Abstract
Context: Epidemiological data suggest a common genetic susceptibility to Type 1 diabetes (T1D) and autoimmune thyroid disease (AITD).
Objective: Our objective was to identify the joint susceptibility genes for T1D and AITD.
Design: We conducted a family based linkage and association study.
Setting: The study took place at an academic medical center.
Participants: Participants included 55 multiplex families (290 individuals) in which T1D and AITD clustered (T1D-AITD families).
Main Outcome Measures: We conducted tests for linkage and family-based associations (transmission disequilibrium test) with four candidate genes, human leukocyte antigen (HLA), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), insulin variable number of tandem repeats (VNTR), and thyroglobulin.
Results: Linkage evidence to HLA appeared when subjects with either T1D or AITD were considered affected [maximum LOD score (MLS), 2.2]. The major HLA haplotype contributing to the shared susceptibility was DR3-DQB1*0201, with DR3 conferring most of the shared risk. The CTLA-4 gene showed evidence for linkage only when individuals with both T1D and AITD were considered affected (MLS, 1.7), and the insulin VNTR showed evidence for linkage when individuals with either T1D or AITD were considered affected (MLS, 1.9); i.e. it may contribute to the familial aggregation of T1D and AITD.
Conclusions: The HLA class II locus contributes to the shared risk for T1D and AITD, and the major HLA haplotype contributing to this association is DR3-DQB1*0201. Additional non-HLA loci contribute to the joint susceptibility to T1D and AITD, and two potential candidates include the CTLA-4 and insulin VNTR loci.
Keywords: Type 1 diabetes, Genetics, HLA, Autoimmune thyroid disease, linkage, association
INTRODUCTION
Autoimmune endocrine diseases are disorders in which immune dysregulation results in immune attack on the endocrine glands. While autoimmune disorders may affect many endocrine glands, the most common autoimmune endocrine disorders are Type 1 diabetes mellitus (T1D) and the autoimmune thyroid diseases (AITD), including Graves' disease and Hashimoto's thyroiditis. Both T1D and AITD are organ specific T-cell mediated diseases in which T-cell infiltration results in dysfunction of the target organ (the pancreatic islets in T1D and the thyroid in AITD). There is a well-known strong association between T1D and AITD (reviewed in (1)). They frequently occur within the same family (2), and in the same individual. When T1D and AITD occur in the same individual the phenotype is considered an autoimmune polyglandular syndrome (APS) variant (3). As summarized in Table 1, approximately 15 to 30% of patients with T1D have thyroid antibodies (TAb's), and up to 50% of such patients progress to clinical AITD (4). Conversely, 2.3% of children with AITD have islet cell antibodies compared with 0% of controls (5). Prevalence rates of Hashimoto's thyroiditis in relatives of type 1 diabetics range from 22% to 44%, compared with a population prevalence of only 5% to 10% (6). Other factors such as age (7), age of onset of puberty, and ethnicity (8) influence the association between T1D and AITD. Indeed, with increasing age, the prevalence of TAb's in diabetic patients rises dramatically (7-9).
Table 1:
Selected studies investigating prevalence of AITD in T1D individuals
| STUDY | REFERENCE | YEAR | STUDY POPULATION | THYROID ANTIBODIES IN T1D PT | THYROID ANTIBODIES IN CONTROL | CLINICAL AITD IN T1D PT |
|---|---|---|---|---|---|---|
| Fialkow | 60 | 1975 | 52 T1D adults | 35 % | 10 % | NA |
| 52 controls (patients' spouses) | ||||||
| Riley | 9 | 1981 | 771 young T1D patients | 17.6 % | 3.3 % | 7.9 % |
| 572 controls | ||||||
| Bright | 5 | 1982 | 182 T1D children | 30 % | 4.3 % | NA |
| 117 controls | ||||||
| Maclaren | 61 | 1985 | 1456 Caucasian T1D patients | 23 % | NA | NA |
| Burek | 8 | 1990 | 159 T1D children | 34 % | NA | 10.7 % |
| Kontiainen | 62 | 1990 | 131 young T1D patients | 22 % | NA | 8 % |
| Dorman | 52 | 1996 | 255 T1D probands | NA | NA | 16 % |
| Roldan | 63 | 1999 | 204 young T1D patients | NA | NA | 4 % |
| Holl | 7 | 1999 | 495 T1D children | 21.8 % | NA | NA |
NA = not available
The similar pathogenesis of T1D and AITD and their tendency to occur together suggest that their etiology may involve shared genetic factors (1). However, so far little is known about the joint genetic etiology of these two diseases. One strong candidate locus is the human leukocyte antigen (HLA), known to influence both diseases, but to different extents (10). Previous HLA studies in Caucasians have suggested that the haplotype DR3-DQB1*0201 may predispose to T1D and AITD when both occur in the same individual (APS variant) (Table 2) (11). So far very few studies have examined the HLA locus in families with T1D and AITD (Table 2). We have recently shown that in families in which both T1D and AITD cluster (“T1D-AITD families”), the HLA locus was linked to both T1D and AITD (12). Moreover, our study demonstrated that HLA-DR3 was associated with both T1D and AITD in these families, but DR4 was specific for T1D (12). Since HLA-DQ alleles have been shown to be the major determinants of genetic susceptibility to T1D (13), while HLA-DR3 is believed to be the primary allele conferring susceptibility to AITD (14;15) it was not clear whether DR, DQ, or both loci contribute to the joint susceptibility to T1D and AITD. Therefore, the aim of the present study was to examine the differential effects of the HLA-DR and HLA-DQB1 genes on susceptibility to T1D and AITD in families in which both diseases cluster. Since our previous study demonstrated that other non-HLA loci must contribute to the joint susceptibility to T1D and AITD, we, therefore, have now tested 3 additional candidate genes for linkage and association with AITD and T1D in the T1D-AITD families: insulin VNTR, CTLA-4, and thyroglobulin.
