Abstract
Probiotic bacteria, including Lactobacillus acidophilus and Bifidobacterium bifidum, have been shown to enhance antibody responses in mammals. The objective of this study was to examine the effects of a probiotic product containing the above bacteria in addition to Streptococcus faecalis on the induction of the chicken antibody response to various antigens, both systemically and in the gut. The birds received probiotics via oral gavage and subsequently were immunized with sheep red blood cells (SRBC) and bovine serum albumin (BSA) to evaluate antibody responses in serum or with tetanus toxoid (TT) to measure the mucosal antibody response in gut contents. Control groups received phosphate-buffered saline. Overall, BSA and SRBC induced a detectable antibody response as early as week 1 postimmunization (p.i.), which lasted until week 3 p.i. Probiotic-treated birds had significantly (P ≤ 0.001) more serum antibody (predominantly immunoglobulin M [IgM]) to SRBC than the birds that were not treated with probiotics. However, treatment with probiotics did not enhance the serum IgM and IgG antibody responses to BSA. Immunization with TT resulted in the presence of specific IgA and IgG antibody responses in the gut. Again, treatment with probiotics did not change the level or duration of the antibody response in the gut. In conclusion, probiotics enhance the systemic antibody response to some antigens in chickens, but it remains to be seen whether probiotics have an effect on the generation of the mucosal antibody response.
The gut and its resident microbiota play a pivotal role in shaping the immune system repertoire (20, 30). Germfree animals have less developed gut-associated lymphoid tissue (GALT), but gut colonization in these animals by members of commensal gut microbiota results in the enhancement and diversification of the antibody-mediated immune response (33, 36). The lamina propria of the gut contains a large population of immunoglobulin A (IgA)-producing plasma cells, while germfree animals possess a very small number of these cells (16). Some of the IgA-producing plasma cells present in the lamina propria originate from CD5+ B, or B1, cells in the peritoneal cavity and are involved in the production of microbiota-specific IgA (24). This IgA-mediated response is T cell independent, does not interfere with the colonization of the gut by microbiota bacteria, and, in fact, may serve as an immune evasion mechanism for gut bacteria (16, 18).
Commensal bacteria present in gut microbiota are in close contact with cells of the immune system. It has recently been demonstrated that resident dendritic cells (DC) in the gut lamina propria have the capacity to directly sample the gut lumen by projecting their dendrites through the tight junctions of epithelial cells (32). The recognition of commensal bacteria or their structural components by Toll-like receptors (TLR) present on the surfaces of DC could lead to the activation and maturation of these cells (31). Differential activation of DC by commensal bacteria promotes the establishment of T-helper 1 (Th1), Th2, and Th3 responses and the secretion of cytokines, some of which are important for antibody production and isotype switching (8, 12, 27).
Commensal bacteria colonize the chicken gut after the chicken hatches, and the composition of the microbiota changes in an age-dependent manner (14). The predominant commensal bacterial species found in young chicks are members of the Lactobacillus spp., but over time, members of the Bifidobacterium spp. predominate (1). Although the notion has not been extensively studied, it is plausible that commensal bacteria present in chicken gut microbiota interact with cells in the immune system and have an influence on the development of the immune response. An equivalent of the mammalian GALT, which contains various cell subsets, including B and T lymphocytes, natural killer (NK) cells, and macrophages, has been described to exist in chickens (28, 22). Immediately after hatching, a chicken's GALT lacks mature B and T cells (4) but is gradually populated by migrating lymphocytes, and by week 2 posthatching, the GALT reaches its functional maturity (4). There is little information available on the process of induction of the immune response in the chicken gut. It appears that antigens that enter the chicken gut are taken up by epithelial cells or specialized intestinal cells that resemble mammalian M cells (28). However, there have been contradictory findings in relation to the fates of antigens and the cells that present them to B and T lymphocytes (28). Nevertheless, the outcome of antigen delivery via the gut may be the induction of an antibody response systemically and locally (22, 28).
