Abstract
In clinical settings, Lactobacillus johnsonii La1 administration has been reported to have a favorable effect on Helicobacter pylori-associated gastritis, although the mechanism remains unclear. We administered, continuously through the water supply, live La1 to H. pylori-infected C57BL/6 mice and followed colonization, the development of H. pylori-associated gastritis in the lamina propria, and the levels of proinflammatory chemokines macrophage inflammatory protein 2 (MIP-2) and keratinocyte-derived cytokine (KC) in the serum and gastric tissue over a period of 3 months. We documented a significant attenuation in both lymphocytic (P = 0.038) and neutrophilic (P = 0.003) inflammatory infiltration in the lamina propria as well as in the circulating levels of anti-H. pylori immunoglobulin G antibodies (P = 0.003), although we did not observe a suppressive effect of La1 on H. pylori colonizing numbers. Other lactobacilli, such as L. amylovorus DCE 471 and L. acidophilus IBB 801, did not attenuate H. pylori-associated gastritis to the same extent. MIP-2 serum levels were distinctly reduced during the early stages of H. pylori infection in the La1-treated animals, as were gastric mucosal levels of MIP-2 and KC. Finally, we also observed a significant reduction (P = 0.046) in H. pylori-induced interleukin-8 secretion by human adenocarcinoma AGS cells in vitro in the presence of neutralized (pH 6.8) La1 spent culture supernatants, without concomitant loss of H. pylori viability. These observations suggest that during the early infection stages, administration of La1 can attenuate H. pylori-induced gastritis in vivo, possibly by reducing proinflammatory chemotactic signals responsible for the recruitment of lymphocytes and neutrophils in the lamina propria.
Helicobacter pylori is a gram-negative spiral motile pathogen with a unique adaptive ability to colonize the hostile acidic environment of the stomach. Clinical manifestations of persistent chronic H. pylori infection range in severity from chronic active gastritis, chronic atrophic gastritis, and peptic ulceration to gastric mucosa-associated lymphoid tissue lymphoma and cancer. Early results from animal and clinical studies suggested that probiotics may contribute to the management of H. pylori infection (Maastricht 2-2000 Consensus) (35). Probiotics have been defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, October 2001). Various Lactobacillus probiotic strains have been demonstrated to exert antagonistic activity against gram-negative pathogens (43) and H. pylori in particular. More specifically, administration of lactic acid- or butyric acid-producing bacteria, such as Lactobacillus salivarius (1, 31), Lactobacillus gasseri (50), and Clostridium butyricum (49), has been shown to prevent H. pylori colonization in germ-free mice. Lactobacillus acidophilus strain LB supernatants have been demonstrated to inhibit Helicobacter felis colonization in conventional specific-pathogen-free BALB/c mice (11). Furthermore, continuous administration of Lactobacillus casei strain Shirota over a 9-month period resulted in reduction of H. pylori colonizing numbers and concomitant attenuation of associated gastritis in C57BL/6 mice infected with H. pylori SS1 (Sydney strain 1) (44). In a similar study involving the same infection model, a mixture of Lactobacillus rhamnosus and L. acidophilus also inhibited H. pylori colonization and reduced the number of animals developing gastritis but did not affect H. pylori-induced apoptosis in the gastric mucosa (30).
Results from a number of clinical trials suggest that administration of lactic acid bacteria (LAB) can have a moderate, yet in some cases significant, inhibitory effect on H. pylori colonization as well as H. pylori-associated gastritis (for a review, see reference 27). In these studies, LAB have been administered to asymptomatic H. pylori-infected patients either alone (9, 14, 26, 37, 40) or as adjunctive agents to conventional eradication therapy (8, 13, 15, 21). However, the majority of studies utilized the urea breath test, a method which may not be suitable for assessment of H. pylori colonization in clinical settings involving lactobacilli, because of the ability of the lactobacilli to reduce H. pylori urease activity (1, 11, 44). Moreover, when more stringent methods, such as quantitative gastric cultures, were employed for evaluation of H. pylori colonization, no significant effect was observed (40). Despite the conflicting reports of the ability of lactobacilli to affect H. pylori colonization, one interesting common observation was the significant attenuation of H. pylori-associated inflammation which was evident with the lactobacillus-administered groups. In particular, Lactobacillus johnsonii La1 (Nestlé, Switzerland) has been shown to exert such an anti-inflammatory effect in two double-blind, placebo-controlled clinical trials, where an acidified milk containing live La1 cells (LC-1) was administered alone (40) or as adjunct to antibiotic eradication therapy (21) to H. pylori-positive asymptomatic volunteers.
Specific CXC family chemokines, such as interleukin-8 (IL-8), growth-related oncogene alpha (GRO-α), gamma interferon-inducible protein 10 (IP-10), and monokine induced by gamma interferon (MIG), have been associated with the development of H. pylori gastritis during early infection stages (18, 54) by driving the chemotaxis of inflammatory cells such as neutrophils (IL-8 and GRO-α) and T lymphocytes (IP-10 and MIG). In rodents, murine macrophage inflammatory protein 2 (MIP-2) and Gro-α/keratinocyte-derived cytokine (KC) have been shown to possess distinct sequence homology to human GRO-α, -β, and -γ chemokines and are regarded as the murine functional homologs of human IL-8 (17, 42).
In the present study, we explored the potential anti-inflammatory effect of La1 on H. pylori-associated gastritis by the H. pylori SS1 strain infection model with C57BL/6 mice, following La1 continuous administration over a period of 3 months. We observed, following histological evaluation, a significant reduction in H. pylori-associated gastritis and reduced levels of proinflammatory chemokines MIP-2 and KC in the serum and in gastric explants from the animals. Similarly, lactobacillus spent culture supernatants significantly reduced H. pylori-induced secretion of IL-8 by human gastric epithelial cells in vitro.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Lactobacillus johnsonii La1, Lactobacillus amylovorus DCE 471, and Lactobacillus acidophilus IBB 801 were part of the lactic acid bacteria of the PROPATH collection, kindly provided by Luc De Vuyst (Free University, Brussels). They were grown in de Man-Rogosa-Sharpe (MRS) broth (Difco Laboratories, Detroit, Mich.) overnight (o/n) at 37°C (approximately 1010 CFU ml−1) prior to use. Respective cell-free spent culture supernatants (LAB-SCS) were prepared by centrifugation of liquid LAB cultures at 10,000 × g for 30 min at 4°C, filtered through a 0.22-μm-pore-size filter (Millipore, Molsheim, France), and checked for the absence of bacteria by plating on MRS agar. They were kept frozen at −20°C until use.
