Abstract
Mycoplasma alligatoris causes acute lethal primary infection of susceptible hosts. A genome survey implicated sialidase and hyaluronidase, potential promoters of CD95-mediated eukaryotic cell death, as virulence factors of M. alligatoris. We used immunofluorescence imaging and flow cytometry to examine the effects of M. alligatoris infection in vitro on CD95 expression and apoptosis by alligator cardiac fibroblasts, a major cell type of a target organ of M. alligatoris infection in vivo. A uniform distribution of CD95 in primary cultured cardiac, skeletal muscle, and embryonic fibroblasts was demonstrated by using polyclonal antibodies against the N or C terminus of mouse or human CD95. Anti-CD95 antibodies reacted on Western blots of fibroblast lysates with a band with the predicted apparent molecular weight of CD95, but soluble CD95 was not detected in plasma from control or M. alligatoris-infected alligators. The proportion of CD95-gated cardiac fibroblasts increased threefold (P < 0.01) 48 h after inoculation with M. alligatoris. Infection induced morphological changes in cardiac fibroblasts, including translocation of CD95 characteristic of apoptosis and an eightfold increase (P < 0.16) in 5-bromo-2′-deoxyuridine (BrdU) incorporation measured in a terminal deoxynucleotide transferase dUTP nick end-labeling apoptosis assay. The proportion of BrdU-gated controls activated with agonistic immunoglobulin M against human CD95 also increased threefold (P < 0.03 for muscle). Heat-inactivated M. alligatoris and sterile M. alligatoris-conditioned culture supernatant had no effect. This is the first report of a CD95 homolog in the class Reptilia and establishes a new model that can be used to test the direct bacterial interaction with upstream components of the CD95 signal transduction pathway.
In contrast to the usually subtle mycoplasmoses, Mycoplasma alligatoris causes acute lethal primary infection of susceptible hosts (7). Pathological changes in affected individuals reflect multisystem inflammatory disease, including necrotic epicarditis, pericarditis, and myocarditis (8, 9). A comparative genome survey approach to the identification of candidate virulence mechanisms revealed that M. alligatoris strain A21JP2T possesses genes for the “spreading factors” sialidase (nanI) and hyaluronidase (nagH), a combination unprecedented among mycoplasmas (10). Functional studies demonstrated expression of the corresponding glycosidase activities, which might degrade host extracellular matrix (ECM) glycans to facilitate the rapid invasiveness of M. alligatoris infection (16, 28, 31, 41, 48). Its attenuated sibling species, Mycoplasma crocodyli strain MP145T (43), also possesses hyaluronidase, so direct ECM damage alone seems insufficient to explain the particular virulence of M. alligatoris. M. crocodyli MP145T does not possess sialidase (10). Desialylation of the eukaryotic cell death inducer CD95 (the fibroblast-associated receptor FasR) by sialidase substantially promoted CD95-mediated apoptosis in B-lineage leukemias and Jurkat T-cell lymphoma (19, 52). In addition, the signal-transducing hyaluronan (HA) receptor CD44 (36), present but inactivated by sialylation on most eukaryotic cells (3, 14, 19, 23, 33, 34), is uniquely modulated by the specific combination of sialidase and hyaluronidase. Sialidase can expose CD44 to promote HA binding; and in many cell types, CD44 binding of low-molecular-weight fragmented HA, such as that generated by hyaluronidase (19, 24, 35, 36), upregulates CD95 (21, 56). Those observations led to the hypothesis that the NanI and NagH glycosidases of M. alligatoris might directly or synergistically potentiate CD95-mediated death of some host cell types during infection, contributing to the fulminant disease observed. In this report we describe the increased CD95 expression and apoptosis of primary cultured cardiac and other fibroblasts following infection with M. alligatoris.
MATERIALS AND METHODS
Cell culture.
Primary cardiac fibroblast cultures were established from the myocardium of an adult alligator that was culture, PCR, and serologically negative for M. alligatoris. Control cell lines were established similarly from adult alligator skeletal muscle and from minced alligator embryo at the 22-day stage of development (20). The cells were cultured as monolayers in Dulbecco's modified Eagle's medium (DMEM; Cellgro/Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Cellgro) plus 50 U/ml penicillin and 50 μg/ml streptomycin (Cellgro) and lifted with 0.05% trypsin plus 0.53 mM EDTA (Cellgro). Cultures were incubated at 28°C in a humidified atmosphere with 5% CO2. The cells were passaged without attempts to transform or immortalize them, in order to retain the in vivo differentiated characteristics of the cell types (11, 12). The source species of each cell line was confirmed by mitochondrial DNA genotyping (26).
CD95.
The CD95 expression by fibroblasts was detected by fluorescence imaging and flow cytometry, as described below, with affinity-purified rabbit polyclonal immunoglobulin G (IgG) primary antibodies against synthetic peptides mapping at either the N terminus (residues 29 to 44) or the C terminus (residues 316 to 335) of CD95 of mouse origin (15 to 20 μg/ml ab13550; Abcam, Cambridge, MA) or human origin (2 μg/ml C-20 [sc-715]; Santa Cruz Biotechnology, Santa Cruz, CA) or immunoaffinity-purified mouse monoclonal IgM primary antibody against the extracellular domain of CD95 of human origin (5 μg/ml CH11; Upstate, Lake Placid, NY) (29). Mouse monoclonal IgG1 primary antibody against the extracellular domain of CD95 of human origin (30 μg/ml DX2; Abcam) was also tested. The primary antibodies were detected by using 2 μg/ml fluorescein isothiocyanate (FITC)-conjugated (sc-2012; Santa Cruz Biotechnology), Alexa Fluor 488-conjugated (A-11008; Molecular Probes, Eugene, OR), or tetramethylrhodamine-conjugated (AP130R; Chemicon International, Temecula, CA) secondary antibodies. Negative controls were exposed only to the respective secondary antibodies (2, 32).
Reduced fibroblast lysates and plasma samples from control and M. alligatoris-infected alligators (9) were probed for the presence of CD95 by using a Western blot assay. Plasma was diluted 1:50 in sample buffer plus reducing agent (NuPAGE LDS; Invitrogen, Carlsbad, CA) and then heated to 70°C for 10 min before electrophoresis through 10% bis-Tris gels (Invitrogen). The positive control was a HeLa epithelial cell lysate. Separated proteins were transferred to nitrocellulose by electroblotting at 30 V for 1 h, and then the blots were blocked in phosphate-buffered saline (PBS) plus 0.1% Tween 20 (PBS-T) and 5% nonfat dry milk (5% PBS-TM) for 1 h at 25°C or overnight at 4°C. The blots were probed with 0.4 μg/ml C-20, 4 μg/ml ab13550, 0.67 μg/ml CH11, or 8 μg/ml DX2 in 2% PBS-TM for 1 h and then washed five times in 2% PBS-TM and incubated with 0.13 μg/ml alkaline phosphatase-conjugated secondary antibodies (Promega, Madison, WI) in 2% PBS-TM for 1 h. The blots were washed three times in PBS-T, followed by two washes in PBS, and were developed with 5-bromo-4-chloro-3-indolyl phosphate plus Nitro Blue Tetrazolium (Sigma-Aldrich, St. Louis, MO).
