + (1.31 · log RIA S/N). The anti-HBs titers measured by EIA correlate well with the S/N assayed by RIA. The annual decay rate of the log anti-HBs level may help in planning booster immunizations for hyporesponders or individuals at risk in adolescence.
Hepatitis B virus (HBV) infection is a global health problem, particularly in areas of endemicity. In Taiwan, before the implementation of hepatitis B control, up to 15% to 20% of the general population were chronic carriers of hepatitis B surface antigen (HBsAg) and perinatal HBV transmission accounted for 40% to 50% of the carrier pool (1, 15, 16). Taiwan launched the world's first nationwide universal vaccination program in 1984, which resulted in a significant reduction in the chronic carrier rate in children from 10% to <1% over 15 years (13). The morbidity and mortality rates associated with the sequelae of chronic HBV infection also declined, as demonstrated by the reduction in the rates of childhood hepatocellular carcinoma (3). We previously reported on the adequate protection against HBV for up to 14 years following universal HBV vaccination in infancy in Taiwan (11). Yet, we also noted the waning immunity to the HBV vaccine and the possible need for a booster dose in the future. We therefore studied the decay rate of HBV surface antibody (anti-HBs) with time.
During our long-term follow-up study, the methods for anti-HBs determination shifted from radioimmunoassay (RIA) to enzyme immunoassay (EIA) due to the trend of reductions in the use of radioisotopes. We found that HBV serologic markers measured by different laboratory methods evolving from RIA during the first 6 years of follow-up to EIA during the last 2 years of follow-up made the interpretation of long-term anti-HBs persistence difficult. Because no quantitative correlation between those two methods has been made before in the literature, in this study we correlated the levels of anti-HBs assayed by RIA with those assayed by EIA in adolescents and also studied the long-term rate of decay of anti-HBs.
MATERIALS AND METHODS
Study population.
One thousand three hundred thirty-seven healthy children (696 boys and 641 girls) were recruited at age 7 years from three primary schools in Taipei, Taiwan, between March and May 1994. All parents of the enrolled children signed an informed consent and provided the child's vaccination history, based on the information in a booklet provided by the health administration authority in Taiwan. In total, 1,337 subjects were divided into an incomplete primary vaccination group (n = 137; primary vaccination, less than three doses) and a complete primary vaccination group (n = 1,200; primary vaccination, three or more doses). Fifty children who received booster doses after age 7 years, consisting of 47 children with anti-HBs levels <10 S/N (where S/N is the ratio of the count in the sample to the count for a negative control) and 3 children with anti-HBs levels ≥10 S/N at age of 7 years, were excluded from the analysis because of the use of diverse booster regimens.
Hepatitis B virus serum markers.
Serum samples were tested for HBsAg, anti-HBs, and hepatitis B core antibody (anti-HBc) by RIA by using the Ausria II, Ausab, and Corab systems (Abbott Laboratories, North Chicago, IL) for subjects at ages 7 to 12 years and by EIA at ages 13 to 16 years. RIA was in widespread use for several years in Taiwanese medical centers before it was replaced by the current EIA methods. To compare EIA and RIA for the detection of anti-HBs in the sera of the adolescents monitored during this program, sera obtained at ages 13 to 15 years were retested for anti-HBs (Ausab) by RIA. The S/Ns for anti-HBs were calculated as described by the manufacturer of the RIA. For quantification of surface antibody in sera, the concentration was detected in mIU/ml by EIA. The protective anti-HBs level was defined as ≥10 S/N for RIA and ≥10 mIU/ml for EIA.
Primary vaccination schedules.
The children were vaccinated with a 5-μg dose of plasma-derived HBV vaccine (Hevac B; Pasteur Institute) at birth and at ages 1, 2, and 12 months. In addition, 0.5 ml (145 IU) of hepatitis B immunoglobulin was given within 24 h after birth if their mothers had hepatitis B e antigen (HBeAg) or reciprocal serum HBsAg titers ≥2,560 by reverse-passive hemagglutinin assay.
Booster dose.
