Abstract
Guanine nucleotide binding proteins (G-proteins) play an important role in mediating signals transduced across the cell membrane by membrane-bound receptors. The precise role of these proteins and their coupled receptors in the physiology of the vestibular neuroepithelium is poorly understood. Although Golfalpha was originally discovered in the olfactory neuroepithelium and striatum, we recently identified this G-protein alpha subunit in a normalized cDNA library constructed from rat vestibular end organs and vestibular nerves including Scarpa's ganglia. In order to further characterize Golfalpha in the rat vestibular periphery, we used in situ hybridization and reverse transcription polymerase chain reaction to determine the anatomic context of this gene expression. Golfalpha was found in both the end organs and the ganglia and could serve unique roles in the physiology of the vestibular neuroepithelium.
Keywords: Efferent vestibular system, G-protein, golfalpha, olfactory, receptor, vestibular
1. Introduction
Many of the pathways required for neurotransmission and neuromodulation of the efferent and afferent systems of the vestibular periphery depend on effectors molecules such as guanine nucleotide binding proteins (G-proteins). Functional G-proteins are heterotrimeric intracellular membrane-bound proteins. They serve to transduce signals from specific membrane receptors to effector molecules such as adenylyl cyclase, phospholipase C, and ion channels. G-proteins regulate cAMP formation, intracellular Ca2+ mobilization, and membrane potential. Heterotrimeric G-proteins are composed of three subunits (α, β, and γ). The β and γ subunits form a heterodimer that dissociates only after denaturing and, thus, represents a functional monomer. G proteins are categorized into four subfamilies according to their α-subunits: Gαs, Gαi/o, Gαq, and Gα12. The Gα subunits bind guanine nucleotides and exist in two activation states. In addition, Gα subunits interact with both receptor and effector molecules and, therefore, are considered to be the functional component of the G-protein complex.
Gαolf (Golfalpha or GNAL) has an 88% amino acid homology to Gαs, and thus is considered a member of the Gαs family. There are three conserved functional domains involved in guanine triphosphate (GTP)-binding affinity, GTPase activity, receptor-dependent GTP binding, and GTP-induced conformational change [12]. Although Golfalpha was originally discovered in the olfactory neuroepithelium and striatum, it has been identified in pancreatic β-cells, testis, spleen, lung and heart (for review see Régnauld et al. [14]). In earlier studies, we found that Golfalpha, Gαs, Gαs2, Gαo, Gαi2, and two splice variants of Gαi2 are expressed in the vestibular periphery [4, 5]. The purpose of the present study was to determine whether Golfalpha is differentially expressed in the vestibular end organs and primary afferent neurons.
2. Materials and methods
This study was performed in accordance with the United States Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Medical College of Wisconsin.
2.1. In situ hybridization
Male and female rats were used. Rats were perfused with 4% paraformaldehyde. The brains and temporal bones were removed. The end organs and the Scarpa's ganglia were dissected out under an operating microscope and all harvested tissues were postfixed for 1 hr in the same fixative. For in situ hybridization, the vestibular end organs (cristae and maculae) and Scarpa's ganglia were cryoprotected in 20% sucrose in phosphate buffer and cut serially at 10 μm using a cryostat. Sections were melted onto Superfrost™ Plus microscope slides and stored desiccated at –80°C until processed.
Preparation of Probes. Plasmids containing a 550 bp fragment of the 5′ coding sequence of rat Golfalpha cDNA obtained from earlier expression studies of G-proteins in the vestibular periphery were used [4]. Digoxigenin-labeled complementary RNA probes were transcribed from linearized templates in 20 μl reaction mixture according to Roche Applied Sciences (Indianapolis, IN) kit #1175 025.
In Situ Hybridization Procedure. Slide-mounted sections were sequentially treated with predigested proteinase K (1 μg/ml) in 0.1 M Tris-HCl, 1 mM EDTA, pH 8.0 for 20 min at 37°C, dipped in DEPC ddH2O (double distilled water treated with 0.1% diethylpyro-carbonate), rinsed for 3 min in 0.1 triethanolamine (pH 8.0), rinsed twice for 2 min each in 2X SSC (1X SSC = 0.15 M NaCl/0.04 M NaCitrate, pH 7.2) and dehydrated in graded ethanols. Slides were air-dried and incubated at 55°C covered with 40 μl/slide of prehybridization mixture (60% deionized formamide, 0.1X hybridization salts (1X hybridization salts = 0.15 M NaCl, 5 mM EDTA, 5 mM PIPES, pH 6.8), 0.1X Denhardt's buffer (100X Denhardt's buffer = 2% each of polyvinylpyrrolidone, ficoll, and bovine serum albumin), 0.2% sodium dodecyl sulfate, and 100 mM dithiothreitol) and 250 μg/ml each of denatured polyA and sheared salmon sperm DNA. After 60 min the sections were covered with 200 μl of prehybridization mixture containing 20–40 ng/slide digoxigenin-labeled cRNA and were incubated at 55°C for 16 hrs. Following hybridization, the slides were rinsed briefly in 4X SSC. Sections were exposed to RNase (100 μg/ml in 0.5 M NaCl, 10 mM Tris, 1 mM EDTA, pH 8.0) for 30 min at 37°C, washed in decreasing concentrations of SSC with the final rinse done in 0.1X SSC at 55°C for 30 min.