Infection studies.
Subconfluent (50 to 70%) fibroblasts in six-well, tissue culture-treated polystyrene plates (Costar 3516; Corning, Corning, NY) were inoculated with 106 or 109 CFU of M. alligatoris strain A21JP2T in the logarithmic phase of growth in American Type Culture Collection medium 988 (SP4) broth supplemented with glucose and 20% FBS. The inoculum replaced 10% of the 2-ml volume of the DMEM. The inoculated cells were incubated for 4, 12, 24, 48, or 96 h at 28°C. Mycoplasmal viability was confirmed by culture of an aliquot of the inoculated DMEM on SP4 agar at the end of the incubation period, and the identities of the mycoplasmas recovered were confirmed by 16S rRNA gene PCR-restriction fragment length polymorphism analysis (8). In addition to untreated controls, negative control fibroblasts were cultured in DMEM plus heat-inactivated M. alligatoris culture or sterile M. alligatoris-conditioned SP4 culture supernatant, which replaced 10% of the volume of the culture medium.
Apoptosis.
Apoptosis of infected fibroblasts was detected by immunofluorescence staining in a species-independent terminal deoxynucleotide transferase (TdT) dUTP nick end-labeling (TUNEL) assay according to the protocols of the supplier (APO-BrdU TUNEL assay kit; Molecular Probes). Briefly, DNA 3′-hydroxyl ends exposed by nucleases as a consequence of apoptosis were labeled with 5-bromo-2′-deoxyuridine (BrdU) 5′-triphosphate (BrdUTP) by TdT. The BrdU incorporated into the DNA was detected with Alexa Fluor 488-conjugated anti-BrdU mouse monoclonal antibody (MAb) PRB-1 (Molecular Probes) by fluorescence imaging and flow cytometry, as described below. Most cells are susceptible to CD95 activation with multivalent agonists such as CD95-specific IgM (44). Apoptosis was induced in positive control fibroblasts by incubation for up to 48 h at 28°C with 10 to 20 ng/ml IgM CH11, an activating α-CD95 antibody previously shown to induce a variety of cell types to undergo CD95-mediated apoptosis (5). Untreated and negative control fibroblasts were cultured as described above for the infection studies.
Fluorescence imaging.
Fibroblasts were cultured as monolayers on coverslips in six-well plates for the CD95 expression and infection studies described above. After media were removed from the wells, the coverslips were rinsed in PBS and then fixed in frozen methanol for 2 min or in 2% paraformaldehyde in PBS for 20 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min, with a final rinse in PBS. For imaging, the fibroblast nuclei were labeled with DAPI (4′,6′-diamidino-2-phenylindole hydrochloride), according to the protocol of the supplier (Vectashield mounting media; Vector Laboratories, Burlingame, CA). Cytoskeletal actin was visualized by labeling with tetramethylrhodamine-conjugated phalloidin, according to the protocol of the supplier (Molecular Probes). Cytoskeletal α-tubulin was visualized by labeling with a 1:500 dilution of mouse ascitic fluid containing primary IgG1 monoclonal DM1A antibody against chick brain α-tubulin (Sigma-Aldrich), detected by using 5 μg/ml tetramethylrhodamine-conjugated goat anti-mouse secondary antibody (Chemicon). Membrane-associated and cytoplasmic β-catenin was visualized by labeling with a 1:1,000 dilution of rabbit serum containing primary antibodies against a synthetic peptide corresponding to the C-terminal region (residues 768 to 781) of human and mouse β-catenin (C2206; Sigma-Aldrich), detected by using 2 μg/ml Alexa Fluor 488-conjugated secondary antibody. The slides were observed with a Leica DMR epifluorescence microscope fitted with an HCX PL APO 63X oil-immersion objective lens (Leica Microsystems, Bannockburn, IL), and the images were recorded by using a Hamamatsu Orca-285 high-resolution digital camera (Hamamatsu, Bridgewater, NJ) and IPlab software (Scanalytics, Fairfax, VA).
Flow cytometry.
To quantitate CD95 expression and apoptosis of untreated, CH11-induced, and M. alligatoris-infected fibroblasts, cell monolayers were rinsed with PBS, lifted with 0.05% trypsin, washed two times with PBS plus 3% FBS (PBS-FBS), and then incubated in 200 to 300 μl of 10 to 20 μg/ml ab13550 in PBS-FBS at 25°C (27) for 1 h or with monoclonal antibody PRB-1 at 25°C, according to the protocol of the supplier. Fibroblasts were washed three times by low-speed centrifugation and resuspension in PBS-FBS and then incubated in 2 μg/ml Alexa Fluor 488-conjugated secondary antibody in PBS-FBS at 25°C (27) for 1 h. Negative controls were incubated with secondary antibodies only. The final volume was adjusted to 500 μl with PBS, and labeling was analyzed by flow cytometry by using a FACSort fluorescence-activated cell sorter and CELLQuest software (BD Biosciences, San Jose, CA). A minimum of 104 cells was counted for each sample. The gate was set to exclude approximately 99.5% of the negative control cells. At least duplicate independent measurements of the effects of each treatment were performed. The effect of treatment on the proportion of CD95-gated cells was analyzed by using the Kruskal-Wallis nonparametric equivalent of a one-way analysis of variance by ranks and by Fisher's protected least significant difference (PLSD) test for post-hoc pairwise comparisons of ranked means, split by fibroblast type, when the effect of treatment was significant (1). Because the PLSD comparisons were confounded by treatment-fibroblast type interaction for the TUNEL assay data (see below), unpaired t tests were used for post-hoc comparisons for that assay.
RESULTS
A comparative genome survey had previously implicated sialidase and hyaluronidase, potential direct or synergistic promoters of CD95-mediated host cell death (12, 14, 36, 38), as virulence factors of M. alligatoris (10). This study established the existence of a CD95 homolog in alligators by use of antibodies against mammalian CD95 and examined the effects of in vitro infection with M. alligatoris on expression of CD95 and apoptosis by primary cultured cardiac fibroblasts, which are a major cell type of a target organ of M. alligatoris infection in vivo (7).
Primary cultured fibroblasts express CD95.