The participants who had no protective levels of anti-HBs (S/N < 10) were selected to be boosted or not boosted at age 7 years based on parental wishes. The same dosage of the neonatal vaccine was used for the booster vaccination (i.e., recombinant hepatitis B vaccines consisting of 5 μg of Ricombivax [Merck]or 20 μg of Engerix [GlaxoSmithKline]).
Anti-HBs decay rate after immunization.
Only samples negative for HBsAg and anti-HBc during follow-up were included in the calculation of the geometric mean titers (GMTs) of anti-HBs. Analysis of variance was used to compare the GMTs of anti-HBs among groups between the ages of 13 and 16 years. Differences in the proportions and numbers of children with protective anti-HBs levels between groups were examined by chi-square test. The GMT for the anti-HBs levels in HBsAg-negative children was computed by the following model: log e (GMT) = A + (B1 · log e [peak level of anti-HBs after vaccination)] + [B2 · log e (time since booster)], where e is the base of the natural logarithmic function and A, B1, and B2 are coefficients of this natural logarithmic function (6).
RESULTS
Basic data and subgrouping of participants.
According to their booster and surface antibody status at age 7 years, 1,150 children who received complete HBV vaccination during infancy were further categorized into three subgroups. By parental choice, group Ia (n = 267) consisted of children who had anti-HBs levels <10 S/N at age 7 and who did not receive any booster dose during the follow-up period, while group Ib (n = 200) consisted of children who had anti-HBs levels <10 S/N at age 7 years and who had received a booster dose at age 7. Group II (n = 683) consisted of children who had anti-HBs levels ≥10 S/N at age 7 years and who did not receive a booster vaccination during the follow-up period. Of the 1,150 participants from the complete vaccination group, only 313 and 227 returned for follow-up at age 15 and 16 years, respectively. The subjects who returned for follow-up at age 15 and 16 years had higher maternal HBsAg carrier rates (relative risks, 1.67 and 2.0, respectively; P < 0.01, χ2 test), and the percentage of subjects who had protective anti-HBs levels at age 7 years (relative risks, 0.81 and 0.66, respectively; P < 0.01, χ2 test) was less than the percentage who did not. Twelve children developed serologic evidence of HBV infection by 16 years of age, but they did not have persistent HBsAg-positive infection during follow-up. Children who had two or more sequential serum samples positive for anti-HBc were classified as documented anti-HBc seroconverters. Among the group I participants, new seroconversion to anti-HBc positivity were observed in 0.8% (2 of 257) of the children in subgroup Ia and in 0.5% (1 of 200) of the children in subgroup Ib. The difference in the rate of seroconversion to anti-HBc positivity between subgroups Ia and Ib was not statistically significant (P = 0.71, χ2 test). Seven (1.0%) of the group II subjects seroconverted to anti-HBc positivity during the ages of 7 to 16 years. At the beginning of the 9-year follow-up, 59.6% of these 1,150 children had protective anti-HBs levels at age 7 years, as determined by RIA. The anti-HBs levels declined with time; the proportion of children with protective anti-HBs levels at age 15 years gradually decreased to 31.4% of the adolescents by RIA and 54.7% of them by EIA. Finally, protective anti-HBs titers were detected in 41.1% of the subjects by EIA at age 16 years, following HBV immunization in infancy.
Correlation between the anti-HBs levels obtained by RIA and EIA.
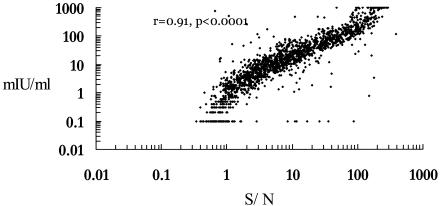
No new HBsAg carrier was detected during the follow-up period. Follow-up serum specimens obtained from 876, 788, and 197 subjects at 13, 14, and 15 years of age, respectively, were tested for anti-HBs levels by both EIA and RIA. The EIA titers and the RIA S/Ns of anti-HBs were compared for each participant; thus, 1,861 pairs of data for 922 participants (477 boys and 445 girls) ages 13 to 15 years were collected. The correlation between the anti-HBs levels measured by RIA and EIA were assessed by a scatter plot. There was a good correlation between the serum anti-HBs levels measured by the RIA and the EIA methods (P < 0.0001; r = 0.91) (Fig. 1). A linear regression equation of RIA to EIA level conversion was formulated as follows: log EIA titer = −0.12 + (1.31 · log RIA S/N) (R2 = 0.82.).