Early-passage H. pylori strain SS1 was kindly provided by R. L. Ferrero (Institut Pasteur, Paris, France). Helicobacter pylori strains CCUG 38770 (Culture Collection, University of Götenburg, Sweden) and 069A were generously provided by T. Wadström (University of Lund, Sweden) and A. L. Servin (INSERM U510, Chātenay-Malabry, France), respectively. All H. pylori strains were routinely cultured under microaerophilic conditions (CampyPak-Plus; Becton-Dickinson, Cockeysville, Md.) at 37°C on Wilkins-Chalgren agar enriched with 7% (vol/vol) horse blood and 1% (vol/vol) VITOX (Oxoid, Basingstoke, United Kingdom). Unless otherwise stated, highly motile bacillary H. pylori cells derived from fresh o/n cultures were used for all in vitro and in vivo procedures.
H. pylori infection of C57BL/6 mice and LAB administration.
Specific-pathogen-free 6- to 8-week-old male C57BL/6 mice were obtained from the Central Animal Facility of the Hellenic Pasteur Institute. They were housed according to relevant Greek national legislation, fed a commercial diet, and given water ad libitum, except as otherwise stated. H. pylori infections by the SS1 strain were carried out as described before (33, 44). Briefly, freshly prepared aliquots (100 μl, 106 CFU) of H. pylori SS1 strain in brain heart infusion broth (Oxoid) were administered to mice via orogastric inoculation three times within a week (days 1, 3, and 5). Accordingly, all noninfected control animals were inoculated with the same volume of plain brain heart infusion broth. LAB cultures were administered through the animals' drinking water, starting from the day of the last H. pylori challenge (day 5), over a period of 3 months. Daily water consumption and LAB viability in the water were monitored closely. Differences in the volumes consumed, possibly due to taste variations or potential osmolality changes following the addition of lactobacilli, between the animal groups were recorded and taken into account for the determination of the administered daily dose. The following groups of animals were included in the study: H. pylori-infected mice administered L. johnsonii La1 (Hp-La1 group; n = 15), L. amylovorus DCE 471 (Hp-DCE471 group; n = 15), or L. acidophilus IBB 801 (Hp-IBB801 group; n = 15). The control groups were H. pylori-infected mice left untreated (HpSS1 group; n = 15) or given nonfermented MRS medium (Hp-MRS group; n = 15). We also included uninfected mice (n = 15), as well as mice administered only the lactobacilli, namely, L. johnsonii La1 (La1 group; n = 15), L. amylovorus DCE 471 (DCE 471 group; n = 15), and L. acidophilus IBB 801 (IBB 801 group; n = 15). Mean daily lactobacillus consumption per animal was calculated to be 1.5 × 108 CFU for La1-administered groups, 2.1 × 108 CFU for DCE 471-administered groups, and 4.6 × 108 CFU for IBB 801-administered groups. At time intervals of 1, 6, and 12 weeks, blood samples were collected and five animals per group were sacrificed by cervical dislocation. All methods describing assessment of H. pylori colonization, LAB isolation, and identification in gastric and intestinal samples as well as evaluation of gastritis and anti-H. pylori immunoglobulin G (IgG) response have been described in detail before (44). Levels of MIP-2 and KC in mouse serum were determined by enzyme-linked immunosorbent assay (ELISA) Quantikine immunoassay kits (R&D Systems, Minneapolis, Minn.), according to the manufacturer's protocols. Analysis of the results with respect to H. pylori-associated gastritis was performed by the Wilcoxon rank sum test. Serum MIP-2 and KC levels were compared between study and control groups by two-tailed unpaired t test with Welch correction.
Determination of proinflammatory chemokines in gastric organ cultures from H. pylori-infected animals administered La1.
MIP-2 and KC secreted by the gastric mucosa were measured following a modification of the protocol of Siegmund et al. (46). Uninfected mice or mice infected with H. pylori were administered La1 as described in the previous section. At selected time points (days 6, 8, 12, and 18) following the first H. pylori challenge, blood samples were collected and eight animals per group were sacrificed by cervical dislocation. Excised stomachs were dissected and turned inside out. They were then washed in cold phosphate-buffered saline supplemented with 100 U/ml penicillin G and 100 μg/ml streptomycin sulfate (Sigma-Aldrich, Steinheim, Germany) and were placed in 24-well flat-bottomed culture plates (Greiner Bio-One, Frickenhausen, Germany) in 2 ml serum-free RPMI 1640 medium (Gibco, Inc., Grand Island, N.Y.) supplemented with penicillin and streptomycin. The culture medium was collected after 24 h of incubation at 37°C, centrifuged at 15,000 × g, and stored at −20°C until analyzed by ELISA (Quantikine immunoassay) for the presence of MIP-2 and KC. Analysis of the results was carried out by two-tailed unpaired t test with Welch correction.
In vitro H. pylori infection of AGS cells and determination of IL-8 levels.
Human gastric adenocarcinoma cell line AGS (ATCC CRL 1739) was maintained in F-12 Coon's modification medium (Euroclone, Ltd., United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 2 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate at 37°C in a 5% CO2 atmosphere. For the infection studies, AGS cells were seeded at a density of 1.7 × 105 cells per well and incubated o/n. Prior to infection with H. pylori, they were washed twice with fresh cell culture medium without antibiotics, and the media were replaced with antibiotic-free complete F-12 (10% fetal bovine serum). H. pylori bacteria were harvested from 18-hour solid cultures and resuspended in antibiotic-free complete F-12 medium. We standardized the H. pylori to AGS populations to achieve a multiplicity of infection of 100. In order to assess the effect of lactobacilli on H. pylori-induced IL-8 secretion, we simultaneously incubated live lactobacilli with H. pylori-infected AGS cells. However, we observed a massive destruction of the epithelial cell monolayer following a 24-hour incubation (data not shown). We therefore incubated H. pylori with LAB-SCS at a 1:1 (vol/vol) ratio for 1 h at 37°C under microaerophilic conditions, which allow for optimum H. pylori viability. Subsequently, the whole bacterial suspension was added to AGS monolayers and further incubated for 24 h (37°C in a 5% CO2 atmosphere). AGS supernatants were collected, centrifuged at 15,000 × g, and kept at −20°C until assayed for IL-8. LAB-SCS were used at their native pH of 4.5, as well as neutralized to pH 6.8 by addition of NaOH. Appropriate controls of acidified nonfermented MRS adjusted to pH 4.5 with 1 N hydrochloric acid or dl-lactic acid (final concentration, 100 mM; Sigma-Aldrich) were also included in the study. The corresponding controls of LAB-SCS at neutralized pH 6.8 were nonfermented MRS broth and MRS initially acidified with lactic acid to pH 4.5 and readjusted to pH 6.8 with 1 N NaOH. In all experiments, we assessed H. pylori morphology by microscopic observation and viability by plating serial dilutions of bacterial cultures on Wilkins-Chalgren agar. IL-8 levels in the AGS culture supernatants were determined by ELISA (Bender Medsystems, Vienna, Austria) according to the manufacturer's protocol. Absorbance at 450 nm (corrected for background at 620 nm) was measured with a Sunrise microtiter plate reader (Tekan, Grödig, Austria). Results were analyzed by two-tailed unpaired t test.