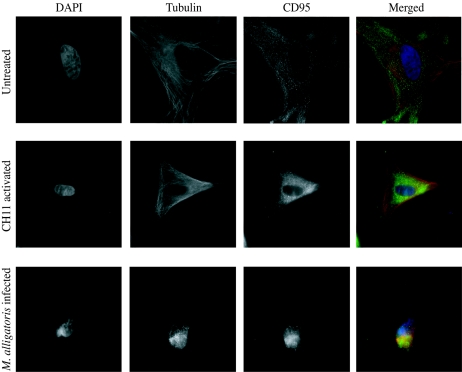
Untreated primary cultured cardiac, skeletal muscle, and embryonic fibroblasts had an approximate doubling time of 10 days. Cardiac fibroblasts could be subcultivated at a ratio of 1:2 for up to six passages, but skeletal muscle and embryonic fibroblasts were passaged more than 10 times. A uniform distribution of CD95 in fixed cardiac, skeletal muscle, and embryonic fibroblasts could be demonstrated by fluorescence microscopy with antibodies against mammalian CD95. The results of fluorescence imaging were similar for antibodies ab13550 against the N terminus of mouse CD95 (Fig. 1) and C-20 against the C terminus of human CD95. The labeling with two different antibodies against synthetic peptides, one mapping at the N terminus of mammalian CD95 and the other mapping at the C terminus of mammalian CD95, was strong evidence of the specificity of the results. The results were also similar when the agonistic antibody CH11 was used against the extracellular domain of human CD95 as the primary antibody for fluorescence imaging; but mouse monoclonal IgG primary antibody DX2 against the extracellular domain of human CD95, previously reported to bind to and stimulate significant levels of apoptosis in Xenopus splenocytes examined by immunohistochemistry (40), did not bind to alligator fibroblasts. Sharper and brighter images were obtained by using Alexa Fluor 488-labeled secondary antibody compared with those obtained with FITC-labeled secondary antibody. These data constitute the first reported demonstration of a CD95 homolog in the class Reptilia. Cytoskeletal actin did not stain well with tetramethylrhodamine-conjugated phalloidin, but cytoskeletal α-tubulin and membrane-associated and cytoplasmic β-catenin was readily visualized with the reagents developed for fluorescence imaging of mammalian cells. The α-tubulin IgG labeling was clearly specific to the cytoskeleton (Fig. 1), and the β-catenin IgG label was partitioned between membrane junctions and the cytosol, as expected (data not shown). These findings help to rule out nonspecific binding of the primary IgGs. In addition, there was no nonspecific binding of three different purified goat polyclonal IgG secondary antibodies used for CD95 imaging or by a panel of goat polyclonal IgG1 (AHS4441; BioSource International, Camarillo, CA), mouse monoclonal IgG2a (ab6124 [F10-44-2]; Abcam), and rat monoclonal IgG2b (ab19622 [IM7]; Abcam) α-CD44 primary antibodies or their respective purified goat polyclonal IgG secondary antibodies (data not shown).
FIG. 1.
Alligator cardiac fibroblasts express CD95. The CD95 of control fibroblasts (upper panels) was labeled with antibody ab13550 against a synthetic peptide mapping at N-terminal residues 29 to 44 of mouse CD95 and visualized by Alexa Fluor 488-conjugated secondary antibody (green). For imaging, the fibroblast nuclei were labeled with 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI; blue). Cytoskeletal α-tubulin was labeled with primary antibody against chick brain α-tubulin and visualized by tetramethylrhodamine-conjugated secondary antibody (red). The apoptotic changes induced by 48 h of activation with the CD95-agonistic monoclonal IgM antibody CH11 (center panels) were less severe than those following 48 h of incubation with 109 CFU M. alligatoris (lower panels).
The α-CD95 antibodies ab13550 and C-20 reacted on Western blots of fibroblast lysates with a band consistent with the predicted apparent molecular mass of glycosylated CD95 of approximately 48 kDa, plus a second strongly reactive 44- to 48-kDa band in the fibroblast lysate which likely represents an alternately glycosylated form of CD95. Some faintly visible bands were also detected in the positive control HeLa cell lysate (Fig. 2). As for the in situ imaging described above, the labeling of a band with a molecular mass consistent with the predicted apparent molecular mass of glycosylated CD95 with two different antibodies against synthetic peptides mapping at the N terminus and at the C terminus of mammalian CD95 was strong evidence of the specificity of the results. No cross-reactive labeling of the putative CD95 antigen was detectable on Western blots probed with anti-α-tubulin IgG or with the panel of α-CD44 IgG primary antibodies or their respective IgG secondary antibodies described above, helping to rule out nonspecific binding of the primary α-CD95 IgGs by a random protein with the same predicted apparent molecular mass as CD95. Subjectively, there were no differences in the intensities of the additional faint bands on Western blots of untreated versus M. alligatoris-infected fibroblast lysates. Monomeric soluble CD95 (13) could not be detected in Western blots of plasma from control or M. alligatoris-infected alligators by probing with any of the four α-CD95 antibodies described, although an unidentified plasma protein with an apparent molecular mass of approximately 30 kDa cross-reacted strongly with ab13550 and CH11 (15, 50).
FIG. 2.