FIG. 1.
Correlation between the anti-HBs levels tested by RIA versus EIA in 1,861 serum samples.
Anti-HBs decay after immunization.
The decay in anti-HBs levels as determined by both laboratory methods is shown in Table 1. From ages 13 to 16 years, the percentage of children with protective anti-HBs levels gradually declined with age (P < 0.05), and the mean anti-HBs titers also decreased gradually with age (P< 0.05) (Table 2). During each year from ages 13 to 16 years, group II participants had the highest GMTs of anti-HBs, while group Ia had the lowest (P < 0.05) (Table 2).
TABLE 1.
Anti-HBs decay from ages 7 to 16 years by different immunoassays (EIA and RIA) in children immunized and boosted with hepatitis B vaccine at age 7 yearsa
| Age (yr) | Laboratory method | Booster group
|
Nonbooster group
|
||
|---|---|---|---|---|---|
| No. | % with protective antibody | No. | % with protective antibody | ||
| 7 | EIAb | 199 | 7.5 | 924 | 76.5 |
| RIAc | 199 | 0.0 | 924 | 72.4 | |
| 8 | EIAb | 197 | 65.0 | 836 | 70.8 |
| RIA | 197 | 60.9 | 836 | 65.4 | |
| 9 | EIAb | 182 | 64.8 | 766 | 76.6 |
| RIA | 182 | 58.8 | 766 | 72.2 | |
| 10 | EIAb | 176 | 59.1 | 712 | 71.6 |
| RIA | 176 | 52.8 | 712 | 65.9 | |
| 11 | EIAb | 171 | 52.0 | 685 | 68.8 |
| RIA | 171 | 45.0 | 685 | 61.0 | |
| 12 | EIAb | 171 | 44.4 | 673 | 59.9 |
| RIA | 171 | 36.8 | 673 | 54.2 | |
| 13 | EIAd | 154 | 43.5 | 603 | 60.7 |
| RIA | 152 | 28.9 | 597 | 47.4 | |
| 14 | EIAd | 140 | 38.6 | 547 | 55.9 |
| RIA | 140 | 25.7 | 545 | 40.7 | |
| 15 | EIAd | 51 | 43.1 | 119 | 59.7 |
| RIA | 71 | 22.5 | 206 | 34.5 | |
| 16 | EIAd | 56 | 35.7 | 146 | 43.2 |
Booster group (group Ib), children who had anti-HBs levels <10 S/N at age 7 years and who received boosters at age 7; nonbooster group, children who did not receive a booster from 7 to 16 years of age, including group Ia (children who had anti-HBs levels <10 S/N at age 7 years and who did not receive any booster during the follow-up) and group II (children who had anti-HBs levels ≧10 S/N at age 7 years and who did not receive a booster vaccination during the follow-up).
Anti-HBs levels were converted from the linear regression equation of the level determined by RIA to the level determined by EIA: log EIA titer = −0.12 + (1.31·log RIA S/N).
Anti-HBs levels measured by RIA.
Anti-HBs levels measured by EIA.
TABLE 2.
Anti-HBs decay by enzyme immunoassays in children immunized and boosted with hepatitis B vaccine at ages 13 to 16 years
| Vaccination groupa | Age (yr)
|
|||
|---|---|---|---|---|
| 13 | 14 | 15 | 16 | |
| Ia | ||||
| No. | 170 | 162 | 35 | 61 |
| % with protective anti-HBs levels | 15.3 | 14.8 | 25.7 | 19.7 |
| GMT (mIU/ml) | 1.4 | 0.9 | 1.2 | 0.9 |
| Ib | ||||
| No. | 154 | 140 | 51 | 56 |
| % with protective anti-HBs levels | 43.5 | 38.6 | 43.1 | 35.7 |
| GMT (mIU/ml) | 7.2 | 5.7 | 6.3 | 4.5 |
| II | ||||
| No. | 433 | 385 | 84 | 85 |
| % with protective anti-HBs levels | 78.5 | 73.2 | 73.8 | 60.0 |
| GMT (mIU/ml) | 32.9 | 26.6 | 22.3 | 18 |
Ia, children who had anti-HBs levels <10 S/N at age 7 years and who did not receive any booster during the follow-up; Ib, children who had anti-HBs levels <10 S/N at age 7 years and who received boosters at age 7 years; II, children who had anti-HBs levels ≧10 S/N at age 7 years and who did not receive a booster vaccination during the follow-up.