RESULTS
Effect of lactobacilli on H. pylori-infected and noninfected animals.
Animals showed no signs of discomfort or distress throughout the whole observation period. No differences in weight between animal groups were recorded over the observation period (data not shown), an indication that there was no adverse effect to the animals by the administration of lactic acid bacteria at such high concentrations.
We followed qualitatively the kinetics of the administered lactobacilli, isolating them from the wet feces of the animals. We identified several other commensal lactobacilli, such as Lactobacillus reuteri, L. gasseri, and Lactobacillus jensenii, in the murine gastric and intestinal microflora by sugar fermentation profiling (AP 150 CHL) and 16S to 23S rRNA typing (data not shown).
Evaluation of chronic and chronic active gastritis in H. pylori-infected animals.
Histopathologic evaluation of the gastric mucosa at 6 weeks postinfection revealed an induction of mild chronic gastritis (Tables 1 and 2) in the antra of animals in the HpSS1 control group. All gastric samples were characterized by mild infiltration of the lamina propria with scattered lymphocytes and neutrophils. At 12 weeks, we observed that two animals developed moderate chronic gastritis (Table 1) without formation of lymphoid follicles. However, there was an increase in active inflammation levels, as four animals developed moderate and one animal marked chronic active gastritis (Table 2). Development of glandular atrophy and intestinal metaplasia was not observed, as the time interval from the onset of infection was too short. No significant difference in levels of H. pylori-associated gastritis between the HpSS1 and Hp-MRS animal groups was observed (data not shown). Uninfected control animals developed no evidence of chronic or chronic active gastritis. A significant attenuation in chronic gastritis at 6 weeks was evident for all lactobacillus-treated H. pylori-infected animal groups, compared to the HpSS1 control (Table 1). This was more pronounced with the Hp-La1 group, where none of the animals developed changes of chronic gastritis (P = 0.003), followed by Hp-IBB801 (one animal with mild gastritis; P = 0.014) and Hp-DCE471 (two animals with mild gastritis; P = 0.048). However, at 12 weeks only the Hp-La1 mice maintained significantly mild changes of chronic gastritis (three animals with mild chronic gastritis; P = 0.038) compared to HpSS1 (Table 1). A trend towards reduced inflammatory lymphocytic infiltration of the gastric mucosa was also observed with the Hp-DCE471 and Hp-IBB801 groups, albeit never reaching statistical significance.
TABLE 1.
Chronic inflammatory infiltrationa in H. pylori-infected mice treated with lactobacilli
| Wk | Group | No. of mice of gradeb:
|
Pc | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| 6 | HpSS1 | 0 | 5 | 0 | 0 | |
| Hp-DCE471 | 3 | 2 | 0 | 0 | 0.047 | |
| Hp-La1 | 5 | 0 | 0 | 0 | 0.003 | |
| Hp-IBB801 | 4 | 1 | 0 | 0 | 0.014 | |
| 12 | HpSS1 | 0 | 3 | 2 | 0 | |
| Hp-DCE471 | 0 | 4 | 1 | 0 | 0.264 | |
| Hp-La1 | 2 | 3 | 0 | 0 | 0.038 | |
| Hp-IBB801 | 0 | 4 | 1 | 0 | 0.264 | |
Lymphocyte infiltration in the antrum.
Histopathology grades according to the updated Sydney system (16) are as follows: normal, 0; mild, 1; moderate, 2; and marked, 3.
Statistical analysis with reference to HpSS1 control group, done by Wilcoxon rank sum test. Significant correlations are depicted in italics.
TABLE 2.
Activity of chronic gastritisa in H. pylori-infected mice treated with lactobacilli
| Wk | Group | No. of mice of gradeb:
|
Pc | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| 6 | HpSS1 | 0 | 5 | 0 | 0 | |
| Hp-DCE471 | 3 | 2 | 0 | 0 | 0.047 | |
| Hp-La1 | 5 | 0 | 0 | 0 | 0.003 | |
| Hp-IBB801 | 4 | 1 | 0 | 0 | 0.014 | |
| 12 | HpSS1 | 0 | 0 | 4 | 1 | |
| Hp-DCE471 | 0 | 4 | 1 | 0 | 0.011 | |
| Hp-La1 | 4 | 1 | 0 | 0 | 0.003 | |
| Hp-IBB801 | 0 | 4 | 1 | 0 | 0.011 | |
Neutrophil infiltration in the antrum.
Histopathology grades according to the updated Sydney system (16) are as follows: normal, 0; mild, 1; moderate, 2; and marked, 3.
Statistical analysis with reference to HpSS1 control group, done by Wilcoxon rank sum test. Significant correlations are depicted in italics.
A pronounced reduction in the neutrophilic polymorphonuclear infiltration of the lamina propria was observed to occur in the antra of all lactobacillus-administered H. pylori-infected animals at 6 and 12 weeks (Table 2). Mice belonging to the Hp-La1 group showed no evidence of active inflammation at 6 weeks, and only one mouse developed mild changes at 12 weeks (P = 0.003). Two animals in the Hp-DCE471 group and one animal in the Hp-IBB801 group developed mild changes of active gastric inflammation at 6 weeks. Moreover, all but one animal in both groups developed mild active inflammatory infiltration at 12 weeks compared to the moderate or marked active inflammation observed for the HpSS1 control group (Table 2). No signs of chronic active inflammatory infiltration were observed for the control animal groups administered just the lactobacilli (data not shown). Therefore, continuous administration of lactobacilli during the early stages of H. pylori infection can affect monocytic (La1) as well as neutrophilic (La1, DCE 471, and IBB 801) polymorphonuclear infiltration in the lamina propria and thus may contribute to the attenuation and delayed onset of H. pylori-associated gastritis.
Effect of lactobacilli on the humoral anti-H. pylori response.