The manipulation of gut microbiota via the administration of probiotics influences the development of the immune response (26). The exact mechanisms that mediate the immunomodulatory activities of probiotics are not clear. However, it has been shown that probiotics stimulate different subsets of immune system cells to produce cytokines, which in turn play a role in the induction and regulation of the immune response (8, 19, 23). Stimulation of human peripheral blood mononuclear cells with Lactobacillus rhamnosus strain GG in vitro resulted in the production of interleukin 4 (IL-4), IL-6, IL-10, tumor necrosis factor alpha, and gamma interferon (35). Other studies have provided confirmatory evidence that Th2 cytokines, such as IL-4 and IL-10, are induced by lactobacilli (8, 19, 31). The outcome of the production of Th2 cytokines is the development of B cells and the immunoglobulin isotype switching required for the production of antibodies. The production of the mucosal IgA response is dependent on other cytokines, such as transforming growth factor β (21). Importantly, various species and strains of lactobacilli are able to induce the production of transforming growth factor β, albeit to various degrees (5).
Probiotics, especially lactobacilli, could modulate the systemic antibody response to antigens in chickens (13, 17). Moreover, the administration of probiotics results in the secretion of cytokines and changes in lymphoid cells in the chicken gut, which may lead to enhanced immunity to Eimeria acervulina (9, 10). However, little is known about the immunomodulatory effects of probiotics on the induction of a systemic antibody response to soluble and cellular antigens as well as on the antibody response in the gut. The objective of the present research was, therefore, to study the immunomodulatory effects of probiotics in chickens on the antibody-mediated immune response, both systemically and locally.
MATERIALS AND METHODS
Chickens and housing.
Newly hatched female crossbred commercial broiler chicks used in the experiments were obtained from Maple Leaf Foods, Inc. (New Hamburg, ON, Canada). The birds were maintained in floor pens on clean wood shavings at the Ontario Ministry of Agriculture and Food Isolation Unit (University of Guelph, ON, Canada). The chicks were provided with free access to water and broiler starter rations. The research complied with University of Guelph Animal Care Committee guidelines.
Experimental design.
To evaluate the systemic antibody response, chicks were wing banded (each with a unique number) and randomly divided into five treatment groups: group I was probiotic treated and immunized (n = 9), group II was probiotic treated and nonimmunized (n = 6), group III was immunized (n = 9), group IV was nontreated and nonimmunized (n = 6), and the fifth group was hyperimmunized as a positive control (n = 2). The chicks in groups I and II were inoculated via oral gavage with 0.5 ml phosphate-buffered saline (PBS) containing 105 bacteria from a commercial probiotic, Interbac (Intervet, Whitby, ON, Canada) on the day of hatching. The chicks in groups I and III were immunized intramuscularly with 0.25 ml of 2% sheep red blood cells (SRBC) (PML Microbiologicals, Mississauga, ON, Canada) in PBS and 0.25 ml PBS containing 100 μg bovine serum albumin (BSA) (Fisher, New Jersey) on day 14 posthatching. PBS was used as a placebo in those groups that did not receive probiotics and/or antigen (II, III, and IV). Blood samples were collected on the day of immunization as well as on days 7, 14, and 21 postimmunization. Birds in the hyperimmunized group were immunized three times every 4 days staring at 2 weeks of age and were bled 4 days after the last immunization. This trial was repeated with the same conditions except that there were 10 birds in groups I and III.
For an assessment of the mucosal antibody response, an immunization protocol as described by Muir and coworkers (29) was employed. Briefly, chicks were randomly wing banded, divided into five groups, and administered probiotics, as described above. Chicks in groups I and III (nine chicks in each group) were immunized intraperitoneally with 0.5 ml of tetanus toxoid (TT) (Intervet Inc., Millsboro, DE) on day 14 of age, followed by a booster immunization via the oral route with 0.5 ml of TT 1 week later. PBS was used as a placebo in groups II, III, and IV (six chicks in groups II and IV), which did not receive probiotics and/or antigen. Samples (gut contents and blood) were obtained on days 0, 7, 14, and 21 after the primary immunization.
Sample collection.