Annual anti-HBs decay rates in boosted and nonboosted children.
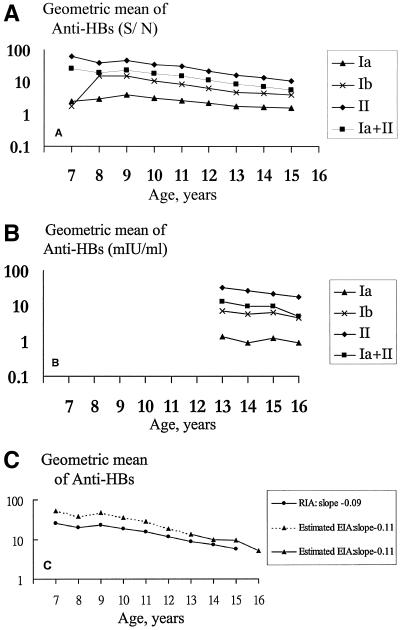
The kinetics of anti-HBs antibody production and persistence were analyzed in serum samples of vaccinees, obtained each year between the ages of 7 and 16 years, who were boosted at age 7 years (group Ib) and subjects who were not boosted during follow-up (group Ia and group II). The logarithmic geometric mean anti-HBs levels for each group in each follow-up year were plotted. Booster doses of vaccine were given to group Ib participants who had received a primary course of HBV vaccine in infancy. Upon assessment of the antibody response, they had developed a large rise in geometric mean anti-HBs levels at age 8 years, and the levels rapidly declined after age 8 years (Fig. 2A). The three groups had a roughly linear decline in log-anti-HBs levels with age; moreover, the trends of anti-HBs in the three groups were parallel to each other (Fig. 2B). The slope (rate) of anti-HBs decay of the nonbooster group (subgroup Ia plus group II) and the booster group (subgroup Ib) determined by RIA are equal to −0.09 (Fig. 2C) and −0.1, respectively. By using the individual anti-HBs S/Ns obtained at each time point, the corresponding EIA titers, according to our proposed logarithmic function, were calculated; and the declines in anti-HBs GMTs during follow-up were determined. The rate of the log anti-HBs GMT change between the ages of 7 and 16 years determined by EIA was −0.11 (Fig. 2C). By converting the logarithmic equation to an exponential equation, the average annual decay of anti-HBs in the nonboosted children is the same as that in boosted children; the average annual decay of anti-HBs from the ages of 7 to 16 years is approximately 20% of the GMT of the previous year.
FIG. 2.
Geometric mean levels of antibody to hepatitis B surface antigen between ages 7 and 16 years. (A) The geometric mean levels determined by RIA are shown for each vaccination group. Of note, group Ib subjects developed a large rise in antibody levels at age 8 years following receipt of a booster, and the antibody levels rapidly waned thereafter. (B) The geometric mean levels determined by EIA are shown for each vaccination group. (C) The slopes of anti-HBs decay of nonboosted children (groups Ia and II) determined by RIA and EIA are equal to −0.09 and −0.11, respectively. Group Ia, children who had anti-HBs levels <10 S/N at age 7 years and who did not receive any booster during the follow-up; group Ib, children who had anti-HBs levels <10 S/N at age 7 years and who received boosters at age 7 years; group II, children who had anti-HBs levels ≥10 S/N at age 7 years and who did not receive booster vaccination during the follow-up.