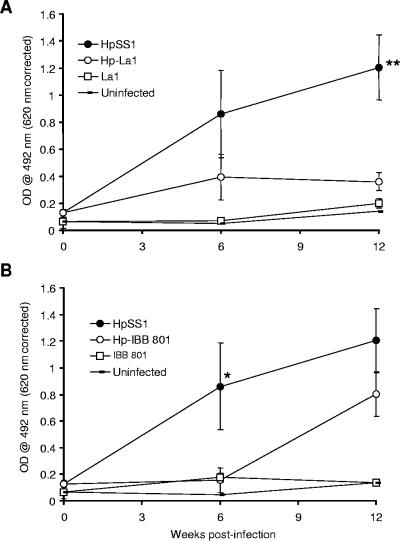
H. pylori-specific IgG antibodies are the dominant antibody class present in the sera of chronically infected mice (22) and may serve as an indicator of successful H. pylori infection. We measured anti-H. pylori IgG antibody titers in serum and observed a trend towards reduced titers in all lactobacillus-treated groups. More specifically, for the Hp-La1 group, we documented a significant reduction in anti-H. pylori titers at 12 weeks (P = 0.003) (Fig. 1A). Interestingly, for the Hp-IBB801 group, titers were significantly reduced at 6 weeks (P = 0.027) but not at 12 weeks (Fig. 1B). Finally, in the Hp-DCE471 group, there was a trend towards reduced anti-H. pylori titers throughout the whole experimental period, but the results never reached significance (data not shown).
FIG. 1.
Anti-H. pylori IgG antibody responses in H. pylori-infected animal groups Hp-La1 (A) or Hp-IBB801 (B) and HpSS1. Uninfected control mice having received La1 (A) or IBB 801 (B) are also depicted. Mouse sera were diluted to 1:50. A statistically significant decrease in anti-H. pylori titers was observed at 12 weeks postinfection for Hp-La1 mice (A) and at 6 weeks for Hp-IBB801 mice (B). A nonsignificant trend towards decreased anti-H. pylori titers was observed for IBB 801 at 12 weeks postinfection. OD, optical density; *, P value of <0.05; **, P value of <0.01.
Effect of lactobacilli on H. pylori colonization.
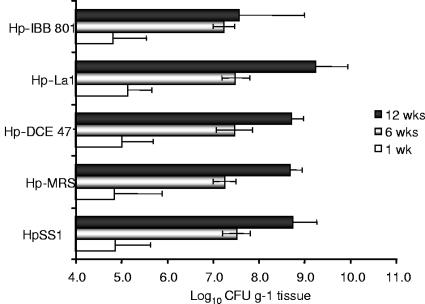
We detected a significant increase in the numbers of H. pylori colonizing bacteria in the HpSS1 control group throughout the 12-week observation period (Fig. 2). In the initial stages of infection (day 6), populations of H. pylori detected in the gastric tissue were in the range of 104 CFU g−1, rising to about 107 CFU g−1 by week 6 and 108 CFU g−1 by week 12. These results were consistent with the histopathologic observations, where H. pylori colonization was evaluated as much heavier (moderate or marked) at 6 and 12 weeks postinfection than at the beginning of the experiment (none or mild). No reduction in H. pylori colonizing levels throughout the whole observation period was determined for the lactobacillus-treated H. pylori-infected animal groups, assessed by either determination of quantitative cultures (Fig. 2) or histological evaluation (data not shown). Furthermore, an equal trend towards an increase in H. pylori colonizing numbers was observed for all animal groups, suggesting that the administered lactobacilli did not suppress H. pylori SS1 strain colonization in vivo.
FIG. 2.
H. pylori colonization in H. pylori-infected mice, following continuous administration of lactobacilli. Each time point (1 week [wk], 6 weeks [wks], and 12 wks) and bar represents the average of five animals. No significant reduction in H. pylori colonization due to administration of lactobacilli was observed for any group at any time point compared to the untreated H. pylori-infected study group.
Effect of lactobacilli on proinflammatory chemokine levels in serum and gastric mucosa.
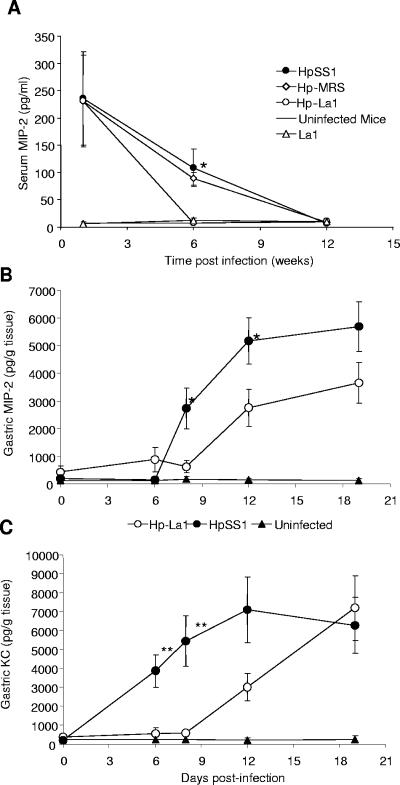
MIP-2 and KC are the dominant IL-8-like chemokines in inflammatory states in rodents and potent murine neutrophil chemoattractants (2, 6). We determined MIP-2 and KC levels in the serum samples from the animals and found significantly reduced levels of MIP-2 (Fig. 3A) but not KC (data not shown) at 6 weeks. More specifically, MIP-2 was high in serum during initial H. pylori infection stages (day 6) before the administration of lactobacilli and remained increased at 6 weeks postinfection for the HpSS1 control animals (P = 0.0412) but not for the Hp-La1 group (Fig. 3A). There was a similar trend towards reduced MIP-2 levels at 6 weeks with the Hp-DCE471 and Hp-IBB801 groups, without reaching significance (data not shown). We did not observe any increase in MIP-2 or KC levels with the lactobacillus-administered uninfected animal groups or the control uninfected mice.
FIG. 3.
Levels of proinflammatory cytokines in H. pylori-infected mice, following continuous administration of La1. (A) Serum MIP-2 protein levels in HpSS1, Hp-MRS, and Hp-La1 animal groups. Results for uninfected control mice and mice administered La1 are also depicted. Each point is the average of five animals. (B and C) Gastric MIP-2 (B) and KC (C) levels in H. pylori-infected mice with or without treatment with La1. Each point is the average of eight animals. Statistical analysis was carried out with respect to the untreated H. pylori controls (two-tailed unpaired t test, Welch correction).
We further assessed the levels of MIP-2 and KC in the gastric mucosae of H. pylori-infected animals during the first 3 weeks of H. pylori infection. We observed a time-dependent increase in the levels of MIP-2 starting at day 8 (Fig. 3B), whereas KC was detected as early as day 6 postinfection (Fig. 3C) in all H. pylori-infected animals. Both chemokines seemed to reach a plateau by day 19 postinfection. However, with the La1-treated H. pylori-infected animals, we observed a distinct delay in induction, reflected by significantly lower levels of both MIP-2 (day 8, P = 0.025; day 12, P = 0.040) and KC (day 6, P = 0.006; day 8, P = 0.008) (Fig. 3B and C, respectively). To ascertain that this observation was not due to reduced H. pylori colonization, we determined H. pylori viability in the gastric samples and found no differences between the groups with or without La1 administration (data not shown).