To assess the systemic antibody response, blood samples were collected from birds via the wing vein on sampling days as described above. Blood samples were kept at room temperature for 2 hours and then at 4°C overnight. Blood samples were centrifuged for 10 min at 580 × g, and serum was isolated and stored at −80°C. To evaluate the mucosal antibody response, chickens at different time points (as described above) were humanely euthanized by cervical dislocation, the abdominal cavity was opened aseptically, and then the entire small intestine, including the duodenum, jejunum, and ileum, was collected. Pancreas, connective tissue, and fat were removed in PBS, and the intestine was cut longitudinally and then cut into 1-cm-long sections. The intestine and the contents were mixed with 5 ml of PBS containing 100 μg/ml of trypsin inhibitor (Sigma Chemical Co., St. Louis, MO). The mixture was transferred into a 50-ml tube, vortexed thoroughly, and then centrifuged at 20,000 × g at 4°C for 30 min. Following centrifugation, the supernatant was removed and stored at −80°C.
Serological analysis.
To determine the antibody response to SRBC, a direct hemagglutination assay was used. Antibody responses to BSA and TT were measured by enzyme-linked immunosorbent assay (ELISA).
A direct hemagglutination assay was performed to measure the total antibody (IgM and IgG) response to SRBC in serum. Briefly, serum samples were incubated at 56°C for 30 min to inactivate the complement. Fifty microliters of PBS containing 0.05% BSA was dispensed into each well of a round-bottomed 96-well microplate. Serum samples (50 μl) were then added and serially double diluted in the wells from columns 2 to 12. The first column (PBS only) of wells was considered blank. Then, 50 μl of 1% SRBC in PBS was added to all wells to make a 100-μl final volume. Subsequently, the plates were shaken for 1 min and incubated for 24 h at 37°C to determine agglutination titers. A positive result was recorded when at least 50% SRBC agglutination was observed. To measure anti-SRBC IgG and IgM antibodies, serum samples were treated with 0.2 M 2-mercaptoethanol (2-ME) for 30 min at 37°C. This treatment inactivates IgM, and as a result, hemagglutination observed after treatment with 2-ME is due mostly to the presence of IgG antibodies. The difference between total antibody and IgG titers determines the IgM titer.
Detection of antibodies against BSA in sera, and against TT in intestinal contents, was performed by an indirect ELISA. For evaluation of serum antibodies, each well of a flat-bottomed 96-well microplate (NUNC Maxisorp, VWR International, Mississauga, ON, Canada) was coated overnight with 100 μl coating buffer (pH 9.6) containing BSA (30 μg/ml) at 4°C. The wells were washed three times with 200 μl of PBS with 0.05% Tween 20 (PBST) with complete decanting between each wash. Subsequently, 100 μl of blocking buffer, composed of PBST containing 0.25% gelatin (HiPure liquid gelatin; Norland Products Inc., New Brunswick, N.J.), was added to each well and incubated for 1 h at 37°C to occupy all unbound sites. Washing was repeated as described above, followed by the addition of 100 μl chicken serum, diluted 1:100 in blocking buffer, to each well. Plates were incubated for 2 h at room temperature and then washed three times, and 100 μl of goat anti-chicken IgG-Fc or goat anti-chicken IgM conjugated with alkaline phosphatase (Cedarlane, Hornby, ON, Canada) (diluted 1:500 in blocking buffer) was added to each well and incubated for 1 h at room temperature (RT) before the plate was washed three times. One hundred microliters p-nitrophenylphosphate solution (KPL, Gaithersburg, MD) was added as the substrate to each well and incubated for 1 h at room temperature in the dark. The absorbance was measured at 405 nm using a microplate reader (Bio-Tek, Winooski, VT). Positive and negative control chicken sera were included in each plate.
An indirect ELISA was also used to evaluate the level of anti-TT IgG, IgA, and IgM antibodies in serum samples. Each well in a microplate was coated overnight with 100 μl of TT solution (Intervet) (diluted 1:50 in coating buffer) at 4°C. Serum samples (100 μl of serum diluted 1:150 in blocking buffer) were added to wells, and plates were incubated for 1 h at RT. After incubation, secondary antibodies were added separately, which included 100 μl of goat anti-chicken IgG-Fc, goat anti-chicken IgA, or goat anti-chicken IgM conjugated with alkaline phosphatase (Cedarlane) (diluted 1:500 in blocking buffer). Incubation was done for 1 h at RT. All the washings, blocking, adding of substrate, and reading of steps were done as described above.