Table 2:
Selected HLA association studies in Caucasian individuals or families with T1D and AITD
| STUDY | YEAR | REFERENCE | COUNTRY | STUDY POPULATION | N | HLA ALLELES | OR or p-value |
|---|---|---|---|---|---|---|---|
| Payami | 1989 | 64 | USA | Patients with both T1D and AITD | 12 | DR4 | p < 0.001 |
| Santamaria | 1994 | 51 | USA | Patients with both T1D and AITD | 39 | DQB1*0201 | p = 0.0005 |
| DQB1*0302 | p = 0.03 | ||||||
| Huang | 1996 | 53 | USA | APS type 2 patients with beta cell autoimmunity | 17 | DR3-DQB1*0201 | p < 0.01 |
| DR4-DQB1*0302 | p < 0.01 | ||||||
| APS type 2 patients without beta cell autoimmunity | 14 | DR3-DQB1*0201 | p < 0.05 | ||||
| Dorman | 1996 | 52 | USA | T1D probands and 1° relatives with AITD | 25 families | DQA1*0501- DQB1*0201 | OR = 2.2 |
| Holl | 1999 | 7 | Germany | T1D children with thyroid antibodies | NS | DR3/DR4 genotype | p = 0.08 |
| Wallaschofski | 2003 | 11 | Germany | APS type 2 patients | 29 | DR3 | p < 0.001 |
| DR4 | p < 0.05 | ||||||
| APS type 3 patients | 83 | DR4 | p < 0.025 |
APS — autoimmune polyglandular syndrome, NS — not specified, OR — odds ratio
SUBJECTS AND METHODS
The families
The project was approved by the institutional review board. Fifty five Caucasian families (290 individuals) were analyzed. Families were ascertained through a patient with T1D who had at least one first degree relative with T1D and at least one additional first degree relative with AITD. T1D was diagnosed based on the American Diabetes Association criteria (16) with age at diagnosis under 15 years. AITD includes Graves' disease and Hashimoto's thyroiditis, both diagnosed as previously described (17).
Genotyping of microsatellite markers in the MHC region
The HLA gene locus is located on chromosome 6p21(34 - 39 cM). We analyzed six microsatellite markers which span the MHC region including one marker that is located very close to the DR locus on its telomeric side (D6S273) and another close to the DQ locus also telomeric to DR (TNF••microsatellite [TNF••-ms]). This enabled us to test for linkage around the MHC region as well as inside the MHC class II gene region. Primers for marker amplification were purchased from ABI (Foster City, CA), and genotyping was performed as previously described (18).
HLA typing
Molecular typing of HLA-DR and HLA-DQB1 was carried out according to the requirements of the American Society for Histocompatibility (19). The major alleles of HLA-DR and HLA-DQB1 were typed using the technique of group-specific PCR-amplification, followed by restriction enzyme digestion, as previously described (20;21).
Genotyping the Insulin VNTR locus
The insulin-VNTR polymorphism, located 5' to the insulin gene, is a tandem repetition of 14- to 15-bp oligonucleotides. It has two main alleles, the shorter class I alleles (28-44 repeats) and the longer class III alleles (138-159 repeats). Family members were typed for the insulin-VNTR class I and class III alleles using the -23 HphI RFLP polymorphism, as previously described (22;23). The -23 HphI RFLP polymorphism was shown to be in extremely tight linkage disequilibrium (>99.7% concordance) with the VNTR in Caucasians
Genotyping the CTLA-4 locus
Linkage analysis for the CTLA-4 locus was performed using the highly informative microsatellite marker D2S325 (24), located 1 cM downstream from the CTLA-4 genes (24).
Genotyping the thyroglobulin locus
We used the microsatellite marker Tgms2 located inside intron 27 of the Tg gene to test for linkage to the Tg locus (25).
Linkage analyses
Two-point linkage analyses: Two-point LOD scores for the different markers studied were computed using LIPED software (26) assuming both dominant and recessive models (27). Twins studies have reported the concordance rates for monozygotice twins to be 30-50% for T1D (28-30) and 30-80% for AITD (31;32) suggesting a penetrance of ∼30-50% for T1D and AITD. Therefore, and in order to choose a conservative estimate, all linkage analyses were performed at an assumed 30% penetrance. In addition, the non-parametric (NPL) LOD scores were also computed using the GeneHunter program.
Heterogeneity testing and multipoint linkage analysis: Multipoint LOD scores were computed by the Allegro program (33) using all the markers spanning the MHC locus. Multipoint linkage analysis yields the maximum information for each family for the area of interest. Using Allegro, we set the inheritance parameters identical to those that gave the maximum LOD scores in the 2-point analyses. Marker placement and distances for the multipoint analysis were obtained from the Genethon maps (34). The order of the markers and recombination fractions in the Genethon maps were verified on our data set. In addition, we tested for heterogeneity in our dataset with multipoint heterogeneity LOD scores (HLOD's), computed by the Allegro program (33) using all the markers spanning the MHC locus.
Affectedness and disease models used in the linkage analyses: Some individuals in our families had T1D, some had AITD, and some had both T1D and AITD (APS variant). Therefore, we analyzed the data using four models:
(1) Model 1 - T1D (n=55 families): Only individuals with T1D were considered as affected, whether they had T1D alone or with AITD. This model tests for linkage to T1D.
(2) Model 2 - AITD (n=55 families): Only individuals with AITD were considered as affected, whether they had AITD alone or with T1D. This model tests for linkage to AITD.
(3) Model 3 - T1D or AITD (n=55 families): Individuals with T1D or AITD (or both) were considered as affected; a locus showing linkage under this model contributes to the clustering of T1D and AITD within the same family.
(4) Model 4 - T1D and AITD (APS variant) (n=31 families): Only individuals with both T1D and AITD were considered as affected. Loci identified using this model contribute only to the combined phenotypes of T1D and AITD in the same individual (considered to be an APS variant (11)), but do not necessarily contribute to the clustering of T1D and AITD in families.
Association analyses
Family based association analyses were performed using the transmission disequilibrium test (TDT). The TDT analysis was performed using Genehunter version 2.0 (35). The TDT compares the rate of transmission of parental alleles to affected offspring with the rate expected if there is no preferential transmission (36). We performed the TDT analyses for offspring affected by T1D alone, offspring affected by AITD alone, and offspring affected by both T1D and AITD (APS variant). A significantly increased transmission of a certain allele to affected offspring indicates association of that allele with the disease phenotype. Conversely, a significantly decreased transmission of a certain allele to affected offspring indicates a negative association of that allele with the disease phenotype, i.e., a protective effect.
RESULTS
Family characteristics
We analyzed 55 families; all families were multiplex for T1D (i.e. >1 affected) and had at least one additional family member with AITD; 21 families (38%) were multiplex for AITD. In 31 families (56%) at least one family member had both T1D and AITD (APS variant (3)). On the average the families had 5.3 members. We had a total of 290 individuals. Of these, 148 individuals were affected; 68 had T1D alone, 45 had AITD alone, and 35 had both T1D and AITD [APS variant] (Table 3). Of the 80 AITD patients (with or without T1D) 62 had Hashimoto's thyroiditis and 18 had Graves' disease.