Effect of lactobacilli on H. pylori-induced IL-8 secretion by gastric epithelial cells.
Upon infection of AGS cells with H. pylori strains CCUG 38770 and 069A, significantly higher levels of IL-8 were induced at 24 h postinfection (636.5 pg ml−1, P < 0.0001, and 605.6 pg ml−1, P < 0.0001, respectively) than for uninfected AGS cells (11.8 pg ml−1). However, H. pylori strain SS1 induced 12-fold-lower IL-8 levels, which did not differ from basal levels produced by uninfected AGS cells. Bacillary and highly motile bacteria yielded higher IL-8 levels, whereas the presence of coccoid forms reduced the levels of induction (data not shown).
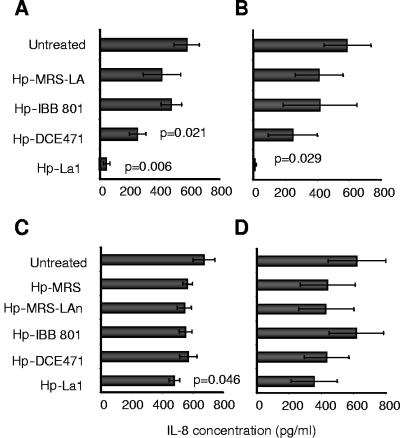
Following treatment with LAB-SCS at their native pH of 4.5, H. pylori strains were significantly affected in their ability to induce IL-8 by AGS cells, with concomitant reduction of their viability. More specifically, La1 supernatant (pH 4.5) reduced viability of H. pylori strains CCUG 38770 and 069A by nearly 5 log10 CFU ml−1 (Table 3) and significantly depressed their ability to induce IL-8 (P = 0.006 and P = 0.029, respectively) (Fig. 4A and B, respectively). A weaker though significant loss in viability (2 to 3 log10 CFU ml−1 [Table 3]) and reduction in IL-8 levels (P = 0.021) were observed when H. pylori CCUG 38770 was treated with DCE 471 (Fig. 4A). In order to exclude potential pH-dependent activity on H. pylori viability, we repeated the experiments with neutralized (pH 6.8) LAB-SCS and observed a significant reduction of IL-8 secretion in the case of H. pylori CCUG 38770 treated with La1 supernatant (P = 0.046) (Fig. 4C). A trend towards reduced IL-8 levels was also observed following treatment of H. pylori 069A with La1 supernatant, yet without reaching significance (Fig. 4D). In all experiments involving treatment with neutralized LAB-SCS, we did not observe any loss of H. pylori viability (Table 3), changes in its morphology and motility, or reduction in its ability to adhere to the gastric epithelial cells (B. Martinez-Gonzalez, unpublished data).
TABLE 3.
Viability of H. pylori strains CCUG 38770 and 069A following 1-h treatment with LAB-SCS at their native pH of 4.5 or neutralized to pH 6.8
| pH | Treatmentb | Viability (log10 CFU ml−1 ± SEM)a of H. pylori strain
|
|
|---|---|---|---|
| CCUG 38770 | 069A | ||
| 4.5 | Untreated | 8.1 ± 0.11 | 7.7 ± 0.11 |
| La1 | 3.3 ± 0.52** | <2** | |
| DCE 471 | 7.5 ± 0.13* | 5.4 ± 0.23** | |
| IBB 801 | 7.8 ± 0.06 | 6.3 ± 0.17* | |
| MRS-LA | 7.9 ± 0.09 | 6.9 ± 0.27 | |
| 6.8 | Untreated | 8.1 ± 0.07 | 7.8 ± 0.20 |
| La1 | 7.9 ± 0.09 | 7.1 ± 0.27 | |
| DCE 471 | 7.8 ± 0.20 | 7.3 ± 0.19 | |
| IBB 801 | 7.7 ± 0.31 | 7.3 ± 0.14 | |
| MRS-LAn | 7.5 ± 0.53 | 7.4 ± 0.17 | |
| MRS | 7.9 ± 0.02 | 7.3 ± 0.26 | |
*, P value of <0.05; **, P value of <0.01 (compared to the respective untreated sample [unpaired t test with Welch correction]).
MRS-LA, acidified non-fermented MRS adjusted to pH 4.5 with dl-lactic acid. MRS-LAn, MRS initially acidified with lactic acid to pH 4.5 and readjusted to pH 6.8 with 1 N NaOH.
FIG. 4.
H. pylori-induced IL-8 secretion by AGS cells, following treatment with LAB-SCS, at their native pH of 4.5 (A and B) or at a neutralized pH of 6.8 (C and D). H. pylori strains depicted are CCUG 38770 (A and C) and 069A (B and D). Statistical analysis was carried out with respect to the untreated H. pylori controls (two-tailed unpaired t test).
DISCUSSION
In the present study, we utilized an established animal model of H. pylori infection to evaluate the effect of continuous lactobacillus administration on H. pylori colonization and development of associated chronic gastritis. This model involves the mouse adapted H. pylori strain SS1, which colonizes the C57BL/6 mouse heavily and leads to the development of appreciable levels of gastritis closely mimicking human H. pylori infection (22, 32, 33, 48). We have applied this infection model in the past and have studied the effect of L. casei Shirota on H. pylori infection over a period of 9 months (44). In the present study, we have limited our observations to a period of 3 months because chronic active gastritis is more pronounced in this particular mouse strain during this period (34, 44).
Continuous administration of lactobacilli La1, DCE 471, and IBB 801 did not reduce the H. pylori colonizing numbers over this experimental period. In fact, bacterial numbers were increased during the course of the study, an observation made independently by determination of viable bacterial counts and histopathologic assessment of density of colonization. There are conflicting reports regarding the antimicrobial properties of lactobacilli. L. johnsonii La1, in particular, has been reported to exert antibacterial activity in vivo in conventional and germ-free mice infected with Salmonella enterica serovar Typhimurium as well as in vitro against a wide range of gram-negative and gram-positive pathogens, but not H. pylori (5). This antibacterial activity was attributed to a yet-unidentified nonbacteriocin substance which acted independently of lactic acid production. Clinical studies involving administration of La1 to asymptomatic H. pylori-positive volunteers, either alone (14, 26) or as adjunct agent to eradication therapy (21, 37), suggested a marginal effect on H. pylori colonization. However, in these studies, evaluation of H. pylori colonization was carried out by [13C]urea breath test and not by direct quantitative cultures, which would have presented a far more accurate result. In a double-blind, placebo-controlled randomized clinical trial in which colonization was evaluated by quantitative bacterial cultures, no difference in H. pylori colonization levels between the placebo- and the La1-administered volunteers was observed (40). Our data from the SS1 mouse model with reference to H. pylori colonization are in accordance with the latter findings and suggest that La1 may not exert an antimicrobial activity on H. pylori in vivo, despite reports about production of substances of a bacteriocin (3) or nonbacteriocin (5) nature. Furthermore, it would seem highly unlikely that an actively secreted bacteriocin produced by La1 would retain activity, with the abundance of proteolytic activity present in the gastric epithelium. Regarding DCE 471 and IBB 801, there have been no previous reports of in vitro or in vivo anti-H. pylori activity. DCE 471 initially isolated from corn steep liquor has been shown to produce the bacteriocin amylovorin 471, with a narrow antibacterial spectrum against gram-positive bacteria (7) and a very strong similarity within its 35-amino-acid N-terminal sequence with lactacin X, a product of L. johnsonii VP 11088 (23). Similarly, IBB 801 has been shown to produce a 6.5-kDa bacteriocin, named acidophilin 801, which displays a narrow inhibitory activity towards related lactobacilli but not gram-negative pathogenic bacteria (55).