To evaluate anti-TT IgA antibodies in intestinal contents, 100 μl of TT solution (Intervet) (diluted 1:50 in coating buffer) was dispensed in each well of a 96-well microplate and incubated at 4°C overnight. Plates were washed, followed by blocking as described above. Washing was repeated, and then 100 μl of intestinal content samples (diluted 1:20 in blocking buffer) was added to each well and plates were incubated at RT for 1 h. Following several washes, 100 μl goat anti-chicken IgA conjugated with alkaline phosphatase (diluted 1:500 in blocking buffer) was added to each well, incubated for 1 h at RT, and washed three times. The addition of substrate and the measurement of the optical density values were performed as described above. The evaluation of anti-TT IgG antibodies in intestinal contents was performed using the same ELISA protocol used for anti-TT IgA in intestinal contents, except that the dilution of antigen was 1:100 and the dilution of samples was 1:50 in blocking buffer.
Statistical analysis.
The antibody response data collected included anti-BSA IgG and IgM antibodies measured in two trials, anti-SRBC total antibody and IgG titers measured in two trials, and gut anti-TT IgG and IgA measured in one trial. The data were subjected to analyses of variance, which used the general linear model procedure of SAS (SAS Institute, 2001). The model included the effects of immunization, treatment with probiotics, time (the number of weeks postimmunization), and replicate experiments (except for experiments with anti-TT IgG and IgA), in addition to a determination of interactions between these variables. The effects and all interactions involving up to three of these effects were included in the initial analysis. Subsequently, a model that omitted nonsignificant interactions was rerun and these results were reported.
Since no antibody could be detected in the nonimmunized groups of chickens in the SRBC trials, the anti-SRBC total antibody data were analyzed using the Freq procedure of SAS (SAS Institute, 2001), treating the negative and positive responses as a binomial distribution, and using McNemar's statistic to determine the significance of the differences in the responses of the immunized groups. Subsequently, only the data from the immunized treatments were subjected to analysis of variance. The model included only the effects of probiotics and time (the number of weeks postimmunization, and replicate effects, in addition to the interactions of these variables); however, the interactions were not significant, and the final model was rerun without them. The least square means (LSM) or adjusted means of antibody titers and optical density values were tested for difference from zero using the one-tailed t test. Statistical significance was considered at a P of ≤0.05.
RESULTS
Systemic antibody responses to SRBC and BSA.
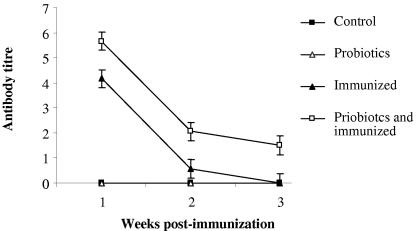
To evaluate the effects of probiotics on the systemic immune response, antibody-mediated responses to SRBC and BSA were assessed. Immunization with SRBC resulted in the appearance of specific anti-SRBC antibody in serum, starting at week 1 postimmunization (Fig. 1). No antibody response against SRBC was detected in the probiotic-treated, nonimmunized birds or the nontreated, nonimmunized birds. There was a gradual decrease in serum antibody titer over time in immunized groups (Fig. 1), which was statistically significant (P ≤ 0.001). By week 1 postimmunization, the immunized birds had the highest level of specific antibody response in serum, but by week 3 postimmunization, anti-SRBC antibody was detectable in 9 out of 19 birds in the immunized, probiotic-treated group and in only 2 out of 19 birds in the immunized group. When the effect of probiotics on the induction of the antibody response was analyzed, it was determined that probiotic treatment significantly increased the antibody response to SRBC at all time points following immunization (P ≤ 0.001). The anti-SRBC antibody titers at weeks 1 and 2 postimmunization in the probiotic-treated, immunized group and the immunization-only group were 5.7 and 4.2 (week 1) and 2 and 0.5 (week 2), respectively. The difference between the two groups was more prominent by week 3 postimmunization. At this time point, chickens in the probiotic-treated, immunized group had significantly more anti-SRBC antibody in their sera than birds in the immunized group (1.5 versus 0.013, respectively).
FIG. 1.