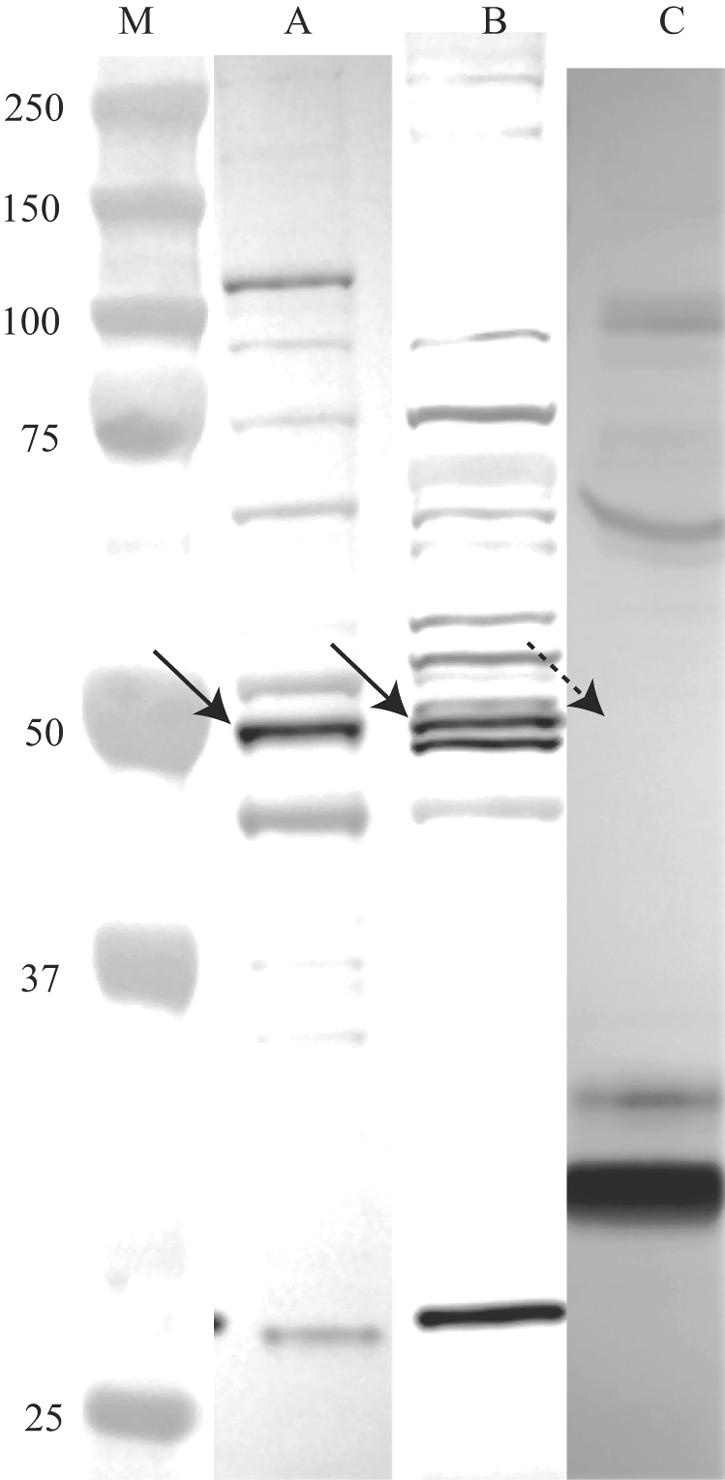
Fibroblast CD95 is detectable by Western blot assay. Antibody ab13550 reacted with a band (solid arrows) in positive control HeLa (lane A) and alligator fibroblast (lane B) cell lysates, consistent with the predicted apparent molecular mass of glycosylated CD95 of approximately 44 to 48 kDa (lane M, molecular mass standards). Monomeric (dashed arrow) or polymeric soluble CD95 could not be detected in plasma from control or M. alligatoris-infected alligators (lane C) by probing with ab13550 (shown) or any of the other antibodies tested in this study. Blots were digitized by using an AlphaImager (Alpha Innotech, San Leandro, CA). Contrast was normalized for better clarity by using the Auto Contrast feature after cropping and alignment with Adobe Photoshop CS 8.0 (Adobe Systems, San Jose, CA).
Mycoplasma alligatoris infection promotes CD95 expression and redistribution.
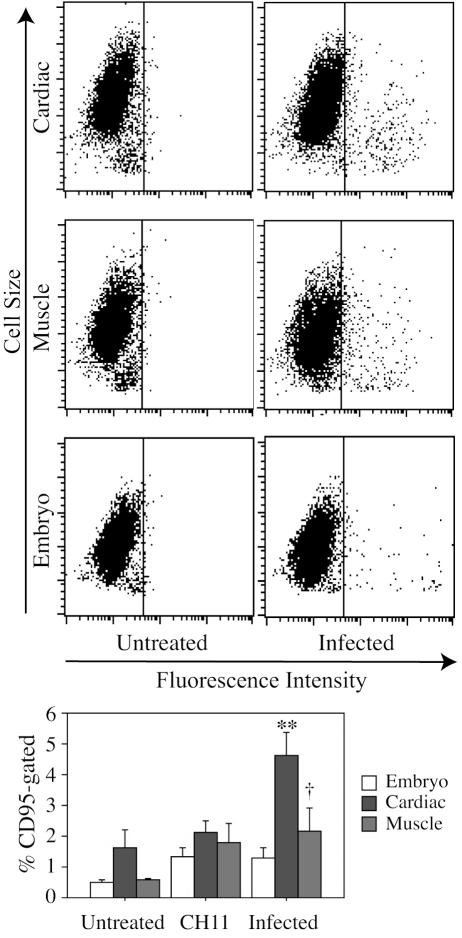
M. alligatoris readily persisted in coculture with the fibroblasts. The proportion of CD95-gated cardiac cells, observed 24 to 48 h after inoculation with 109 CFU of M. alligatoris, increased approximately threefold (P < 0.01) compared to the proportion of CD95-gated untreated control cells (Fig. 3). The effects were smaller after shorter incubation periods or with less inoculum. There was an almost fourfold increase (P < 0.08) in the proportion of CD95-gated skeletal muscle cells but less than a threefold increase (P < 0.12) in the proportion of CD95-gated embryonic fibroblasts following 24 to 48 h incubation with M. alligatoris. The window of opportunity for measurement was narrow, accounting for some of the variability observed, because usually less than the necessary minimum of 104 fibroblasts remained to be analyzed by flow cytometry beyond the 48-h incubation with M. alligatoris. For comparison, no increase in primary rat α-CD44 MAb IgG2b IM7 binding to alligator fibroblasts was detectable by flow cytometry after 48 h of incubation with α-CD95 MAb CH11 or infection with M. alligatoris, ruling out the possibility that nonspecific IgG binding might be increased in IgM-induced or M. alligatoris-infected fibroblasts when binding is measured by using rabbit polyclonal α-CD95 IgG ab13550 as the primary antibody in flow cytometry. Subjectively, translocation of the CD95 label away from the lamellipodium was a characteristic effect of infection of many cardiac and skeletal muscle cells examined (Fig. 1). The β-catenin label remained partitioned between membrane junctions and the cytosol, as expected, while in contrast, the ab13550 label seemed to become focused toward the center of the cell body. Induction by incubation with the CD95-agonistic antibody CH11 had similar effects on the redistribution of CD95 (Fig. 1) and the proportion of CD95-gated muscle cells (P < 0.17), but the increase in CD95-gated induced cardiac cells from untreated controls was proportionately less than that for infected cells (Fig. 3). Incubation with heat-inactivated M. alligatoris culture or M. alligatoris-conditioned SP4 culture supernatant had no effects on CD95 expression or distribution.
FIG. 3.
Mycoplasma alligatoris infection promotes CD95 expression of cardiac fibroblasts. Subconfluent fibroblasts were incubated for 48 h with 109 CFU of M. alligatoris, and then CD95 expression was measured with primary antibody ab13550 by flow cytometry (n = 3 replicates for embryo fibroblasts; n = 4 replicates for cardiac and muscle cells) (upper panel). Control fibroblasts were incubated with either IgM CH11 or medium only. The gate was set to exclude approximately 99.5% of negative control cells. Bars, 1 standard error. Statistical significance of the increase from untreated controls is indicated (**, P < 0.01; †, P < 0.10) (lower panel).