We evaluated anti-H. pylori IgG titers in our mice primarily as a sign of successful H. pylori infection, as IgG is the dominant antibody class present in the sera of chronically infected mice (22). IgG levels in the La1-treated animals were significantly lower than levels in untreated, H. pylori-infected controls. For humans, significant decrease in IgG antibody titers has been suggested as an indicator for successful eradication of H. pylori (4, 20). In the past, we observed L. casei strain Shirota-mediated decrease in anti-H. pylori titers in C57BL/6 mice infected with the SS1 strain, but we attributed that effect to the inhibition of H. pylori colonization (44). Others have also documented a reduction in IgG antibodies following administration of lactobacilli to H. pylori-infected mice, as a result of decreased H. pylori colonization (1). However, in the present study, administration of La1 and to a lesser extent IBB 801 or DCE 471 significantly reduced IgG titers without concomitant decrease in H. pylori colonizing numbers. This may suggest that administration of lactobacilli and La1 in particular could potentially affect the humoral immune response towards H. pylori through possible induction of TH2 cellular subsets participating primarily in humoral responses. However, we did not evaluate IgG1/IgG2a subtype ratios in the sera of our mice because commercially available IgG2a sera, used for the isotype determination raised in BALB/c mice, fail to detect or grossly underestimate levels of IgG2c present in C57BL/6 mice (36).
In our experiments, although we did not observe any anti-H. pylori activity, a distinct attenuation in the neutrophilic polymorphonuclear inflammatory infiltration of the laminae propriae of the lactobacillus-administered animals was evident. Neutrophilic infiltration in the lamina propria is regarded as a hallmark of H. pylori-associated gastritis, and the density of intraepithelial neutrophilic infiltration has been well linked to the extent of mucosal damage and the intensity of H. pylori infection (47). Invading neutrophils can be a very sensitive indicator of H. pylori presence and disappear within days of cure of the infection. Furthermore, in the case of La1-treated animals there was an equally significant reduction in the levels of intramucosal infiltrating lymphocytes. This is an important observation, as chronic inflammatory cells have been shown to disappear slowly following H. pylori eradication and usually persist for a long time before they fall to the expected or “normal” levels in humans (25). These results suggest that lactobacilli and La1 in particular may exert an anti-inflammatory activity without necessarily affecting H. pylori colonization. Similar anti-inflammatory properties with concomitant reduction in proinflammatory cytokine expression, without a profound effect on pathogen colonization, have been observed with a murine Helicobacter hepaticus-induced inflammatory bowel disease model following administration of Lactobacillus spp. (41).
In humans, the recruitment and activation of inflammatory cells in the H. pylori-infected gastric mucosa is mediated by proinflammatory CXC chemokines, such as IL-8, GRO-α, IP-10, and MIG (12, 18, 45). Moreover, successful H. pylori eradication has been shown to result in significant reduction of mucosal IL-8 and GRO-α to nondetectable levels (54). Although mice lack a sequence homolog of human IL-8, chemokines such as MIP-2 and KC show distinct sequence homology to human GRO-α, -β, and -γ chemokines and are regarded as the murine functional homologs of human IL-8 (17, 42). Both have been reported to be very potent neutrophil attractants and activators, thus functioning as the major proinflammatory CXC chemokines in mice (2, 6, 19). A putative murine homolog of the human IL-8 receptor β, to which both mouse MIP-2 and KC bind with high affinity (28), has also been cloned.
Gastric transcriptional induction of these chemokines at the early stages of H. pylori infection in vitro and in vivo has been documented (18, 24, 38, 39), but there is no information describing levels of gastric and systemic MIP-2 and KC chemokines during early infection in the H. pylori SS1 model. We determined significantly higher levels of MIP-2 protein in the sera of the H. pylori-infected controls than in the sera of the lactobacillus-treated animals during the early stages of infection, namely, week 1 and week 6 postinfection. However, there was no difference in the levels of MIP-2 in serum at week 12 postinfection, by which time the H. pylori-infected control animals had already developed marked chronic active gastritis. Furthermore, in separate experiments we followed the kinetics of MIP-2 and KC protein secretion in murine gastric explants during the initial 3 weeks of H. pylori infection. Our findings indicate potential differences in the temporal expression of these two chemokines in the gastric mucosa, as the expression of KC preceded that of MIP-2 in the first days following H. pylori infection. A similar temporal pattern of KC and MIP-2 expression has been documented for inflammatory conditions induced by surgical injury in C57BL/6 mice (19), as a consequence of temporally ordered contribution of nonmyeloid and myeloid cell types involved in the expression of these two chemokines (2). Relevant spatial differences in the expression levels of GRO-α and IL-8 chemokines have been demonstrated for H. pylori-infected individuals (18). With the La1-treated animals, we observed a significant delay in the production of both MIP-2 and KC, as gastric mucosal levels of both chemokines were very low during the first 10 days postinfection. This could suggest a potential effect of La1 on temporal and/or spatial expression of these two chemokines by targeting specific cellular types. However, from this study we cannot draw any such conclusions, since we did not characterize the specific cellular populations expressing MIP-2 and KC in our mice.