Table 3:
Characteristics of the 55 T1D-AITD families
| TOTAL NUMBER | FEMALES | MALES | F/M RATIO | |
|---|---|---|---|---|
| All family members | 290 | 151 | 139 | 1.1 / 1 |
| Unaffected individuals | 142 | 65 | 77 | 0.8 / 1 |
| Affected individuals: | 148 | 86 | 62 | 1.4 / 1 |
| T1D only | 68 | 29 | 39 | 0.7 / 1 |
| T1D and AITD | 35 | 22 | 13 | 1.7 / 1 |
| AITD only | 45 | 35 | 10 | 3.5 / 1 |
| Mean age of onset of T1D (years) | 8.3 | 8.9 | 7.7 |
Linkage and association analyses of the HLA region
Two-point linkage analysis and NPL analysis: Six markers spanning the HLA locus were analyzed for linkage with T1D and AITD. When we analyzed for model 1 (T1D) the 2-point maximum LOD score (MLS) was 7.43 at marker D6S273 for the recessive model, at 30% penetrance and a θ (θ indicates the recombination fraction at which the MLS was obtained) of 0.05. Analysis for model 2 (AITD) gave a 2-point MLS of 0.95 at marker TNF••-ms (recessive model, 30% penetrance, ••=0.1). When we analyzed for model 3 (all T1D or AITD patients considered affected) the 2-point MLS was 2.21 at marker D6S273 (recessive model, 30% penetrance, ••=0.2), and considering only individuals that had both T1D and AITD as affected (APS variant, Model 4) gave an MLS of 1.4 at marker TNF••-ms (recessive model, 30% penetrance, ••=0.01). Similar results were obtained by the NPL analysis. The maximum NPL score for model 1 (T1D) was 4.8, obtained at marker TNF••-ms, the maximum NPL score for model 2 (AITD) was 0.6 (at marker TNF••-ms), the maximum NPL score for model 3 (T1D or AITD) was 3.6,(at marker TNF••-ms), and the maximum NPL score for model 4 (T1D+AITD) was 0.8 between markers TNF••-ms and D6S273. Thus, both the parametric and the nonparametric (NPL) linkage analyses showed positive LOD scores for model 3 (T1D or AITD) suggesting that the HLA locus contributes to the familial clustering of T1D and AITD. However, the LOD scores were significantly higher for T1D (model 1) reflecting the well-known major influence of HLA on the etiology of T1D (37).
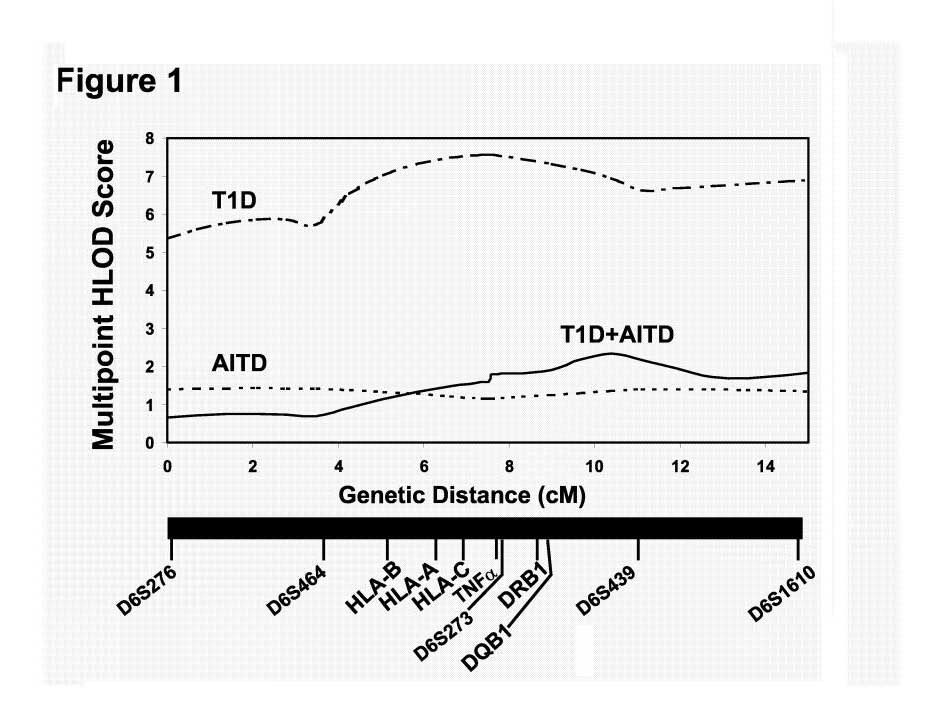
Heterogeneity testing and Multipoint Analysis: As for the 2-point analysis, multipoint linkage analyses using Allegro also showed positive LOD scores for models 1 (T1D) and 3 (T1D or AITD) (Figure 1). For affectedness T1D (model 1) the maximum multipoint LOD score when allowing for heterogeneity (HLOD) was 7.57 between markers TNF••-ms and D6S273 (Figure 1). For affectedness AITD (model 2) the HLOD was 1.0-1.5 throughout the HLA region. Multipoint linkage analysis for affectedness T1D or AITD (model 3) gave a maximum multipoint HLOD score of 2.2 between markers D6S273 and D6S439 (Figure 1), and the maximum multipoint HLOD score for affectedness T1D+AITD (model 4) was 1.6 between markers D6S464 and TNF••-ms. These results support our previous data (12) showing that the clustering of AITD with T1D in families is partially determined by the HLA locus. However, the LOD score for HLA was highest for T1D alone, and when we added the AITD-affected individuals to the analysis the LOD score decreased from 7.57 to 2.2. This decrease in LOD score when individuals with AITD were added to the analysis shows that HLA contributes significantly less to the inherited susceptibility to AITD in T1D families. Therefore, other non-HLA genes must contribute to the strong familial clustering of T1D and AITD.
Figure 1:
Multipoint LOD score analysis for the MHC region on chromosome 6p21. The X axis shows the relative positions in centimorgans (cM) of the 6 microsatellite markers used in the linkage analysis, and the Y axis shows the multipoint heterogeneity LOD (HLOD) score. The multipoint HLOD score was computed using Allegro. The multipoint HLOD scores are computed using all the markers simultaneously and allowing for genetic heterogeneity (i.e. assuming only a subset of the families is linked at the marker locus). The maximum multipoint HLOD scores were 7.57 when considering T1D as affected (model 1), 1.5 when considering AITD as affected (model 2), and 2.2 when considering individuals with either T1D or AITD (or both) as affected (model 3).
Family based association studies (Figure 2): All family members were typed for DR and DQB1 alleles and a TDT test was performed. TDT analysis examining transmission to the proband showed preferential transmission of the haplotypes DR3-DQB1*0201 (p=0.00016) and DR4-DQB1*0302 (p=0.0004) to offspring affected with T1D alone (Figure 2, Table 4). The same haplotypes were preferentially transmitted to probands affected with both T1D and AITD (APS variant). However, only the DR3-DQB1*0201 haplotype was preferentially transmitted to offspring affected with AITD alone (p=0.02, Figure 2, Table 4). In contrast, the DR4-DQB1*0302 haplotype showed preferential nontransmission to offspring with AITD alone (p=0.01, Table 4). Preferential nontransmission was also seen with the haplotypes DR2-DQB1*0602 and DR4-DQB1*0301 to offspring with T1D alone and offspring with both T1D and AITD (APS variant). This was not seen in offspring with AITD alone; however the numbers were very small. As a control for the TDT analysis we tested the transmission of the same haplotypes to unaffected offspring. The analysis demonstrated no significant deviation from expected transmission (Table 4). All remaining haplotypes that were found among the 290 family members were rare and, therefore, excluded from the analysis.