Mycoplasma alligatoris infection promotes apoptosis.
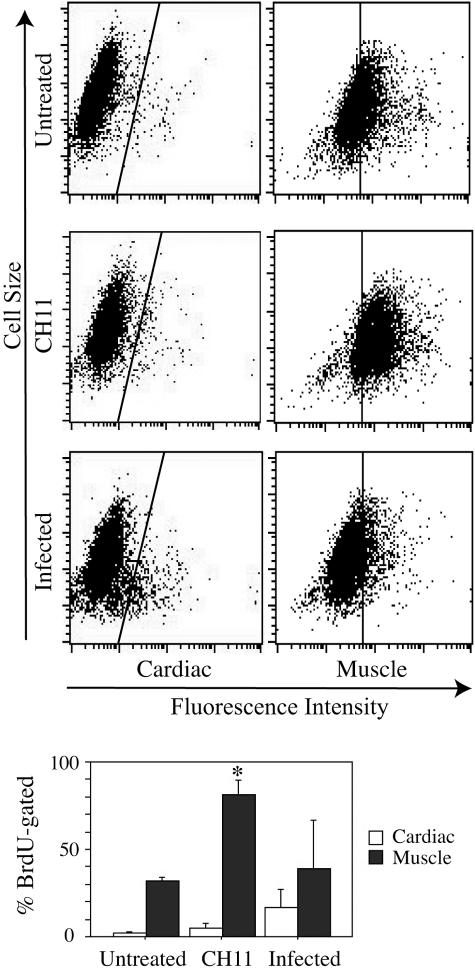
For all fibroblast types, fluorescence imaging revealed morphological changes characteristic of apoptosis, including decreased cell size, rounding, disordered α-tubulin, and nuclear condensation and fragmentation of M. alligatoris-infected cells (Fig. 1). Less severe changes were also evident in positive control fibroblasts activated by the CD95-agonistic antibody CH11 (Fig. 1). A greater than eightfold increase (P < 0.16) in the proportion of BrdU-gated infected cardiac fibroblasts compared to the increase in the proportion of BrdU-gated untreated controls was demonstrated by flow cytometry (Fig. 4). Activation by CH11 increased the proportion of BrdU-gated skeletal muscle fibroblasts approximately threefold (P < 0.03) compared to the increase in the proportion of BrdU-gated untreated control cells. The proportional increase was also threefold for CH11-activated cardiac cells, although that effect did not approach statistical significance. Incubation with heat-inactivated M. alligatoris culture or M. alligatoris-conditioned SP4 culture supernatant did not induce apoptosis. Also, sham induction either with irrelevant mouse IgM MAb against rat nuclear lamin A/C (sc-7293; Santa Cruz Biotechnology) as a negative isotype control for CH11 or with the irrelevant IgG1 MAb against α-tubulin did not influence fibroblast CD95 expression or apoptosis.
FIG. 4.
Mycoplasma alligatoris promotes apoptotic death of cardiac fibroblasts. Subconfluent fibroblasts were incubated for 48 h with 109 CFU M. alligatoris, and then the TUNEL assay was performed with primary antibody PRB-1 by flow cytometry (n = 4 replicates for cardiac cells, and n = 2 replicates for muscle cells) (upper panel). Control fibroblasts were incubated with either IgM CH11 or medium only. The gate was set to exclude approximately 99.5% of negative control cells. Bars, 1 standard error. Statistical significance of the increase from untreated controls is indicated (*, P < 0.05) (lower panel).
DISCUSSION
Sialidase and hyaluronidase were recognized through a genome survey as candidate factors responsible for the remarkably high virulence of M. alligatoris. Because its attenuated sibling species M. crocodyli also possesses hyaluronidase, we sought to identify specific mechanisms besides ECM degradation by which that combination of glycosidases might directly or synergistically promote the particular virulence of M. alligatoris. Several lines of prior evidence suggested that the eukaryotic cell signal-transducing HA receptor, CD44, is modulated by the combination of sialidase and hyaluronidase, leading to CD95-mediated cell death, consistent with the pathogenic effects of M. alligatoris infection in vivo. Desialylation by sialidase was required to activate the HA binding by CD44 of many diverse cell types (33, 34, 56). Treatment with hyaluronidase-fragmented HA induced expression of CD95 and promoted CD95-mediated apoptosis (21, 56). Although elements of the CD95 signaling pathway have been demonstrated previously by immunological methods in the cartilagenous ray Torpedo, the bony fish Ictalurus and Sparus, and the amphibian Xenopus (17, 38-40, 49), the present study established for the first time that reptile cells express a CD95 homolog which can be imaged and quantitated by using antibodies against mammalian CD95. In addition, incubation with activating α-CD95 IgM CH11 against mammalian CD95 induced reptilian fibroblasts to undergo apoptosis, which could be imaged by immunofluorescence and quantitated by TUNEL assay, evidence that the relationship of CD95-mediated signal transduction with apoptosis is the same in alligator fibroblasts as in other cell types. The data also support the fact that CH11 and ab13550 recognize distinct evolutionarily conserved epitopes on the N terminus of CD95, because prior incubation with CH11 did not block subsequent labeling by ab13550. The antigenic similarities between the N- and C-terminal epitopes of reptilian and mammalian CD95 homologs provide additional evidence that the apoptosis receptors are evolutionarily conserved across vertebrate species (49).
The heart is a major target organ of M. alligatoris infection in vivo (9). Cardiac fibroblasts are the major noncardiomyocyte heart cell type and are developmentally and functionally distinct from fibroblasts in other organs and tissues (11, 12). They originate during embryonic development from migratory proepicardium cells and undergo fibroblast growth factor- and platelet-derived growth factor-mediated differentiation to acquire their phenotype. They have central roles in homeostatic maintenance of the highly differentiated normal heart ECM. Cardiac fibroblasts also serve as intermediate sensors and amplifiers of signals from immune cells and myocytes. During infectious myocarditis and other forms of heart injury, cardiac fibroblasts undergo phenotypic and functional transitions, activated by cytokines and growth factors released from other cells and by the fibroblasts themselves, as part of normal tissue healing. Apoptosis of activated fibroblasts terminates the normal heart injury repair process (11). The in vitro infection with M. alligatoris tended to increase the proportion of BrdU-gated fibroblasts more in cardiac cells than in muscle cells, which are comparatively less injured during infection (9), a possible clue to one reason why certain cell types or organs are more severely affected by in vivo infection with M. alligatoris. Both de novo synthesis and externalization of preformed CD95 initially sequestered within the fibroblasts may have contributed to the increased cell surface expression of CD95 measured by flow cytometry following induction by agonistic antibody or by infection (5, 44).