Consistent with our in vivo observations that La1 administration reduced gastric mucosal levels of MIP-2 and KC, we also observed a significant decrease in IL-8 levels secreted by human gastric epithelial cells infected with H. pylori in vitro. IL-8 is the chief mediator of inflammatory responses during H. pylori infection in humans, the mucosal levels of which have been correlated with cellular inflammatory infiltration in the antrum (54). We observed that cell-free spent culture supernatants of La1 and DCE 471 at their native pH of 4.5 could dramatically reduce levels of IL-8 released in vitro by H. pylori-infected AGS cells. However, this was not a direct effect on IL-8 secretion, as La1 and in part DCE 471 induced stress-related morphological changes (U-shaped and coccoid formations) and significantly reduced H. pylori viability. When we repeated the experiments using supernatants neutralized to pH 6.8, we observed that only La1 still caused a significant reduction in IL-8 secretion by gastric epithelial cells. As we did not observe any reduction in H. pylori viability or changes in bacterial morphology and the binding efficiency of H. pylori to AGS cells (B. Martinez-Gonzalez, unpublished data), this could suggest that an La1-secreted compound(s) could potentially interfere with IL-8 induction directly. Indeed, previous reports indicate that La1-derived lipoteichoic acid or peptidoglycan trace contaminants in the lipoteichoic acid preparations can antagonize, in a dose-dependent manner, IL-8 production by human intestinal epithelial HT-29 cells in response to lipopolysaccharide and gram-negative bacteria (52). In H. pylori infection, NF-κB-dependent IL-8 induction is mediated by intracellular Nod1 through recognition of meso-diaminopimelate-containing tripeptidoglycan, transferred inside the epithelial cells through the type IV secretion system (51). Nod proteins have been proposed to function as intracellular sensors by which epithelial cells can discriminate pathogenic from nonpathogenic bacteria (10, 29). Consequently, an La1-secreted compound(s) may interfere with H. pylori peptidoglycan recognition by Nod1 or direct activation of Nod2 signaling. This could lead to attenuation of H. pylori-associated gastritis through possible downregulation of TH1 responses (53).
We observed a pronounced anti-inflammatory effect exerted by lactobacilli and La1 in particular on H. pylori-associated neutrophilic and lymphocytic infiltration and were able to document significantly reduced proinflammatory chemokine levels in the gastric mucosa during the early stages of H. pylori infection. It would be of great interest to further explore the role of such probiotic strains in the complex regulation of proinflammatory signal strength during early infection and identify the clinical potential in the induction of cellular inflammatory processes.
Acknowledgments
We thank Ingolf Nes and A. L. Servin for their helpful comments during the manuscript preparation. We also acknowledge the help and support of the staff of the Central Animal House of Institut Pasteur Hellenique and in particular A. Marandidou.
This study was financed by a grant from the Commission of the European Communities, specifically, RTD program “Quality of Life and Management of Living Resources,” QLK1-2001-01179, “PROPATH—Molecular analysis and mechanistic elucidation of the functionality of probiotics and prebiotics in the inhibition of pathogenic microorganisms to combat gastrointestinal disorders and to improve human health.”
The study does not necessarily reflect the views of the Commission of the European Communities and in no way anticipates the Commission's future policy in this area.
REFERENCES
- 1.Aiba, Y., N. Suzuki, A. M. Kabir, A. Takagi, and Y. Koga. 1998. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am. J. Gastroenterol. 93:2097-2101. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. A., J. A. Major, A. Chudyk, and T. A. Hamilton. 2004. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J. Leukoc. Biol. 75:641-648. [DOI] [PubMed] [Google Scholar]
- 3.Avonts, L., and L. De-Vuyst. 2001. Antimicrobial potential of probiotic lactic acid bacteria. Meded. Fac. Landbouwkd. Toegep. Biol. Wet. Univ. Gent 66:543-545. [PubMed] [Google Scholar]
- 4.Bergey, B., P. Marchildon, J. Peacock, and F. Megraud. 2003. What is the role of serology in assessing Helicobacter pylori eradication? Aliment. Pharmacol. Ther. 18:635-639. [DOI] [PubMed] [Google Scholar]
- 5.Bernet-Camard, M.-F., V. Liévin, D. Brassart, J.-R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozic, C. R., L. F. Kolakowski, Jr., N. P. Gerard, C. Garcia-Rodriguez, C. von Uexkull-Guldenband, M. J. Conklyn, R. Breslow, H. J. Showell, and C. Gerard. 1995. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 154:6048-6057. [PubMed] [Google Scholar]
- 7.Callewaert, R., H. Holo, B. Devreese, J. Van Beeumen, I. Nes, and L. De Vuyst. 1999. Characterization and production of amylovorin L471, a bacteriocin purified from Lactobacillus amylovorus DCE 471 by a novel three-step method. Microbiology 145:2559-2568. [DOI] [PubMed] [Google Scholar]
- 8.Canducci, F., A. Armuzzi, F. Cremonini, G. Cammarota, F. Bartolozzi, P. Pola, G. Gasbarrini, and A. Gasbarrini. 2000. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment. Pharmacol. Ther. 14:1625-1629. [DOI] [PubMed] [Google Scholar]
- 9.Cats, A., E. J. Kuipers, M. A. R. Bosschaert, R. G. J. Pot, C. M. J. E. Vandenbroucke-Grauls, and J. G. Kusters. 2003. Effect of frequent consumption of a Lactobacillus casei-containing milk drink in Helicobacter pylori-colonized subjects. Aliment. Pharmacol. Ther. 17:429-435. [DOI] [PubMed] [Google Scholar]
- 10.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 11.Coconnier, M.-H., V. Lievin, E. Hemery, and A. L. Servin. 1998. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 64:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree, J. E., J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, J. N. Primrose, and I. J. Lindley. 1994. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 47:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremonini, F., S. Di Caro, M. Covino, A. Armuzzi, M. Gabrielli, L. Santarelli, E. C. Nista, G. Cammarota, G. Gasbarrini, and A. Gasbarrini. 2002. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am. J. Gastroenterol. 97:2744-2749. [DOI] [PubMed] [Google Scholar]
- 14.Cruchet, S., M. C. Obregon, G. Salazar, E. Diaz, and M. Gotteland. 2003. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 19:716-721. [DOI] [PubMed] [Google Scholar]
- 15.De Francesco, V., V. Stoppino, C. Sgarro, and D. Faleo. 2000. Lactobacillus acidophilus administration added to omeprazole/amoxycillin-based double therapy in Helicobacter pylori eradication. Dig. Liver Dis. 32:746-747. [DOI] [PubMed] [Google Scholar]
- 16.Dixon, M. F., R. M. Genta, J. H. Yardley, P. Correa, et al. 1996. Classification and grading of gastritis. The updated Sydney system. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll, K. E. 1994. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp. Lung Res. 20:473-490. [DOI] [PubMed] [Google Scholar]