Figure 2:
Chi squared values obtained in the transmission disequilibrium test (TDT) for the transmission of key HLA Class 2 haplotypes to affected offspring. Bars to the right of the Y-axis show preferential transmission and bars to the left of the Y-axis show preferential nontransmission. The haplotype DR3-DQB1*0201 was preferentially transmitted to offspring affected by T1D, AITD, or both, while the DR4-DQB1*0302 haplotype was specific for T1D (* P < 0.05, **P < 0.01, *** P < 0.001).
Table 4:
TDT analysis of selected HLA haplotypes
| Haplotype |
Offspring with T1D alone |
Offspring with T1D and AITD |
Offspring with AITD alone |
Unaffected offspring |
||||
|---|---|---|---|---|---|---|---|---|
| T/U* | p-value | T/U | p-value | T/U | p-value | T/U | p-value | |
| DR3-DQB1*0201 | 30/7 | 0.00016 | 24/4 | 0.0002 | 7/1 | 0.03 | 16/25 | NS* |
| DR3-other DQ | 1/2 | NS | 1/2 | NS | 0/1 | NS | 0/1 | NS |
| other DR-DQB1*0201 | 4/11 | 0.07 | 2/7 | NS | 2/4 | NS | 10/5 | NS |
| DR4-DQB1*0302 | 26/6 | 0.0004 | 19/3 | 0.0006 | 0/6 | 0.01 | 26/18 | NS |
| DR4-other DQ | 0/10 | 0.0015 | 0/7 | 0.008 | 1/2 | NS | 5/6 | NS |
| other DR-DQ/b1*0302 | 4/2 | NS | 1/1 | NS | 0/0 | NS | 2/5 | NS |
NS - not significant; T/U - transmitted/untransmitted
Analysis of individual alleles within associated haplotypes (Table 4): In order to examine which allele was the primary allele conferring susceptibility within each associated haplotype, we compared the TDT results for haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 with haplotypes containing one, but not both, of their constituent alleles. As previously mentioned, the haplotype DR3-DQB1*0201 was preferentially transmitted to offspring affected with T1D, AITD, or both. Haplotypes containing DR3 without DQB1*0201 were extremely rare among the families. In contrast, haplotypes containing DQB1*0201 without DR3 were more prevalent, and these haplotypes trended towards preferential nontransmission to affected offspring (p = 0.07, Table 4). This suggested that within the haplotype DR3-DQ*0201, it was the DR3 conferring most of the risk to T1D and AITD. The haplotype DR4-DQB1*0302 was preferentially transmitted to offspring with T1D. Haplotypes containing DR4 without DQB1*0302 were preferentially not transmitted to offspring with T1D (p=0.0015, Table 4), whereas haplotypes containing DQB1*0302 without DR4, showed no preferential transmission pattern (Table 4). This suggested that within the haplotype DR4-DQ*0302, it was the DQB1*0302 that conferred most of the risk to T1D.
Linkage analyses of non-HLA candidate genes
CTLA-4 locus: Linkage analysis for the CTLA-4 locus was performed using the microsatellite marker D2S325. Analysis for model 1 (T1D) and for model 2 (AITD) gave low positive LOD scores (0.3 and 0.9, respectively)(Table 5), while analysis for model 3 (T1D or AITD) gave negative 2-point LOD scores (MLS=0, at θ=0.5, Table 5). This suggested that CTLA-4 does not contribute to the familial clustering of T1D and AITD in our dataset. However, when considering only individuals that had both T1D and AITD as affected (APS variant, model 4) the MLS was 1.7 (recessive model, 30% penetrance, ••=0.05), suggesting evidence for linkage (Table 5). Since AITD includes both GD and HT and most of the patients with T1D+AITD in our families had T1D+HT we re-analyzed the data considering individuals that had both T1D and HT as affected. The MLS when considering individuals that had both T1D and HT as affected was 2.1 (recessive model, 30% penetrance, ••=0.05), suggesting that T1D+HT is the distinct phenotype which is linked with CTLA-4 in our T1D-AITD families. This suggests that patients with T1D and AITD/HT (APS variant) may be a genetically distinct subgroup of diabetics influenced by CTLA-4. Therefore, these data may help explain previous inconsistent studies of CTLA- 4 in T1D with some reporting significant linkage/association of CTLA-4 to T1D (38;39) while others reporting no (40;41) or very weak (42) associations. These inconsistent results of previous studies could be explained by noting that the T1D datasets were likely collected without regard to the AITD status of the probands. Because approximately 10-20% of T1D patients have AITD (1), the presence or absence of AITD in such patients can represent a significant source of variation from study to study. Our results showed that in the subset of T1D patients who also have AITD (APS variant) there is strong linkage to CTLA-4.
Table 5:
Two-point LOD scores for three candidate loci in 55 T1D-AITD families
| Locus/Gene | Location | Marker | MLS for T1D (Model 1) | MLS for AITD (Model 2) | MLS for T1D or AITD (Model 3) | MLS for T1D and AITD (APS variant, Model 4) |
|---|---|---|---|---|---|---|
| CTLA-4 | 2q | D2S325 | 0.3 (θ=0.2) | 0.9 (θ=0.2) | 0 (θ=0.5) | 1.7 (θ=0.05)* |
| Ins-VNTR | 11q | HphI RFLP | 0.7 (θ=0.05) | 0.6 (θ=0.05) | 1.9 (θ=0.01)** | 0.2 (θ=0.1) |
| Thyroglobulin | 8q | Tg-ms2 | 0.1 (θ=0.1) | 0.1 (θ=0.1) | 0.4 (θ=0.05) | 0 (θ=0.5) |
NPL= 0.8
NPL=1.1
Insulin VNTR locus: Analysis for model 1 (T1D) gave a 2-point maximum LOD score (MLS) of 0.7, and analysis for model 2 (AITD) gave a 2-point MLS of 0.6 (Table 5) suggesting that there was little information for linkage. When we analyzed considering all T1D or AITD patients as affected (model 3) the 2-point LOD scores was 1.9 (recessive model, 30% penetrance, ••=0.01) showing evidence for linkage (Table 5). These results, may suggest that, in addition to HLA, the insulin VNTR or a nearby gene in linkage disequilibrium may also contributes to the familial clustering of T1D and AITD.