LSM of serum anti-SRBC antibody titer as determined by a direct hemagglutination assay. Error bars represent standard errors of the LSM. The groups were as follows: control (nonimmunized, nontreated), probiotics (nonimmunized, treated with probiotics), immunized (immunized with SRBC, nontreated), and probiotics and immunized (immunized with SRBC, treated with probiotics).
The addition of 2-ME resulted in the abrogation of hemagglutination in many of the samples, except in two samples from birds in the probiotic-treated, immunized group at week 3, indicating that IgG isotype switching had occurred only in this group. However, due to the small number of positive samples, the data were not statistically analyzed. Collectively, these data indicate that treatment with probiotics enhanced the antibody response to SRBC, which was primarily of the IgM isotype, and prolonged the presence of this antibody response in serum. In addition, treatment with probiotics induced the IgG response in a subset of birds at later time points after immunization.
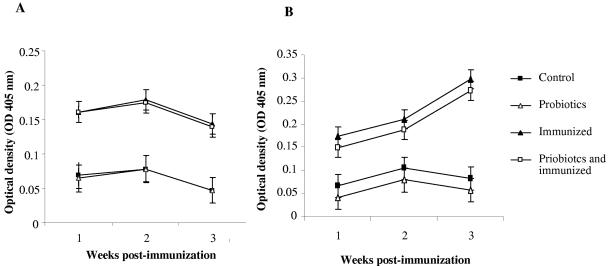
Immunization with BSA resulted in the appearance of anti-BSA antibodies of the IgM and IgG isotypes in serum, unlike in nonimmunized birds. This immunization protocol elicited an IgG response as early as week 1 postimmunization, which peaked by week 2 and lasted until week 3 (Fig. 2A). There was a significant difference between IgG responses in immunized and nonimmunized groups (P ≤ 0.001). However, combining probiotics with immunization did not significantly enhance the serum IgG response to BSA compared to the anti-BSA IgG response in the immunized group. An IgM response was elicited by BSA, which gradually increased from week 1 and peaked by week 3 postimmunization (Fig. 2B). Treatment with probiotics did not enhance the IgM-mediated response to BSA. In contrast, this treatment appeared to reduce the response, although the effect only approached significance (P < 0.06).
FIG. 2.
LSM of serum anti-BSA antibody as determined by ELISA. Error bars represent standard errors of the LSM. The groups were as follows: control (nonimmunized, nontreated), probiotics (nonimmunized, treated with probiotics), immunized (immunized with BSA, nontreated), and probiotics and immunized (immunized with BSA, treated with probiotics). (A) Anti-BSA IgG antibody response; (B) anti-BSA antibody IgM response.
Antibody response to TT.
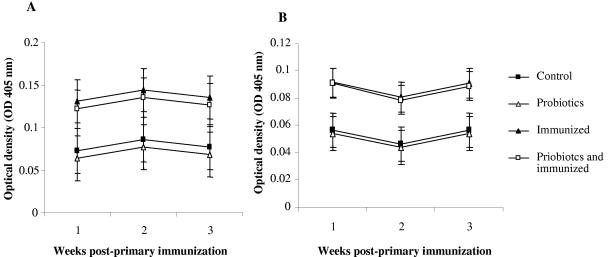
Tetanus toxoid was employed to assess IgA- and IgG-antibody-mediated responses in the gut. Immunization had a significant effect on the appearance of anti-TT IgA and IgG in the gut (P ≤ 0.001 and P ≤ 0.001, respectively). Both isotypes appeared in the gut as early as week 1 after the primary immunization and lasted for the duration of sampling, which ended at week 3 after the primary immunization (Fig. 3A and B). However, no significant change was observed in antibody responses between different sampling time points. Moreover, combining probiotics with immunization did not change antibody levels in the gut compared to those in birds that were immunized but not treated with probiotics. No specific anti-TT antibody responses of IgA, IgM, and IgG isotypes were detected in the sera of immunized birds (data not shown).
FIG. 3.
LSM of gut anti-TT antibody as determined by ELISA. Error bars represent standard errors of the LSM. The groups were as follows: control (nonimmunized, nontreated), probiotics (nonimmunized, treated with probiotics), immunized (immunized with TT, nontreated), and probiotics and immunized (immunized with TT, treated with probiotics). (A) Anti-TT IgA antibody response; (B) anti-TT antibody IgG response.