- 18.Eck, M., B. Schmausser, K. Scheller, A. Toksoy, M. Kraus, T. Menzel, H. K. Müller-Hermelink, and R. Gillitzer. 2000. CXC chemokines Groα/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin. Exp. Immunol. 122:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endlich, B., D. Armstrong, J. Brodsky, M. Novotny, and T. A. Hamilton. 2002. Distinct temporal patterns of macrophage-inflammatory protein-2 and KC chemokine gene expression in surgical injury. J. Immunol. 168:3586-3594. [DOI] [PubMed] [Google Scholar]
- 20.Fanti, L., R. Ieri, G. Mezzi, P. A. Testoni, S. Passaretti, and M. Guslandi. 2001. Long-term follow-up and serologic assessment after triple therapy with omeprazole or lansoprazole of Helicobacter-associated duodenal ulcer. J. Clin. Gastroenterol. 32:45-48. [DOI] [PubMed] [Google Scholar]
- 21.Felley, C. P., I. Corthesy-Theulaz, J. L. Rivero, P. Sipponen, M. Kaufmann, P. Bauerfeind, P. H. Wiesel, D. Brassart, A. Pfeifer, A. L. Blum, and P. Michetti. 2001. Favourable effect of an acidified milk (LC-1) on Helicobacter pylori gastritis in man. Eur. J. Gastroenterol. Hepatol. 13:25-29. [DOI] [PubMed] [Google Scholar]
- 22.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1998. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 66:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frémaux, C., C. Ahn, and T. R. Klaenhammer. 1993. Molecular analysis of the lactacin F operon. Appl. Environ. Microbiol. 59:3906-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genta, R. M., G. M. Lew, and D. Y. Graham. 1993. Changes in the gastric mucosa following eradication of Helicobacter pylori. Mod. Pathol. 6:281-289. [PubMed] [Google Scholar]
- 26.Gotteland, M., and S. Cruchet. 2003. Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J. Antimicrob. Chemother. 51:1317-1319. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton-Miller, J. M. 2003. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int. J. Antimicrob. Agents 22:360-366. [DOI] [PubMed] [Google Scholar]
- 28.Heinrich, J. N., and R. Bravo. 1995. The orphan mouse receptor interleukin (IL)-8R beta binds N51. Structure-function analysis using N51/IL-8 chimeric molecules. J. Biol. Chem. 270:4987-4989. [DOI] [PubMed] [Google Scholar]
- 29.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 30.Johnson-Henry, K. C., D. J. Mitchell, Y. Avitzur, E. Galindo-Mata, N. L. Jones, and P. M. Sherman. 2004. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 49:1095-1102. [DOI] [PubMed] [Google Scholar]
- 31.Kabir, A. M., Y. Aiba, A. Takagi, S. Kamiya, T. Miwa, and Y. Koga. 1997. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut 41:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, D. H., S. W. Kim, Y. J. Song, T. Y. Oh, S. U. Han, Y. B. Kim, H. J. Joo, Y. K. Cho, D. Y. Kim, S. W. Cho, M. W. Kim, J. H. Kim, and K. B. Hahm. 2003. Long-term evaluation of mice model infected with Helicobacter pylori: focus on gastric pathology including gastric cancer. Aliment. Pharmacol. Ther. 18:14-23. [DOI] [PubMed] [Google Scholar]
- 33.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 34.Mähler, M., C. Janke, S. Wagner, and H. J. Hedrich. 2002. Differential susceptibility of inbred mouse strains to Helicobacter pylori infection. Scand. J. Gastroenterol. 37:267-278. [DOI] [PubMed] [Google Scholar]
- 35.Malfertheiner, P., F. Mégraud, C. O'Morain, A. P. S. Hungin, R. Jones, A. Axon, D. Y. Graham, G. Tytgat, and The European Helicobacter Pylori Study Group (EHPSG). 2002. Current concepts in the management of Helicobacter pylori infection—The Maastricht 2-2000 Consensus Report. Aliment. Pharmacol. Ther. 16:167-180. [DOI] [PubMed] [Google Scholar]
- 36.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BLr6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 37.Michetti, P., G. Dorta, P. H. Wiesel, D. Brassart, E. Verdu, M. Herranz, C. Felley, N. Porta, M. Rouvet, A. L. Blum, and I. Corthésy-Theulaz. 1999. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion 60:203-209. [DOI] [PubMed] [Google Scholar]
- 38.Obonyo, M., D. G. Guiney, J. Harwood, J. Fierer, and S. P. Cole. 2002. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 70:3295-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obonyo, M., D. G. Guiney, J. Fierer, and S. P. Cole. 2003. Interactions between inducible nitric oxide and other inflammatory mediators during Helicobacter pylori infection. Helicobacter 8:495-502. [DOI] [PubMed] [Google Scholar]
- 40.Pantoflickova, D., I. Corthesy-Theulaz, G. Dorta, M. Stolte, P. Isler, F. Rochat, M. Enslen, and A. L. Blum. 2003. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment. Pharmacol. Ther. 18:805-813. [DOI] [PubMed] [Google Scholar]
- 41.Peña, J. A., A. B. Rogers, Z. Ge, V. Ng, S. Y. Li, J. G. Fox, and J. Versalovic. 2005. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect. Immun. 73:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schall, T. J. 1994. The chemokines, p. 419-460. In A. Thomson (ed.), The cytokine handbook, 2nd ed. Academic Press, Inc., San Diego, Calif.
- 43.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 44.Sgouras, D., P. Maragkoudakis, K. Petraki, B. Martinez-Gonzalez, E. Eriotou, S. Michopoulos, G. Kalantzopoulos, E. Tsakalidou, and A. Mentis. 2004. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 70:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma, S. A., M. K. R. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 46.Siegmund, B., H. A. Lehr, G. Fantuzzi, and C. A. Dinarello. 2001. IL-1 beta-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 98:13249-13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolte, M., and S. Eidt. 1992. Chronic erosions of the antral mucosa: a sequela of Helicobacter pylori-induced gastritis. Z. Gastroenterol. 30:846-850. [PubMed] [Google Scholar]
- 48.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, M., H. Taguchi, H. Yamaguchi, T. Osaki, and S. Kamiya. 2000. Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J. Med. Microbiol. 49:635-642. [DOI] [PubMed] [Google Scholar]
- 50.Ushiyama, A., K. Tanaka, Y. Aiba, T. Shiba, A. Takagi, T. Mine, and Y. Koga. 2003. Lactobacillus gasseri OLL2716 as a probiotic in clarithromycin-resistant Helicobacter pylori infection. J. Gastroenterol. Hepatol. 18:986-991. [DOI] [PubMed] [Google Scholar]
- 51.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 52.Vidal, K., A. Donnet-Hughes, and D. Granato. 2002. Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria. Infect. Immun. 70:2057-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe, T., A. Kitani, P. J. Murray, and W. Strober. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5:800-808. [DOI] [PubMed] [Google Scholar]
- 54.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamfir, M., R. Callewaert, P. C. Cornea, L. Savu, I. Vatafu, and L. De Vuyst. 1999. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB 801. J. Appl. Microbiol. 87:923-931. [DOI] [PubMed] [Google Scholar]