Thyroglobulin locus: We used the microsatellite Tgms2, located inside intron 27 of Tg, to test for linkage to the Tg locus. Analysis for all models gave low LOD scores (< 0.5) (Table 5). These low LOD scores cannot show or exclude evidence for linkage at the Tg locus in our 55 T1D-AITD families.
DISCUSSION
Despite the well-known association between T1D and AITD, there have been relatively few reports on the shared genetic susceptibility for these two disorders. Previously, we analyzed 40 families in which T1D and AITD cluster, and demonstrated linkage of HLA with both T1D and AITD. Our results also had suggested that HLA-DR3 contributed to both diseases, whereas HLA-DR4 contributed to T1D but not to AITD. In this study, we have expanded our dataset to 55 families and examined the contribution of HLA-DR and -DQ, and three non-HLA genes; CTLA-4, insulin VNTR, and thyroglobulin, to the genetic association between T1D and AITD. Our linkage analysis of the HLA locus confirmed again the evidence for linkage of HLA to T1D and AITD in the T1D-AITD families. The maximum multipoint HLOD was 7.57 for T1D (model 1), 1.5 for AITD (model 2), and 2.2 when all individuals with T1D or AITD were considered affected (model 3) [Figure 1]. These results suggested that the clustering of AITD with T1D in families is partially determined by the HLA locus. The finding of low positive LOD scores when considering AITD as affected (MLS=1.5) is interesting since most previous studies by us and others found strong evidence against linkage (negative LOD scores) with the HLA locus in families with AITD but without T1D (17;43-47). Since it was possible that these positive LOD scores for AITD reflected the contribution of individuals with AITD+T1D (APS variant) we re-analyzed the data when considering individuals with only AITD (and not T1D) as affected. The analysis showed an MLS of 0.8 at marker TNFα-ms (data not shown), supporting our conclusion that in the T1D-AITD families the HLA locus is linked with AITD. Thus, it is possible that the AITD phenotype that is seen in T1D families has a different genetic etiology, and potentially a different pathogenesis, than the AITD phenotype seen in families in which only AITD clusters and, the sporadic AITD phenotype.
When we considered both T1D and AITD as affected the maximum LOD score was 2.2 which is significantly lower than the MLS obtained when considering only T1D as affected (MLS=7.57) (Figure 1). One possible explanation for this observation is that in our T1D-AITD families, T1D may show strong evidence of linkage to the HLA locus while AITD does not. Thus, considering AITD-affected individuals as affected introduces genetic heterogeneity into the data because another non-HLA locus contributes to the familial clustering of T1D and AITD at least in some of the families.
Our TDT analysis revealed preferential transmission of HLA haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 to offspring affected with T1D alone. The same was true for offspring affected with both T1D and AITD (APS variant). However, when looking at offspring affected with AITD alone, only DR3-DQB1*0201 was preferentially transmitted, whereas DR4-DQB1*0302 was preferentially not transmitted. These data may suggest that DR3-DQB1*0201 haplotype confers susceptibility to both diseases, whereas the haplotype DR4-DQB1*0302 is specific to T1D. Indeed, it is well known that the DR4-DQB1*0302 haplotype shows the strongest association with T1D (48-50). It is also likely that in offspring with both T1D and AITD (APS offspring), the major haplotype is DR3-DQB1*0201, while the preferential transmission of the DR4-DQB1*0302 haplotype reflects the strong influence of this haplotype on the T1D component.
In order to look for differential effects of HLA-DR and HLA-DQB1 alleles on susceptibility to T1D and AITD, we compared the haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 to other haplotypes containing one, but not both, of their constituent alleles (Table 4). This analysis has suggested that within the DR3-DQB1*0201 haplotype DR3 was the primary allele conferring most of the risk to both T1D and AITD, whereas DQB1*0201, in linkage disequilibrium with DR3, may have a secondary role. Similarly, our data suggested that DQB1*0302 was the primary allele conferring susceptibility to T1D whereas DR4, in linkage disequilibrium, may have a secondary role. However, we cannot exclude the possibility that these associations may reflect the effects of another nearby gene or genes which may be the causative gene and is in linkage disequilibrium with the specific HLA alleles that we found to be associated with T1D and/or AITD.
One potential weakness of our study is the relatively small number of families used in TDT analysis, particularly when analyzing family members affected by AITD only. Therefore, additional studies in independent dataset are needed to confirm these data. However, despite the small numbers we were able to identify haplotypes which were associated with T1D and/or AITD in our dataset.
Results similar to our own have been reported in two previous studies looking at HLA associations in Caucasian families with both T1D and AITD. Santamaria et al (51) compared 39 subjects with both T1D and AITD to 17 AITD-only affected siblings of T1D probands. They showed that individuals with both T1D and AITD were more likely to have alleles DQB1*0201 and DQB1*0302, whereas individuals with AITD only were more likely to have DQB1*0201 but not DQB1*0302. Dorman et al (52) studied 25 T1D families in which at least one parent and one offspring had Hashimoto's thyroiditis and found a two-fold increase in the prevalence of DQA1*0501-DQB1*0201 among family members with Hashimoto's compared to those without. No difference in the prevalence of DQA1*0301-DQB1*0302 among these two groups was observed. Similarly, our data showed that the haplotype DR3-DQB1*0201 contributed to the shared susceptibility to T1D and AITD whereas the haplotype DR4-DQB1*0302 was T1D specific. We have extended these observations and present data that may suggest that DR3 and not DQB1*0201 is most likely the primary allele contributing to this joint susceptibility to T1D and AITD.
Our study also demonstrated that the haplotypes DR3-DQB1*0201 and DR4- DQB1*0302 predispose to the combined phenotype of T1D and AITD in the same individual, considered a variant of APS (3). Two other studies investigated HLA associations in Caucasian patients with variants of APS. Huang et al (53) HLA-typed 31 unrelated patients with APS2 (autoimmune adrenalitis plus at least one other autoimmune disorder) and divided the patients into two subgroups; 17 patients whose autoimmune phenotype included T1D and/or islet-cell or glutamic acid decarboxylase antibodies, and 14 patients without such evidence of beta-cell autoimmunity. In the former group, the haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 were more frequent compared to controls. However, in the APS2 patients lacking beta-cell autoimmunity, only the haplotype DR3-DQB1*0201 was increased, lending further evidence to the notion that DR3-DQB1*0201 is associated with multiple endocrine organ autoimmunity. Wallaschofski et al (11) reported slightly different findings. In this study, 112 unrelated APS patients were divided into 29 patients with APS2 (as defined above) and 83 patients with APS3 (one autoimmune endocrinopathy other than autoimmune adrenalitis plus at least one other autoimmune disorder). Of note, 21 (25%) of the APS3 patients had T1D and 82 (99%) had AITD. The haplotypes DR3- DQB1*0201 and DR4-DQB1*0302 were both increased in the APS2 patients compared to controls, but only DR4-DQB1*0302 was increased in the APS3 patients. The authors concluded that the extended haplotype DR4-DQA1*0301-DQB1*0302 is associated with APS2 and APS3. These results are different than ours, which showed that both haplotypes DR3-DQB1*0201 and DR4-DQB1*0302 contributed to the APS variant consisting of T1D and AITD. However, Wallaschofski et al did not present subgroup analysis looking specifically at APS patients with the T1D+AITD phenotype.