DISCUSSION
Administration of probiotic bacteria or their products may have immunomodulatory effects (26). Specifically, these bacteria may enhance the antibody response (11, 15). The present study was, therefore, aimed at elucidating the effects of probiotics on the development of systemic and mucosal immune responses in chickens. We used three antigens, BSA, SRBC, and TT, which have previously been reported to elicit antibody-mediated responses in chickens (7, 25, 29). When treated with probiotics, immunized birds mounted a significantly greater antibody response to SRBC, predominantly of the IgM isotype, in their sera than that of the immunized birds that did not receive probiotics.
Previous work with chickens has demonstrated that probiotics enhance the systemic antibody response to soluble antigens, such as trinitrophenyl (TNP)-keyhole limpet hemocyanin (KLH) and KLH alone, which, like SRBC, are classified as thymus-dependent immunogens (13, 17). In one study, chickens that were fed fermented liquid feed supplemented with various lactobacilli showed enhanced IgM and IgG responses to TNP (17). In another study, the administration of probiotics containing Lactobacillus acidophilus and Lactobacillus casei enhanced the serum IgA response to KLH, while the treatment did not influence the IgG response to this antigen (13). Our results extend the previous findings for chickens, which were obtained with soluble antigens, by showing that probiotics may also enhance the specific antibody response to a thymus-dependent cellular antigen. However, the exact mechanisms of the enhancement of immune responsiveness conferred by probiotics in our studies remain to be discovered. It is possible that some of these effects are mediated by cytokines secreted by immune system cells stimulated with probiotic bacteria (8, 19, 23). In this regard, Th2 cytokines, such as IL-4 and IL-10, play an important role, and indeed probiotic bacteria could stimulate the expression of these cytokines (8, 19, 31). Given the recent discovery of IL-4 and IL-10 in chickens (2, 34), it will be interesting to determine whether probiotics can steer cytokine production in this species, resulting in a polarized Th2-like response.
In the present study, we did not detect anti-SRBC IgG in the sera of chickens in the immunized group, while these antibodies were present only in 2/19 of birds in the probiotic-treated, immunized group. This difference might be due to the fact that we used a primary immunization protocol; other studies have demonstrated that after the secondary immunization with SRBC, antibodies of the IgG isotype predominate in serum (6). In addition, the age of the chicken and the route of administration of SRBC may have a role in the quantity and quality of antibody responses to this antigen (6, 7). In this study, we immunized young chicks (2 weeks of age) via the intramuscular route, whereas in other studies, older chickens, ranging from 28 to >100 days of age, were immunized through the intravenous route (6, 7). Also, it should be noted that the occurrence of hemagglutination observed in the anti-SRBC antibody detection assay is primarily mediated by IgM and, to a lesser extent, by IgG antibodies. Therefore, given the relatively low sensitivity of the direct hemagglutination test for the detection of IgG, it is possible that the sera of immunized birds in this study contained specific anti-SRBC IgG antibodies which could not be detected.
When levels of serum and gut antibodies to BSA and TT, respectively, were assessed for the immunization-only group and the probiotic-treated, immunized group, no significant difference was detected. For the anti-BSA response, there was a tendency for the probiotic-treated and immunized group to even have lower levels of specific IgM in their sera. In fact, there have been reports that combining probiotics with immunization may not enhance specific antibodies and could even result in the reduction of the antibody response in serum or in the gut contents (3, 9, 10). The main reason for this phenomenon is not known, but it is conceivable that several parameters are involved in determining the efficacy of probiotics in the stimulation of the immune response, and as a result, the immune-enhancing effects of probiotics may not be generalized. For example, Huang and coworkers (13) demonstrated that probiotics containing L. acidophilus and L. casei enhanced the serum IgA response to KLH, but that the treatment did not influence the IgG response to this antigen. In a different study, egg layer and broiler chickens treated with probiotics responded differently to TNP, with layer chickens mounting a significantly higher antibody response than broiler chickens, indicating that the genetic background of chickens plays an important role in the mediation of immunomodulatory activities of probiotics (17). Taken together, these findings support the notion that the immunomodulatory activities of probiotics in enhancing the antibody response are highly dependent on the antigen, immunization regimen, type and number of species of bacteria present in probiotics, and genetic background of the host.