DISCUSSION
During long-term follow-up to the age of 13 to 16 years, the longitudinal quantification of antibody titers of the three vaccination groups showed that those vaccinees with protective anti-HBs levels at age 7 years (group II) still retained the highest anti-HBsGMT. Among vaccinees who did not retain protective anti-HBs levels at age 7 years, the anti-HBs levels of those boosted (group Ib) after booster vaccination were higher than those not boosted (group Ia), followed by the same decline rate, resulting in curves parallel with those for the nonboosted group 2 years after the booster vaccination (Fig. 2A). Hyporesponders (vaccinees with initial anti-HBs levels less than 10 S/N at age 7) after booster vaccination would never gain higher anti-HBs levels than the vaccine responders (group II).
Our previous study suggests that routine booster vaccination may not be necessary to provide protection against chronic HBV infection before age 15 years in Taiwan (11), as the maintenance of HBsAg-specific memory confers protection against a clinical breakthrough infection even in the absence of detectable antibodies (5, 17, 19). However, the possibility of a need for a booster dose exists, particularly when the child becomes a sexually active adolescent. The results of our 9-year follow-up study extended to adolescents after primary vaccination in Taiwan show that the rate of long-term protection against HBV infection is high. Since new seroconversion to anti-HBc positivity is always low in hyporesponders, booster vaccination did not seem to provide additional protection. Although the smaller number of vaccinees at ages 15 and 16 years may affect the results for an accurate comparison of HBV infection, the result still has relevance. Since those who were monitored to age 16 years had a higher maternal HBsAg carrier rate than those who were lost to follow-up, the results may overestimate the risk of HBV infection. Yet, we have obtained a very low annual anti-HBc seroconversion rate (0‰) at the ages of 15 to 16 years, suggesting that the annual incidence of HBV infection at this age is indeed very low. Further large-scale longitudinal studies of HBV status among adolescents may elucidate whether primary HBV vaccination in infancy can provide protection in adolescence when the risk of HBV transmission is higher through increased sexual activities and other parenteral exposures.
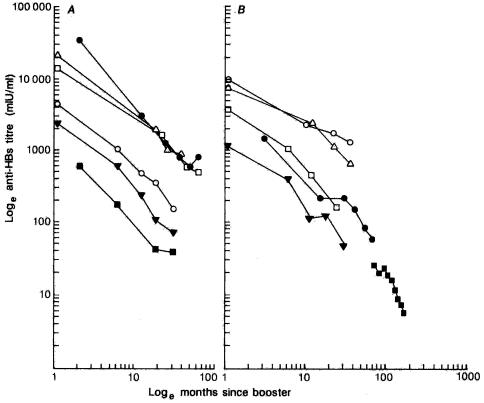
The persistence of protective anti-HBs levels after immunization was first addressed by Jilg et al. in 1984 (10). Different mathematical models have recently been proposed to describe the rate of antibody decline, and best fits between the calculated and the observed anti-HBs levels were obtained with a bilogarithmic function (log10 level and log10 time since receipt of the booster) of anti-HBs decay (2, 4, 6, 7, 8, 12). All previous studies show that the rate of antibody decay is independent of the vaccine type, protocol, gender, and age of the vaccine recipients. The slope of anti-HBs decay plotted on a bilogarithmic scale was equal to −1.0 (6) (Fig. 3). The original definition of the time since receipt of the booster in those studies was that time that had lapsed since receipt of the last dose of the primary vaccination series. Hence, in our study, the time since receipt of the booster was replaced by the time since the shot was given at 12 months of age, and we calculated the decay rate for 924 children who neither received the booster at age of 7 years nor developed serologic evidence of HBV infection through the follow-up period. The slope of the line of the plot of the log anti-HBs GMTs against the log time since receipt of the booster is equal to −1.6; the decline rate here was much faster than that in the previous studies. However, the previous studies were based on a limited number of subjects and/or serum samples; a selected cluster of vaccine recipients (newborns, homosexual males, adolescents, and young adults), which introduced parameters which are known to influence the immune response. In addition, the follow-up period of the present study was between the ages of 7 and 16 years, reflecting the antibody decay rate after a much longer duration of follow-up in children and young adolescents after primary vaccination in infancy; this has never been investigated before. Moreover, the large cohort of 1,150 vaccinees in this study exceeded the number of subjects in all previously published studies. In our previous study (14) we quantified the anti-HBs titers in 39 noncarrier vaccinees with low concentrations of anti-HBs (S/N < 10) at the age of 7 years and showed that 36 (92%) of the 39 children had quantitative titers <10 mIU/ml. Although detailed quantification of the hepatitis B vaccine-induced antibodies was not performed, the good correlation of anti-HBs levels obtained by EIA and RIA in the present study suggests that either EIA or RIA is probably equally sensitive to the detection of declines in antibody levels.