Our data showed that the CTLA-4 gene also contributed to the development of both T1D and AITD in the same individual (APS variant). Three other studies have examined the CTLA-4 gene in APS patients. Kemp et al. found an association of CTLA-4 with an APS variant in which the main components were vitiligo with AITD or T1D (54). Another study from Japan found an association between the G allele of the CTLA-4 A/G49 SNP and younger T1D patients with AITD (55). In contrast Donner et al. found no association between CTLA-4 and an APS variant consisting of Addison's disease and AITD or T1D (56). Therefore, it is likely that the CTLA-4 gene contributes to the expression of only certain APS variants. Since, as noted above, distinct HLA class 2 haplotypes have also been shown to be associated with certain APS variants, we hypothesize that specific combinations of HLA and CTLA-4 alleles, as well as alleles of other genes, predispose to specific APS phenotypes.
Our linkage analysis showed that the insulin VNTR locus may contribute to the clustering of T1D and AITD within families, a finding of some surprise given that this locus has so far been associated only with T1D. These results may imply that the insulin VNTR locus may harbor a gene, which contributes to the familial clustering of T1D and AITD. A recent study have shown no association of the VNTR polymorphism with GD (57). Therefore, if our data of linkage of T1D and AITD to the insulin VNTR locus are confirmed they may suggest that another gene in this locus and not the VNTR polymorphism itself the autoimmunity locus in this region.
In conclusion, our data have shown the HLA haplotype DR3-DQB1*0201 contributes to the genetic susceptibility to T1D and AITD, whereas DR4-DQB1*0302 is specific for T1D. Within these haplotypes, the DR3 and DQB1*0302 alleles may play the primary roles, respectively. In addition, the insulin VNTR locus may contribute to the clustering of T1D and AITD in families, and the CTLA-4 locus may play a role in the development of T1D and AITD in the same individual (a variant of APS). Thus, several genes are involved in joint susceptibility to T1D and AITD. However, we do not know if these genes interact in conferring risk for T1D and AITD. We have previously shown an additive effect on the odds ratio for HLA-DR3 and the G allele of the CTLA-4 A/G49 SNP suggestive of an interaction between the HLA-DR genes and the CTLA-4 gene in predisposing to GD (58). Another study suggesting interaction between HLA and CTLA-4 was also recently reported (59). Thus, it is possible that the autoimmunity genes contributing to the joint susceptibility to T1D and AITD interact and that their interactions may influence disease phenotype and severity. The molecular basis for the interactions between susceptibility genes in complex diseases is unknown. These interactions could represent the cumulative effect of increased statistical risk, or alternatively, there may be molecular interactions between the susceptibility genes or their products, which ultimately determine disease phenotype.
Acknowledgments
We would like to thank the human biological data interchange (HBDI, Philadelphia, PA, USA) for making families from their repository available for our studies. We would also like to thank Dr. Terry Davies for helpful discussions.
Abbreviations
- AITD
autoimmune thyroid diseases
- APS
autoimmune polyglandular syndrome
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- HLA
human leukocyte antigen
- LOD
logarithm of odds
- MLS
maximum LOD score
- NS
not significant
- T1D
Type 1 diabetes
- TDT
transmission disequilibrium test
- VNTR
variable number of tandem repeats
Footnotes
Financial Support Information: This work was supported in part by: DK61659 and DK067555 from NIDDK (to YT), and DK31775, NS27941 & MH48858 (to DAG).
REFERENCES
- 1.Levin L, Tomer Y. The etiology of autoimmune diabetes and thyroiditis: evidence for common genetic susceptibility. Autoimmun Rev. 2003;2:377–386. doi: 10.1016/s1568-9972(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP, Jenkins RC. Disease associations with autoimmune thyroid disease. Thyroid. 2002;12:977–988. doi: 10.1089/105072502320908312. [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350:2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 4.Kordonouri O, Klinghammer A, Lang EB, Gruters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25:1346–1350. doi: 10.2337/diacare.25.8.1346. [DOI] [PubMed] [Google Scholar]
- 5.Bright GM, Blizzard RM, Kaiser DL, Clarke WL. Organ-specific autoantibodies in children with common endocrine diseases. J Pediatr. 1982;100:8–14. doi: 10.1016/s0022-3476(82)80227-8. [DOI] [PubMed] [Google Scholar]
- 6.McCanlies E, O'Leary LA, Foley TP, Kramer MK, Burke JP, Libman A, Swan JS, Steenkiste AR, McCarthy BJ, Trucco M, Dorman JS. Hashimoto's thyroiditis and insulin-dependent diabetes mellitus: differences among individuals with and without abnormal thyroid function. J Clin Endocrinol Metab. 1998;83:1548–1551. doi: 10.1210/jcem.83.5.4769. [DOI] [PubMed] [Google Scholar]
- 7.Holl RW, Bohm B, Loos U, Grabert M, Heinze E, Homoki J. Thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Effect of age, gender and HLA type. Horm Res. 1999;52:113–118. doi: 10.1159/000023446. [DOI] [PubMed] [Google Scholar]
- 8.Burek CL, Rose NR, Guire KE, Hoffman WH. Thyroid autoantibodies in black and in white children and adolescents with type 1 diabetes mellitus and their first degree relatives. Autoimmunity. 1990;7:157–167. doi: 10.3109/08916939008993388. [DOI] [PubMed] [Google Scholar]
- 9.Riley WJ, Maclaren NK, Lezotte DC, Spillar RP, Rosenbloom AL. Thyroid autoimmunity in insulin-dependent diabetes mellitus: the case for routine screening. J Pediatr. 1981;99:350–354. doi: 10.1016/s0022-3476(81)80316-2. [DOI] [PubMed] [Google Scholar]
- 10.Tomer Y, Greenberg DA, Davies TF. The genetic susceptibility to type 1 (insulin dependent) diabetes mellitus and autoimmune thyroid diseases: From epidemiological observations to gene mapping. In: Volpe R, editor. Contemporary Endocrinology: Autoimmune Endocrinopathies. Humana Press; Totowa, NJ: 1999. pp. 57–90. [Google Scholar]
- 11.Wallaschofski H, Meyer A, Tuschy U, Lohmann T. HLA-DQA1*0301-associated susceptibility for autoimmune polyglandular syndrome type II and III. Horm Metab Res. 2003;35:120–124. doi: 10.1055/s-2003-39059. [DOI] [PubMed] [Google Scholar]