Inappropriate apoptosis is important in the pathogenesis of many infectious diseases. Host animal damage results directly from induced death of epithelial or parenchymal cells, in some cases exacerbated by tumor necrosis factor alpha-mediated apoptosis of phagocytes and immune system cells that might otherwise limit bacterial invasion (22, 42). Damage also results indirectly from proinflammatory interleukin 1 and gamma interferon responses, especially when apoptosis is so extensive that the rate of cell death exceeds the rate of clearance by phagocytes (42, 58). CD95-mediated apoptosis is distinct from necrosis in the morphological and biochemical changes that occur (6, 18, 55), and its comparative “immunological silence” causes less inflammation and tissue damage than necrosis does if phagocytosis is able to keep pace with apoptosis (22, 42). A pathway to necrotic cell death also originates from CD95. Activation of an intracellular caspase cascade following CD95 agonist binding leads to apoptosis, but mitochondrial oxygen radical production also stimulated by CD95 agonist binding leads to necrosis, with the type of death determined individually in each cell by the rate of procaspase activation (18, 55). Translocation of CD95 toward the center of the cell body following M. alligatoris infection or activation by the agonistic antibody CH11 may reflect or may result in its concentration into lipid rafts in the plasma membrane where signal transduction cofactors are constitutively localized, a mechanism for regulating the efficiency of CD95 signaling (44). The significance of an increased proportion of CD95-gated primary cultured fibroblasts following inoculation with M. alligatoris is therefore the correlated association with at least three potential mechanisms of host damage in vivo (apoptosis, necrosis, and inflammation), consistent with the acute, severe inflammatory, and necrotizing disease caused by M. alligatoris infection.
In the so-called hyaluronan-mediated escape from apoptosis by lung, mammary, and gastric carcinomas (57), fragmented HA paradoxically reduced cell surface CD95 expression by cultured carcinoma cells, which then resisted agonist-induced apoptosis. The effect may result from a switch, linked to metastasis in vivo, to the upregulated secretion of soluble CD95 (10), with the subsequent inhibition of apoptosis by competitive binding of agonists by the soluble form of the receptor. However, we were unable to detect soluble CD95 in the plasma of control or M. alligatoris-infected alligators by Western blot analyses, from which we conclude that upregulated secretion of soluble CD95 is not an effect of M. alligatoris infection.
Diverse bacteria are able to modulate apoptosis, but no mechanisms have been previously proposed for direct effects on upstream components of the CD95 signal transduction pathway by any species of bacteria. Apoptosis induced by most extracellular or facultative intracellular pathogens has been attributed to nonspecific damage of host plasma or reticular membranes (e.g., Listeria, Neisseria, Staphylococcus, and Streptococcus) or direct activation of caspases (e.g., Legionella, Salmonella, and Shigella) (22). Inoculation with Mycoplasma bovis but not Mycoplasma bovirhinis or Mycoplasma bovoculi induced apoptosis of isolated bovine peripheral blood monocytes (54) and increased the rate of spontaneous apoptosis in human lymphoid and epithelial tumor cell lines (51) by unknown mechanisms. Mycoplasmal endonucleases were proposed to enhance sensitivity to nucleosomal DNA degradation in mycoplasma-infected cell lines either directly (4) or synergistically with various other inducers of apoptosis (45, 46, 51). Purified arginine deiminase from Mycoplasma arginini induced apoptosis of lymphatic cell lines through nutrient depletion (37). Membrane lipoproteins from Mycoplasma salivarium and Mycoplasma fermentans induced caspase-dependent necrotic and apoptotic cell death in lymphocytes, monocytes/macrophages, and Toll-like receptor-transfected HEK293 cell lines (29, 30, 37). In addition, heat-inactivated M. fermentans was cytocidal for the human myelomonocytic cell line U937 by a mechanism that specifically excluded CD95 involvement (47). Infection with live M. fermentans did not induce apoptosis in U937 cells but, instead, protected against tumor necrosis factor alpha-induced apoptosis through reduction of caspase-8 activity (25). M. alligatoris infection therefore is the first mycoplasmosis shown to influence an upstream element of the CD95 signal transduction pathway.
Our data show that apoptosis and cell death follow M. alligatoris infection and a positive correlation between the proportion of CD95-gated fibroblasts and apoptosis. The lack of an effect of heat-inactivated M. alligatoris culture or sterile M. alligatoris-conditioned culture supernatant on fibroblast CD95 expression or apoptosis suggests an enzymatic, mycoplasmal cell-associated mechanism of interaction with the fibroblasts' extracellular signal receptors. The sialidase and hyaluronidase of M. alligatoris are both cell-associated enzymes (10). Elucidation of a causal pathway, possibly involving CD44, between the glycosidases and the effects described here will require a series of additional studies such as enzyme inhibitor and factorial glycosidase gene knockout and complementation analyses. For example, primary pulmonary fibroblasts, which represent a second target organ of M. alligatoris infection in vivo (9), were protected from M. alligatoris-induced apoptosis in a dose-dependent fashion by 2,3-didehydro-2-deoxy-N-acetylneuraminic acid, a potent inhibitor of M. alligatoris sialidase (53). Incubation with purified Clostridium perfringens sialidase alone did not induce fibroblast apoptosis (our unpublished data). Those preliminary observations suggest that sialidase is necessary, but not sufficient alone, for M. alligatoris to induce apoptosis of pulmonary fibroblasts. Because the M. alligatoris genome includes only single copies of nanI and nagH, in contrast to other invasive species of bacteria that possess multiple sialidase and hyaluronidase genes, it should constitute the model most easily testable by gene inactivation and complementation approaches for demonstration of potential bacterial glycosidase modulation of the CD95 signal transduction and apoptosis pathways.
Acknowledgments
Embryonic alligator fibroblasts were a gift from Carlos Romero, Department of Pathobiology, University of Florida. The mitochondrial DNA genotyping and flow cytometry were performed by the Interdisciplinary Center for Biotechnology Research at the University of Florida.
This work was supported by Public Health Service grant 1R15HG02389-01A1 from the National Human Genome Research Institute.
REFERENCES
- 1.Abacus Concepts, Inc. 1996. StatView reference. Abacus Concepts, Inc., Berkeley, Calif.