FIG. 3.
Studies of decay rate in anti-HBs titers after the last dose of primary vaccination series. (A) Healthy adults: Gesemann and Scheiermann (6c) (220 subjects; ▵), Goudeau et al. (8a) (178 subjects; •), Grob et al. (8b) (86 subjects; ○), Laplanche et al. (10a) and Crosnier et al. (6a) (80 subjects; □), Oon et al. (13a) (31 subjects; ▪), and Zachoval et al. (21) (195 subjects; ▾). (B) Patients and children: Benhamou et al. (1a) and Couroucé et al. (4) (69 hemodialysis patients; □), Grob et al. (8b) (62 hemodialysis patients; ▾), Zanetti et al. (22) (67 anti-human immunodeficiency virus-negative hemophiliacs; ○), Dentico et al. (6b) (40 Italian children; ▵), Yvonnet et al. (20) (125 Senegalese infants; •), and our results by the RIA method (924 Taiwanese children; ▪). Anti-HBs titers were measured in S/N by the RIA method for our results.
Serologic markers have been used for the diagnosis and monitoring of HBV infection. In recent years, the detection of HBV serologic markers by EIA has replaced RIA because of advantages that include the longer shelf-lives of EIA systems and the absence of radioisotope safety issues, the need for licensing, and the need to adhere to disposal regulations for RIA but not for EIA. Epidemiologic and laboratory studies of passive immunization with hepatitis B immune globulin and active immunization with plasma-derived vaccine pointed to a serum level of 10 mIU/ml determined by EIA or 10 S/N by RIA as the titer of anti-HBs that confers protection against clinically significant infection (18). However, no quantitative relationship between RIA and EIA for the determination of anti-HBs levels has previously been proposed. From our long-term study with 922 adolescents, we found that the anti-HBs titers measured by EIA correlated well with the S/N values obtained by RIA. This finding suggests that S/N could represent accurate anti-HBs titers.
With a waning humoral response, the memory of cell-mediated immunity may still be detectable after booster immunization (9). However, the assay of cell-mediated immunity is tedious, costly, and not applicable to a large population. Determination of serum levels of anti-HBs after hepatitis B vaccination is the simplest reliable test available to predict the waning of protection yielded by vaccination and to plan whether or not a booster is needed. This annual decay rate of anti-HBs in nonboosted children after primary immunization in infancy may serve as the reference of planning for booster vaccination of adolescents at risk. In addition, by obtaining routine postvaccination assessment of HBV serology after the first booster dose, one may schedule revaccination on the basis of this anti-HBs titer decay curve. As long as strong evidence of protection against HBV infection in individuals is needed, this decay rate could help to cut down on the costs of retesting and enable long-term planning for HBV booster vaccinations.
In conclusion, the anti-HBs titers measured by EIA correlated well with the S/N values assayed by RIA. With anti-HBs quantification and determination of the corresponding number of years since receipt of the booster, the annual decay rate of anti-HBs provides a means of calculation of a time-related anti-HBs titer, which will help us plan the booster schedule, if a booster is needed.
Acknowledgments
We acknowledge the contributions of many medical professionals in the follow-up of these children, with specific credit to Su-Mein Huang for technical assistance.
This work was supported in part by grants from the Department of Health of the Republic of China (grants 88-DC1003, 89-DC1023, 90-DC1019, 91-DC1047, 92-DC1016, and 93-DC1027).