In conclusion, this study provides evidence that the administration of probiotics to broiler chickens early in life enhances antibody responses, at least systemically, to cellular antigens, such as SRBC. It remains, however, to be determined whether probiotics can influence immune responsiveness at mucosal surfaces. Based on the data presented here, the gut antibody response to soluble antigens, such as TT, may not be enhanced by probiotics. Studies are under way by our group to examine the effects of probiotics on the immune response to gut bacteria. These studies should provide more insights into the immunomodulatory activities of probiotics and may be used in the future for the development of new products with an immune-enhancing ability.
Acknowledgments
This study was funded by the Ontario Ministry of Agriculture and Food (Food Safety Research and Poultry Research Programs), the Poultry Industry Council, and the SCIDF.
REFERENCES
- 1.Amit-Romach, E., D. Sklan, and Z. Uni. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 83:1093-1098. [DOI] [PubMed] [Google Scholar]
- 2.Avery, S., L. Rothwell, W. D. Degan, V. E. Schijns, J. Young, J. Kaufman, and P. Kaiser. 2004. Characterization of the first nonmammalian T2 cytokine gene cluster: the cluster contains functional single-copy genes for IL-3, IL-4, IL-13, and GM-CSF, a gene for IL-5 that appears to be a pseudogene, and a gene encoding another cytokinelike transcript, KK34. J. Interferon Cytokine Res. 24:600-610. [DOI] [PubMed] [Google Scholar]
- 3.Balevi, T., U. S. Ucan, B. Coskun, V. Kurtoglu, and I. S. Cetingul. 2001. Effect of dietary probiotic on performance and humoral immune response in layer hens. Br. Poult. Sci. 42:456-461. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shira, E., D. Sklan, and A. Friedman. 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27:147-157. [DOI] [PubMed] [Google Scholar]
- 5.Blum, S., D. Haller, A. Pfeifer, and E. J. Schiffrin. 2002. Probiotics and immune response. Clin. Rev. Allergy Immunol. 22:287-309. [DOI] [PubMed] [Google Scholar]
- 6.Boa-Amponsem, K., S. E. Price, M. Picard, P. A. Geraert, and P. B. Siegel. 2000. Vitamin E and immune responses of broiler pureline chickens. Poult Sci. 79:466-470. [DOI] [PubMed] [Google Scholar]
- 7.Boa-Amponsem, K., S. E. Price, E. A. Dunnington, and P. B. Siegel. 2001. Effect of route of inoculation on humoral immune response of White Leghorn chickens selected for high or low antibody response to sheep red blood cells. Poult Sci. 80:1073-1078. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, H. R., H. Frokiaer, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171-178. [DOI] [PubMed] [Google Scholar]
- 9.Dalloul, R. A., H. S. Lillehoj, T. A. Shellem, and J. A. Doerr. 2003. Intestinal immunomodulation by vitamin A deficiency and lactobacillus-based probiotic in Eimeria acervulina-infected broiler chickens. Avian Dis. 47:1313-1320. [DOI] [PubMed] [Google Scholar]
- 10.Dalloul, R. A., H. S. Lillehoj, T. A. Shellem, and J. A. Doerr. 2003. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 82:62-66. [DOI] [PubMed] [Google Scholar]
- 11.de Vrese, M., P. Rautenberg, C. Laue, M. Koopmans, T. Herremans, and J. Schrezenmeir. 2004. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur. J. Nutr. 44:406-413. [DOI] [PubMed] [Google Scholar]
- 12.Di Giacinto, C., M. Marinaro, M. Sanchez, W. Strober, and M. Boirivant. 2005. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 174:3237-3246. [DOI] [PubMed] [Google Scholar]
- 13.Huang, M. K., Y. J. Choi, R. Houde, J. W. Lee, B. Lee, and X. Zhao. 2004. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult. Sci. 83:788-795. [DOI] [PubMed] [Google Scholar]
- 14.Hume, M. E., L. F. Kubena, T. S. Edrington, C. J. Donskey, R. W. Moore, S. C. Ricke, and D. J. Nisbet. 2003. Poultry digestive microflora biodiversity as indicated by denaturing gradient gel electrophoresis. Poult. Sci. 82:1100-1107. [DOI] [PubMed] [Google Scholar]