- 12.Levin L, Ban Y, Concepcion E, Davies TF, Greenberg DA, Tomer Y. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum Immunol. 2004;65:640–647. doi: 10.1016/j.humimm.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Ilonen J, Sjoroos M, Knip M, Veijola R, Simell O, Akerblom HK, Paschou P, Bozas E, Havarani B, Malamitsi-Puchner A, Thymelli J, Vazeou A, Bartsocas CS. Estimation of genetic risk for type 1 diabetes. Am J Med Genet. 2002;115:30–36. doi: 10.1002/ajmg.10341. [DOI] [PubMed] [Google Scholar]
- 14.Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet. 2000;95:432–437. doi: 10.1002/1096-8628(20001218)95:5<432::aid-ajmg5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, Tomer Y. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves' disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 16.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 17.Tomer Y, Barbesino G, Greenberg DA, Concepcion ES, Davies TF. Mapping the major susceptibility loci for familial Graves' and Hashimoto's diseases: Evidence for genetic heterogeneity and gene interactions. J Clin Endocrinol Metab. 1999;84:4656–4664. doi: 10.1210/jcem.84.12.6216. [DOI] [PubMed] [Google Scholar]
- 18.Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF. Common and unique susceptibility loci in Graves and Hashimoto diseases: Results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73:736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh SG, Bodmer JG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Hansen JA, Mach B, Mayr WR, Parham P, Petersdorf EW, Sasazuki T, Schreuder GM, Strominger JL, Svejgaard A, Terasaki PI. Nomenclature for factors of the HLA system, 2000. Hum Immunol. 2001;62:419–468. doi: 10.1016/s0198-8859(01)00229-4. [DOI] [PubMed] [Google Scholar]
- 20.Westman P, Kuismin T, Partanen J, Koskimies S. An HLA-DR typing protocol using group-specific PCR-amplification followed by restriction enzyme digests. Eur J Immunogenet. 1993;20:103–109. doi: 10.1111/j.1744-313x.1993.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 21.Sengar DP, Goldstein R. Comprehensive typing of DQB1 alleles by PCR RFLP. Tissue Antigens. 1994;43:242–248. doi: 10.1111/j.1399-0039.1994.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 22.Huxtable SJ, Saker PJ, Haddad L, Walker M, Frayling TM, Levy JC, Hitman GA, O'Rahilly S, Hattersley AT, McCarthy MI. Analysis of parent-offspring trios provides evidence for linkage and association between the insulin gene and type 2 diabetes mediated exclusively through paternally transmitted class III variable number tandem repeat alleles. Diabetes. 2000;49:126–130. doi: 10.2337/diabetes.49.1.126. [DOI] [PubMed] [Google Scholar]
- 23.Vankova M, Vrbikova J, Hill M, Cinek O, Bendlova B. Association of insulin gene VNTR polymorphism with polycystic ovary syndrome. Ann N Y Acad Sci. 2002;967:558–565. doi: 10.1111/j.1749-6632.2002.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 24.Tomer Y, Greenberg DA, Barbesino G, Concepcion ES, Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab. 2001;86:1687–1693. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 25.Tomer Y, Greenberg DA, Concepcion E, Ban Y, Davies TF. Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab. 2002;87:404–407. doi: 10.1210/jcem.87.1.8291. [DOI] [PubMed] [Google Scholar]
- 26.Ott J. A computer program for linkage analysis of general human pedigrees. Am J Hum Genet. 1976;28:528–529. [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg DA, Abreu P, Hodge SE. The power to detect linkage in complex disease by means of simple LOD- score analyses. Am J Hum Genet. 1998;63:870–879. doi: 10.1086/301997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnett AH, Eff C, Leslie RDG, Pyke DA. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981;20:87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- 29.Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, Leslie RD. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–362. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 30.Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. BMJ. 1995;311:913–917. doi: 10.1136/bmj.311.7010.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brix TH, Kyvik KO, Hegedus L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–539. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 32.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population- based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 33.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A. Allegro, a new computer program for multipoint linkage analysis. Nat Genet. 2000;25:12–13. doi: 10.1038/75514. [DOI] [PubMed] [Google Scholar]
- 34.Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gapay G, Morissete J, Weissenbach J. A comprehensive genetic map of the human genome base on 5,264 microsatellites. Nature. 1996;380:152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- 35.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 36.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus. Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 37.Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235–249. doi: 10.1038/sj.gene.6363875. [DOI] [PubMed] [Google Scholar]
- 38.Marron MP, Raffel LJ, Garchon HJ, Jacob CO, SerranoRios M, Martinez Larrad MT, Teng WP, Park Y, Zhang ZX, Goldstein DR, Tao YW, Beaurain G, Bach JF, Huang HS, Luo DF, Zeidler A, Rotter JI, Yang MCK, Modilevsky T, Maclaren NK, She JX. Insulin-dependent diabetes mellitus (IDDM) is assocaitedwith CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet. 1997;6:1275–1282. doi: 10.1093/hmg/6.8.1275. [DOI] [PubMed] [Google Scholar]
- 39.Nistico L, Buzzetti R, Pritchard LE, Auwera B, Giovannini C, Bosi E, Larrad MT, Rios MS, Chow CC, Cockram CS, Jacobs K, Mijovic C, Bain SC, Barnett AH, Vandewalle CL, Schuit F, Gorus FK, Tosi R, Pozzilli P, Todd JA. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Begian Diabetes Registry. Hum Mol Genet. 1996;5:1075–1080. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 40.Ban Y, Taniyama M, Tozaki T, Yanagawa T, Yamada S, Maruyama T, Kasuga A, Tomita M, Ban Y. No association of type 1 diabetes with a microsatellite marker for CTLA-4 in a Japanese population. Autoimmunity. 2001;34:39–43. doi: 10.3109/08916930108994124. [DOI] [PubMed] [Google Scholar]