REFERENCES
- 1.Beasley, R. P., L. Y. Hwang, C. C. Lin, M. L. Leu, C. E. Stevens, W. Szmuness, et al. 1982. Incidence of hepatitis B virus infections in preschool children in Taiwan. J. Infect. Dis. 146:198-204. [DOI] [PubMed] [Google Scholar]
- 1a.Benhamou, E., A.-M. Courouce, A. Laplanche, P. Jungers, J. F. Tron, and J. Crosnier. 1986. Long-term results of hepatitis B vaccination in patients on dialysis. N. Engl. J. Med. 314:1710-1711. [DOI] [PubMed] [Google Scholar]
- 2.Bryan, J. P., M. H. Sjogren, P. Macarthy, B. Cox, T.-C. Kao, and P. L. Perine. 1991. Dosing schedule for recombinant hepatitis B vaccine. J. Infect. Dis. 163:1384-1385. [DOI] [PubMed] [Google Scholar]
- 3.Chang, M. H., C. J. Chen, M. S. Lai, H. M. Hsu, T. C. Wu, M. S. Kong, et al. 1997. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N. Engl. J. Med. 336:1855-1859. [DOI] [PubMed] [Google Scholar]
- 4.Couroucé, A. M., P. Jungers, E. Benhamou, A. Laplanche, and J. Crosnier. 1984. Hepatitis B vaccine in dialysis patients. N. Engl. J. Med. 311:1515-1516. [DOI] [PubMed] [Google Scholar]
- 5.Coursaget, P., D. Leboulleux, M. Soumare, et al. 1994. Twelve-year follow-up study of hepatitis B immunization of Senegalese infants. J. Hepatol. 21:250-254. [DOI] [PubMed] [Google Scholar]
- 6.Coursaget, P., B. Yvonnet, W. Gilks, C. C. Wang, N. E. Day, J. P. Chiron, et al. 1991. Scheduling of revaccination against hepatitis B virus. Lancet 337:1180-1183. [DOI] [PubMed] [Google Scholar]
- 6a.Crosnier, J., P. Jungers, A.-M. Courouce, et al. 1981. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units. I. Medical staff. Lancet i:455-459. [DOI] [PubMed] [Google Scholar]
- 6b.Dentico, P., R. Buongiorno, A. Zavoianni, A. Volpe, G. Pastore, and O. Schiraldi. 1990. Immunogenicity of HEVAC-B Pasteur vaccine in infants: comparison of 2 and 5 mcg, p. 325-332. In P. Coursaget and M. J. Tong (ed.), Progress in hepatitis B immunization. Editions John Libbey Eurotext, Montrouge, France.
- 6c.Gesemann, M., and N. Scheiermann. 1989. Timing of booster doses of hepatitis B vaccine. Lancet ii:1274. [Google Scholar]
- 7.Geseman, M., and N. Scheiermann. 1995. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine 13:443-447. [DOI] [PubMed] [Google Scholar]
- 8.Goilav, C., H. Prinsen, and P. Piot. 1990. Protective efficacy of a recombinant DNA vaccine against hepatitis B in male homosexuals: results at 36 months. Vaccine 8:S50-S52. [DOI] [PubMed] [Google Scholar]
- 8a.Goudeau, A., F. Dubois, and P. Asou. 1990. Long-term persistence of anti-HBs after hepatitis B immunization in adults, p. 123-137. In P. Coursaget and M. J. Tong (ed.), Progress in hepatitis B immunization. Editions John Libbey Eurotext, Montrouge, France.