- 2.Alpatov, R., G. C. Munguba, P. Caton, J. H. Joo, Y. Shi, Y. Shi, M. E. Hunt, and S. P. Sugrue. 2004. Nuclear speckle-associated protein Pnn/DRS binds to the transcriptional corepressor CtBP and relieves CtBP-mediated repression of the E-cadherin gene. Mol. Cell. Biol. 24:10223-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolazzi, A., A. Nocks, A. Aruffo, F. Spring, and I. Stamenkovic. 1996. Glycosylation of CD44 is implicated in CD44-mediated cell adhesion to hyaluronan. J. Cell Biol. 132:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendjennat, M., A. Blanchard, M. Loutfi, L. Montagnier, and E. Bahraoui. 1999. Role of Mycoplasma penetrans endonuclease P40 as a potential pathogenic determinant. Infect. Immun. 67:4456-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, M., K. Macdonald, S. W. Chan, J. P. Luzio, R. Simari, and P. Weissberg. 1998. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282:290-293. [DOI] [PubMed] [Google Scholar]
- 6.Bortner, C. D., N. B. E. Oldenburg, and J. A. Cidlowski. 1995. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 5:21-26. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. R. 2002. Mycoplasmosis and immunity of fish and reptiles. Front. Biosci. 7:d1338-d1346. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. R., J. M. Farley, L. A. Zacher, J. M. Carlton, T. L. Clippinger, J. G. Tully, and M. B. Brown. 2001. Mycoplasma alligatoris sp. nov., from American alligators. Int. J. Syst. Evol. Microbiol. 51:419-424. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. R., M. F. Nogueira, T. R. Schoeb, K. A. Vliet, R. A. Bennett, G. W. Pye, and E. R. Jacobson. 2001. Pathology of experimental mycoplasmosis in American alligators. J. Wildl. Dis. 37:671-679. [DOI] [PubMed] [Google Scholar]
- 10.Brown, D. R., L. A. Zacher, and W. G. Farmerie. 2004. Spreading factors of Mycoplasma alligatoris, a flesh-eating mycoplasma. J. Bacteriol. 186:3922-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, R. D., S. K. Ambler, M. D. Mitchell, and C. S. Long. 2005. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu. Rev. Pharmacol. Toxicol. 45:657-687. [DOI] [PubMed] [Google Scholar]
- 12.Camelliti, P., T. K. Borg, and P. Kohl. 2005. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65:40-51. [DOI] [PubMed] [Google Scholar]
- 13.Cascino, I., G. Papoff, A. Eramo, and G. Ruberti. 1996. Soluble Fas/Apo-1 splicing variants and apoptosis. Front. Biosci. 1:d12-d18. [DOI] [PubMed] [Google Scholar]
- 14.Catterall, J. B., L. M. Jones, and G. A. Turner. 1999. Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin. Exp. Metastasis 17:583-591. [DOI] [PubMed] [Google Scholar]
- 15.Chan, F. K., H. J. Chun, L. Zheng, R. M. Siegel, K. L. Bui, and M. J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288:2351-2354. [DOI] [PubMed] [Google Scholar]
- 16.Corfield, T. 1992. Bacterial sialidases—roles in pathogenicity and nutrition. Glycobiology 2:509-521. [DOI] [PubMed] [Google Scholar]
- 17.Cuesta, A., M. A. Esteban, and J. Meseguer. 2003. Identification of a FasL-like molecule in leucocytes of the teleost fish gilthead seabream (Sparus aurata L.). Dev. Comp. Immunol. 27:21-27. [DOI] [PubMed] [Google Scholar]
- 18.Denecker, G., D. Vercammen, M. Steemans, T. Vanden Berghe, G. Brouckaert, G. Van Loo, B. Zhivotovsky, W. Fiers, J. Grooten, W. Declercq, and P. Vandenabeele. 2001. Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell Death Differ. 8:829-840. [DOI] [PubMed] [Google Scholar]
- 19.Dorrie, J., K. Sapala, and S. J. Zunino. 2002. Interferon-gamma increases the expression of glycosylated CD95 in B-leukemic cells: an inducible model to study the role of glycosylation in CD95-signalling and trafficking. Cytokine 18:98-107. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, M. W., L. S. Honig, and H. C. Slavkin. 1984. Differentiation of cultured palatal shelves from alligator, chick, and mouse embryos. Anat. Rec. 209:231-249. [DOI] [PubMed] [Google Scholar]
- 21.Fujii, K., Y. Fujii, S. Hubscher, and Y. Tanaka. 2001. CD44 is the physiological trigger of Fas up-regulation on rheumatoid synovial cells. J. Immunol. 167:1198-1203. [DOI] [PubMed] [Google Scholar]
- 22.Gao, L., and Y. Abu Kwaik. 2000. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2:1705-1719. [DOI] [PubMed] [Google Scholar]
- 23.Gee, K., M. Kozlowski, and A. Kumar. 2003. Tumor necrosis factor-alpha induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J. Biol. Chem. 278:37275-37287. [DOI] [PubMed] [Google Scholar]
- 24.Gee, K., M. Kryworuchko, and A. Kumar. 2004. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch. Immunol. Ther. Exp. (Warsaw) 52:13-26. [PubMed] [Google Scholar]
- 25.Gerlic, M., J. Horowitz, and S. Horowitz. 2004. Mycoplasma fermentans inhibits tumor necrosis factor alpha-induced apoptosis in the human myelomonocytic U937 cell line. Cell Death Differ. 11:1204-1212. [DOI] [PubMed] [Google Scholar]
- 26.Glenn, T. C., J. L. Staton, A. T. Vu, L. M. Davis, J. R. Bremer, W. E. Rhodes, I. L. Brisbin, Jr., and R. H. Sawyer. 2002. Low mitochondrial DNA variation among American alligators and a novel non-coding region in crocodilians. J. Exp. Zool. 294:312-324. [DOI] [PubMed] [Google Scholar]
- 27.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Hynes, W. L., and S. L. Walton. 2000. Hyaluronidases of gram-positive bacteria. FEMS Microbiol. Lett. 183:201-207. [DOI] [PubMed] [Google Scholar]
- 29.Into, T., K. Kiura, M. Yasuda, H. Kataoka, N. Inoue, A. Hasebe, K. Takeda, S. Akira, and K. Shibata. 2004. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell Microbiol. 6:187-199. [DOI] [PubMed] [Google Scholar]
- 30.Into, T., Y. Nodasaka, A. Hasebe, T. Okuzawa, J. Nakamura, N. Ohata, and K. Shibata. 2002. Mycoplasmal lipoproteins induce Toll-like receptor 2- and caspases-mediated cell death in lymphocytes and monocytes. Microbiol. Immunol. 46:265-276. [DOI] [PubMed] [Google Scholar]
- 31.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joo, J. H., R. Alpatov, G. C. Munguba, M. R. Jackson, M. E. Hunt, and S. P. Sugrue. 2005. Reduction of Pnn by RNAi induces loss of cell-cell adhesion between human corneal epithelial cells. Mol. Vis. 11:133-142. [PubMed] [Google Scholar]
- 33.Katoh, S., T. Miyagi, H. Taniguchi, Y. Matsubara, J. Kadota, A. Tominaga, P. W. Kincade, S. Matsukura, and S. Kohno. 1999. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J. Immunol. 162:5058-5061. [PubMed] [Google Scholar]
- 34.Katoh, S., Z. Zheng, K. Oritani, T. Shimozato, and P. W. Kincade. 1995. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J. Exp. Med. 182:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim, H. R., M. A. Wheeler, C. M. Wilson, J. Iida, D. Eng, M. A. Simpson, J. B. McCarthy, and K. M. Bullard. 2004. Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res. 64:4569-4576. [DOI] [PubMed] [Google Scholar]
- 36.Lesley, J., R. Hyman, N. English, J. B. Catterall, and G. A. Turner. 1997. CD44 in inflammation and metastasis. Glycoconj. J. 14:611-622. [DOI] [PubMed] [Google Scholar]
- 37.Lo, S.-C. 2002. Apoptotic, antiapoptotic, clastogenic and oncogenic effects, p. 403-416. In S. Razin and R. Herrmann (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum, New York, N.Y.