- 41.Yanagawa T, Maruyama T, Gomi K, Taniyama M, Kasuga A, Ozawa Y, Terauchi M, Hirose H, Maruyama H, Saruta T. Lack of association between CTLA-4 gene polymorphism and IDDM in Japanese subjects. Autoimmunity. 1999;29:53–56. doi: 10.3109/08916939908995972. [DOI] [PubMed] [Google Scholar]
- 42.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 43.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf) 2002;57:81–88. doi: 10.1046/j.1365-2265.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 44.Bode HH, Dorf ME, Forbes AP. Familial lymphocytic thyroiditis: analysis of linkage with histocompatibility and blood group. J Clin Endocrinol Metab. 1973;37:692–697. doi: 10.1210/jcem-37-5-692. [DOI] [PubMed] [Google Scholar]
- 45.Roman SH, Greenberg DA, Rubinstein P, Wallenstein S, Davies TF. Genetics of autoimmune thyroid disease: lack of evidence for linkage to HLA within families. J Clin Endocrinol Metab. 1992;74:496–503. doi: 10.1210/jcem.74.3.1740483. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins BR, Ma JT, Lam KS, Wang CC, Yeung RT. Analysis of linkage between HLA haplotype and susceptibility to Graves' disease in multiple-case Chinese families in Hong Kong. Acta Endocrinol (Copenh) 1985;110:66–69. doi: 10.1530/acta.0.1100066. [DOI] [PubMed] [Google Scholar]
- 47.Sakai K, Shirasawa S, Ishikawa N, Ito K, Tamai H, Kuma K, Akamizu T, Tanimura M, Furugaki K, Yamamoto K, Sasazuki T. Identification of susceptibility loci for autoimmune thyroid disease to 5q31-q33 and Hashimoto's thyroiditis to 8q23-q24 by multipoint affected sib-pair linkage analysis in Japanese. Hum Mol Genet. 2001;10:1379–1386. doi: 10.1093/hmg/10.13.1379. [DOI] [PubMed] [Google Scholar]
- 48.Michelsen B, Lernmark A. Molecular cloning of a polymorphic DNA endonuclease fragment associates insulin-dependent diabetes mellitus with HLADQ. J Clin Invest. 1987;79:1144–1152. doi: 10.1172/JCI112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caillat-Zucman S, Garchon HJ, Timsit J, Assan R, Boitard C, Djilali-Saiah I, Bougneres P, Bach JF. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest. 1992;90:2242–2250. doi: 10.1172/JCI116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kockum I, Sanjeevi CB, Eastman S, Landin-Olsson M, Dahlquist G, Lernmark A. Complex interaction between HLA DR and DQ in conferring risk for childhood type 1 diabetes. Eur J Immunogenet. 1999;26:361–372. doi: 10.1046/j.1365-2370.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 51.Santamaria P, Barbosa JJ, Lindstrom AL, Lemke TA, Goetz FC, Rich SS. HLA-DQB1-associated susceptibility that distinguishes Hashimoto's thyroiditis from Graves' disease in type I diabetic patients. J Clin Endocrinol Metab. 1994;78:878–883. doi: 10.1210/jcem.78.4.8157715. [DOI] [PubMed] [Google Scholar]
- 52.Dorman J, Kramer MK, O'Lear LA, Burke JP, McCanlies E, McCarthy BJ, Trucco M, Swan JS, Steenkiste A, Koehler AN, Foley TP. Molecular epidemiology of autoimmune thyroid disease. Gac Med Mex. 1996;133(Supp 1):97–103. [PubMed] [Google Scholar]
- 53.Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK. Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab. 1996;81:2559–2563. doi: 10.1210/jcem.81.7.8675578. [DOI] [PubMed] [Google Scholar]
- 54.Kemp EH, Ajjan RA, Waterman EA, Gawkrodger DJ, Cork MJ, Watson PF, Weetman AP. Analysis of a microsatellite polymorphism of the cytotoxic T-lymphocyte antigen-4 gene in patients with vitiligo. Br J Dermatol. 1999;140:73–78. doi: 10.1046/j.1365-2133.1999.02610.x. [DOI] [PubMed] [Google Scholar]
- 55.Takara M, Komiya I, Kinjo Y, Tomoyose T, Yamashiro S, Akamine H, Masuda M, Takasu N. Association of CTLA-4 gene A/G polymorphism in Japanese type 1 diabetic patients with younger age of onset and autoimmune thyroid disease. Diabetes Care. 2000;23:975–978. doi: 10.2337/diacare.23.7.975. [DOI] [PubMed] [Google Scholar]
- 56.Donner H, Braun J, Seidl C, Rau H, Finke R, Ventz M, Walfish PG, Usadel KH, Badenhoop K. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab. 1997;82:4130–4132. doi: 10.1210/jcem.82.12.4406. [DOI] [PubMed] [Google Scholar]
- 57.Tait KF, Collins JE, Heward JM, Eaves I, Snook H, Franklyn JA, Barnett AH, Todd JA, Maranian M, Compston A, Sawcer S, Gough SC. Evidence for a Type 1 diabetes-specific mechanism for the insulin gene-associated IDDM2 locus rather than a general influence on autoimmunity. Diabet Med. 2004;21:267–270. doi: 10.1111/j.1464-5491.2004.01129.x. [DOI] [PubMed] [Google Scholar]
- 58.Ban Y, Concepcion ES, Villanueva R, Greenberg DA, Davies TF, Tomer Y. Analysis of immune regulatory genes in familial and sporadic Graves' disease. J Clin Endocrinol Metab. 2004;89:4562–4568. doi: 10.1210/jc.2003-031693. [DOI] [PubMed] [Google Scholar]
- 59.Einarsdottir E, Soderstrom I, Lofgren-Burstrom A, Haraldsson S, Nilsson-Ardnor S, Penha-Goncalves C, Lind L, Holmgren G, Holmberg M, Asplund K, Holmberg D. The CTLA4 region as a general autoimmunity factor: an extended pedigree provides evidence for synergy with the HLA locus in the etiology of type 1 diabetes mellitus, Hashimoto's thyroiditis and Graves' disease. Eur J Hum Genet. 2003;11:81–84. doi: 10.1038/sj.ejhg.5200903. [DOI] [PubMed] [Google Scholar]
- 60.Fialkow PJ, Zavala C, Nielsen R. Thyroid autoimmunity: increased frequency in relatives of insulin-dependent diabetes patients. Ann Intern Med. 1975;83:170–176. doi: 10.7326/0003-4819-83-2-170. [DOI] [PubMed] [Google Scholar]
- 61.Maclaren NK, Riley WJ. Thyroid, gastric, and adrenal autoimmunities associated with insulin-dependent diabetes mellitus. Diabetes Care 8 Suppl. 1985;1:34–38. doi: 10.2337/diacare.8.1.s34. [DOI] [PubMed] [Google Scholar]
- 62.Kontiainen S, Schlenzka A, Koskimies S, Rilva A, Maenpaa J. Autoantibodies and autoimmune diseases in young diabetics. Diabetes Res. 1990;13:151–156. [PubMed] [Google Scholar]
- 63.Roldan MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with Type 1 diabetes mellitus. Diabetes Nutr Metab. 1999;12:27–31. [PubMed] [Google Scholar]
- 64.Payami H, Joe S, Thomson G. Autoimmune thyroid disease in Type 1 diabetes. Genetic Epidemiology. 1989;6:137–141. doi: 10.1002/gepi.1370060126. [DOI] [PubMed] [Google Scholar]