- 8b.Grob, P. J., A. Dufek, and H. I. Joller-Jemelka. 1985. Hepatitis B immunisation—when is a booster injection necessary? Schweiz. Med. Wochenschr. 115:394-402. [PubMed] [Google Scholar]
- 9.Huang, L. M., B. L. Chiang, C. Y. Lee, P. I. Lee, W. K. Chi, and M. H. Chang. 1999. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology 29:954-959. [DOI] [PubMed] [Google Scholar]
- 10.Jilg, W., M. Schmidt, F. Deinhardt, and R. Zachoval. 1984. Hepatitis B vaccination: how long does protection last? Lancet ii:458. [DOI] [PubMed] [Google Scholar]
- 10a.Laplanche, A., A.-M. Courouce, E. Benhamou, and P. Jungers. 1987. Timing of hepatitis B revaccination in healthy adults. Lancet i:1206-1207. [DOI] [PubMed] [Google Scholar]
- 11.Lin, Y. C., M. H. Chang, Y. H. Ni, H. Y. Hsu, and D. S. Chen. 2003. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J. Infect. Dis. 187:134-138. [DOI] [PubMed] [Google Scholar]
- 12.Mintai, Z., L. Kezhou, D. Lieming, and R. A. Smego, Jr. 1993. Duration and efficacy of immune response to hepatitis B vaccine in high-risk Chinese adolescents. Clin. Infect. Dis. 16:165-167. [DOI] [PubMed] [Google Scholar]
- 13.Ni, Y. H., M. H. Chang, L. M. Huang, H. L. Chen, H. Y. Hsu, T. Y. Chiu, et al. 2001. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann. Intern. Med. 135:796-800. [DOI] [PubMed] [Google Scholar]
- 13a.Oon, C. J., K. T. Goh, K. L. Tan, S. H. Chan, and G. K. Lim. 1990. Experience of hepatitis B prevention and control programme in Singapore, p. 475-489. In P. Coursaget and M. J. Tong (ed.), Progress in hepatitis B immunization. Editions John Libbey Eurotext, Montrouge, France.
- 14.Shih, H. H., M. H. Chang, H. Y. Hsu, P. I. Lee, Ni, Y. H., and D. S. Chen. 1999. Long-term immune response of universal hepatitis B vaccination in infancy: a community-based study in Taiwan. Pediatr. Infect. Dis. J. 18:427-432. [DOI] [PubMed] [Google Scholar]
- 15.Stevens, C. F., R. P. Beasley, J. J. Tsui, and W. C. Lee. 1975. Vertical transmission of hepatitis B antigen in Taiwan. N. Engl. J. Med. 292:771-774. [DOI] [PubMed] [Google Scholar]
- 16.Tsen, Y. J., M. H. Chang, H. Y. Hsu, C. Y. Lee, J. L. Sung, and D. S. Chen. 1991. Seroprevalence of hepatitis B virus infection in children in Taipei, 1989: five years after a mass hepatitis B vaccination program. J. Med. Virol. 34:96-99. [DOI] [PubMed] [Google Scholar]
- 17.Wainwright, R. B., L. R. Bulkow, A. J. Parkinson, C. Zanis, and B. J. McMahon. 1997. Protection provided by hepatitis B vaccine in a Yupik Eskimo population: results of a 10-year study. J. Infect. Dis. 175:674-677. [DOI] [PubMed] [Google Scholar]
- 18.West, D. J., and G. B. Calandra. 1996. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine 14:1019-1027. [DOI] [PubMed] [Google Scholar]
- 19.West, D. J., B. Watson, J. Lichtman, T. M. Hesley, and K. Hedberg. 1994. Persistence of immunologic memory for twelve years in children given hepatitis B vaccine in infancy. Pediatr. Infect. Dis. J. 13:745-747. [DOI] [PubMed] [Google Scholar]
- 20.Yvonnet, B., P. Coursaget, J. Chotard, et al. 1987. Hepatitis B vaccine in infants from an endemic area: long-term anti-HBs persistence and revaccination. J. Med. Virol. 22:315-321. [DOI] [PubMed] [Google Scholar]
- 21.Zachoval, R., W. Jilg, B. Lorbeer, M. Schmidt, and F. Deinhardt. 1984. Passive/active immunisation against hepatitis B. J. Infect. Dis. 150:112-117. [DOI] [PubMed] [Google Scholar]
- 22.Zanetti, A. R., E. Tanzi, L. Romano, D. Mari, M. Colombo, and P. M. Mannucci. 1990. Anti-pre-S2 and anti-HBs responses in hemophiliacs vaccinated with a plasma-derived vaccine. A four year follow-up study, p. 79-86. In P. Coursaget and M. J. Tong (ed.) Progress in hepatitis B immunization. Editions John Libbey Eurotext, Montrouge, France.