- 38.Long, S., M. Wilson, E. Bengten, L. W. Clem, N. W. Miller, and V. G. Chinchar. 2004. Identification and characterization of a FasL-like protein and cDNAs encoding the channel catfish death-inducing signaling complex. Immunogenetics 56:518-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loredana, R., D. Barbara, L. Annamaria, T. Stefania, M. C. Maria, F. Silvana, A. Piero, and P. Marina. 2003. Role of apoptosis and Fas/FasL system in the oogenesis of the spotted ray Torpedo marmorata. Mol. Reprod. Dev. 66:54-59. [DOI] [PubMed] [Google Scholar]
- 40.Mangurian, C., R. O. Johnson, R. McMahan, R. H. Clothier, and L. N. Ruben. 1998. Expression of a Fas-like proapoptotic molecule on the lymphocytes of Xenopus laevis. Immunol. Lett. 64:31-38. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita, O., and A. Okabe. 2001. Clostridial hydrolytic enzymes degrading extracellular components. Toxicon 39:1769-1780. [DOI] [PubMed] [Google Scholar]
- 42.Menaker, R. J., and N. L. Jones. 2003. Fascination with bacteria-triggered cell death: the significance of Fas-mediated apoptosis during bacterial infection in vivo. Microbes Infect. 5:1149-1158. [DOI] [PubMed] [Google Scholar]
- 43.Mohan, K., C. M. Foggin, P. Muvavarirwa, J. Honywill, and A. Pawandiwa. 1995. Mycoplasma-associated polyarthritis in farmed crocodiles (Crocodylus niloticus) in Zimbabwe. Onderstepoort J. Vet. Res. 62:45-49. [PubMed] [Google Scholar]
- 44.Muppidi, J. R., and R. M. Siegel. 2004. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 5:182-189. [DOI] [PubMed] [Google Scholar]
- 45.Paddenberg, R., A. Weber, S. Wulf, and H. G. Mannherz. 1998. Mycoplasma nucleases able to induce internucleosomal DNA degradation in cultured cells possess many characteristics of eukaryotic apoptotic nucleases. Cell Death Differ. 5:517-528. [DOI] [PubMed] [Google Scholar]
- 46.Paddenberg, R., S. Wulf, A. Weber, P. Heimann, L. A. Beck, and H. G. Mannherz. 1996. Internucleosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasma endonucleases. Eur. J. Cell Biol. 71:105-119. [PubMed] [Google Scholar]
- 47.Rawadi, G., S. Roman-Roman, M. Castedo, V. Dutilleul, S. Susin, P. Marchetti, M. Geuskens, and G. Kroemer. 1996. Effects of Mycoplasma fermentans on the myelomonocytic lineage. Different molecular entities with cytokine-inducing and cytocidal potential. J. Immunol. 156:670-678. [PubMed] [Google Scholar]
- 48.Rood, J. R. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 49.Sakamaki, K., C. Takagi, K. Kominami, S. Sakata, Y. Yaoita, H. Y. Kubota, M. Nozaki, S. Yonehara, and N. Ueno. 2004. The adaptor molecule FADD from Xenopus laevis demonstrates evolutionary conservation of its pro-apoptotic activity. Genes Cells 9:1249-1264. [DOI] [PubMed] [Google Scholar]
- 50.Siegel, R. M., J. K. Frederiksen, D. A. Zacharias, F. K. Chan, M. Johnson, D. Lynch, R. Y. Tsien, and M. J. Lenardo. 2000. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 288:2354-2357. [DOI] [PubMed] [Google Scholar]
- 51.Sokolova, I. A., A. T. Vaughan, and N. N. Khodarev. 1998. Mycoplasma infection can sensitize host cells to apoptosis through contribution of apoptotic-like endonuclease(s). Immunol. Cell Biol. 76:526-534. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki, O., Y. Nozawa, and M. Abe. 2003. Sialic acids linked to glycoconjugates of Fas regulate the caspase-9-dependent and mitochondria-mediated pathway of Fas-induced apoptosis in Jurkat T cell lymphoma. Int. J. Oncol. 23:769-774. [PubMed] [Google Scholar]
- 53.Usuki, S., P. Hoops, and C. C. Sweeley. 1988. Growth control of human foreskin fibroblasts and inhibition of extracellular sialidase activity by 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. J. Biol. Chem. 263:10595-10599. [PubMed] [Google Scholar]
- 54.Vanden Bush, T. J., and R. F. Rosenbusch. 2002. Mycoplasma bovis induces apoptosis of bovine lymphocytes. FEMS Immunol. Med. Microbiol. 32:97-103. [DOI] [PubMed] [Google Scholar]
- 55.Vercammen, D., G. Brouckaert, G. Denecker, M. Van de Craen, W. Declercq, W. Fiers, and P. Vandenabeele. 1998. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasuda, M., K. Nakano, K. Yasumoto, and Y. Tanaka. 2002. CD44: functional relevance to inflammation and malignancy. Histol. Histopathol. 17:945-950. [DOI] [PubMed] [Google Scholar]
- 57.Yasuda, M., Y. Tanaka, K. Fujii, and K. Yasumoto. 2001. CD44 stimulation down-regulates Fas expression and Fas-mediated apoptosis of lung cancer cells. Int. Immunol. 13:1309-1319. [DOI] [PubMed] [Google Scholar]
- 58.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]