- 15.Isolauri, E., Y. Sutas, P. Kankaanpaa, H. Arvilommi, and S. Salminen. 2001. Probiotics: effects on immunity. Am. J. Clin. Nutr. 73:444S-450S. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, H. Q., M. C. Thurnheer, A. W. Zuercher, N. V. Boiko, N. A. Bos, and J. J. Cebra. 2004. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine 22:805-811. [DOI] [PubMed] [Google Scholar]
- 17.Koenen, M. E., J. Kramer, R. van der Hulst, L. Heres, S. H. M Jeurissen, and W. J. A. Boersma. 2004. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 45:355-366. [DOI] [PubMed] [Google Scholar]
- 18.Kroese, F. G., R. de Waard, and N. A. Bos. 1996. B-1 cells and their reactivity with the murine intestinal microflora. Semin. Immunol. 8:11-18. [DOI] [PubMed] [Google Scholar]
- 19.Lammers, K. M., P. Brigidi, B. Vitali, P. Gionchetti, F. Rizzello, E. Caramelli, D. Matteuzzi, and M. Campieri. 2003. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 38:165-172. [DOI] [PubMed] [Google Scholar]
- 20.Lanning, D., X. Zhu, S. K. Zhai, and K. L. Knight. 2000. Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol. Rev. 175:214-228. [PubMed] [Google Scholar]
- 21.Lebman, D. A., and J. S. Edmiston. 1999. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 15:1297-1304. [DOI] [PubMed] [Google Scholar]
- 22.Lillehoj, H. S., and J. M. Trout. 1996. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 9:349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maassen, C. B., C. van Holten-Neelen, F. Balk, M. J. den Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 24.Macpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 25.Mast, J., and B. M. Goddeeris. 1999. Development of immunocompetence of broiler chickens. Vet. Immunol. Immunopathol. 70:245-256. [DOI] [PubMed] [Google Scholar]
- 26.McCracken, V. J., and H. R. Gaskins. 1999. Probiotics and the immune system, p. 85-112. In G. W. Tannock (ed.), Probiotics, a critical review. Horizon Scientific Press, Norfolk, United Kingdom.
- 27.Mohamadzadeh, M., S. Olson, W. V. Kalina, G. Ruthel, G. L. Demmin, K. L. Warfield, S. Bavari, and T. R. Klaenhammer. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 102:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir, W. I., W. L. Bryden, and A. J. Husband. 2000. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 24:325-342. [DOI] [PubMed] [Google Scholar]
- 29.Muir, W. I., A. J. Husband, and W. L. Bryden. 2002. Dietary supplementation with vitamin E modulates avian intestinal immunity. Br. J. Nutr. 87:579-585. [DOI] [PubMed] [Google Scholar]
- 30.Noverr, M. C., and G. B. Huffnagle. 2004. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 12:562-568. [DOI] [PubMed] [Google Scholar]
- 31.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 32.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 4:361-367. [DOI] [PubMed] [Google Scholar]
- 33.Rhee, K. J., P. Sethupathi, A. Driks, D. K. Lanning, and K. L. Knight. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 172:1118-1124. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell, L., J. R. Young, R. Zoorob, C. A. Whittaker, P. Hesketh, A. Archer, A. L. Smith, and P. Kaiser. 2004. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 173:2675-2682. [DOI] [PubMed] [Google Scholar]
- 35.Schultz, M., H. J. Linde, N. Lehn, K. Zimmermann, J. Grossmann, W. Falk, and J. Scholmerich. 2003. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J. Dairy Res. 70:165-173. [DOI] [PubMed] [Google Scholar]
- 36.Tlaskalova-Hogenova, H., R. Stepankova, T. Hudcovic, L. Tuckova, B. Cukrowska, R. Lodinova-Zadnikova, H. Kozakova, P. Rossmann, J. Bartova, D. Sokol, D. P. Funda, D. Borovska, Z. Rehakova, J. Sinkora, J. Hofman, P. Drastich, and A. Kokesova. 2004. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93:97-108. [DOI] [PubMed] [Google Scholar]