Following the final wash in 0.1 × SSC, the slides processed were washed in Tris buffered saline (TBS; 3 × 10 min) and incubated with alkaline phosphatase conjugated sheep anti-digoxigenin antibody (Roche Applied Sciences; 1:500 in TBS containing 0.3% TRITON X 100 and 2.5% normal sheep serum) for 16–24 hrs at room temperature. Sections were washed in TBS (3 × 30 min), followed by a wash in buffer 3 (100 mM Tris containing 100 mM NaCl, 50 mM MgCl2, pH 9.5) for 20 min. Subsequently, sections were incubated in NBT/BCIP (freshly prepared 200 μl NBT/BCIP (vial 4 in 10 mL buffer 3, see Roche Applied Sciences kit # 1175 041; NBT: nitroblue tetrazodium; BCIP: 5-bromo-4-chloro-3-indoyl-phosphate, 4 toluidine salt) for 16–24 hrs. When the color reaction product reached the desired intensity, slides were washed in 10 mM Tris, 1 mM EDTA, pH 8.0 5 times, 2 min each. Slides were air-dried and mounted with Permount or DPX.
Controls. Slide-mounted sections were treated with RNase (100 μg/ml) for 30 min at 37°C prior to the proteinase K treatment. Greatly diminished or no specific digoxigenin signal was expected in these sections suggesting that hybridization was to single-stranded RNA and not other cellular components.
Analysis. Sections of cristae, maculae and Scarpa's ganglia processed using the in situ hybridization protocol were studied using a Zeiss Axioskop 2 photomicroscope (Carl Zeiss AG, Jena, Germany). Cells within the vestibular end organs and ganglia were determined to be digoxigenin positive or negative.
2.2. Reverse transcription polymerase chain reaction
Total RNA was isolated from separate pools of vestibular end organs and Scarpa's ganglia (Invitrogen, Carlsbad, CA). Approximately 1 μg total RNA was used to synthesize cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). In addition to controlling for genomic DNA contamination by developing a primer design that spanned an intron, true negative control experiments were included in which all reagents except for reverse transcriptase were added to the reaction (RT-). One-tenth of each cDNA reaction was added to a 50 μl polymerase chain reaction containing 1.5 mM MgCl2. Cycling conditions consisted of 94°C denature for 30 sec, 58°C anneal for 30 sec, and 72°C polymerization for 45 sec, for a total of 35 cycles. Four μl aliquots of amplified DNA were electrophoretically resolved on a 1% agarose gel stained with GelStar (Cambrex Bio Science Rockland, New Jersey), and visualized using ultraviolet light. Amplimers based on rat Golfalpha subunit mRNA were intron spanning and from exon 5 (5′ TACCAGCTGATCGACTGTGC 3′, forward) and from exon 10 (5′ TGGCATACTCCGGGAAATAG 3′, reverse). The expected amplicon size is 445 base pairs and was tested by restriction endonuclease mapping using BamHI to verify its authenticity.
3. Results
3.1. In situ hybridization
Digoxigenin reaction product was visualized in virtually all Scarpa's ganglion somata, indicating in situ hybridization to Golfalpha mRNA (Fig. 1(A)). Pretreatment with RNase showed no digoxigenin reaction product in these same Scarpa's ganglion somata (Fig. 1(B)). No digoxigin reaction product was seen within the cristae or the maculae.
Fig. 1.
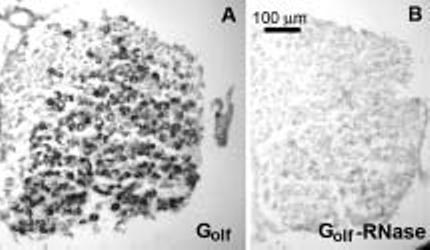
Localization of Golfalpha in Scarpa's ganglion using in situ hybridization. (A) Photomicrographs illustrating hybridization with digoxigenin labeled Golfalpha cRNA to a 10 μm thick section of Scarpa's ganglion. (B) Hybridization with Golfalpha cRNA after treating the section with RNase. Because of the high sequence homology of the Golfalpha and Gαs mRNA, the hybridization was repeated at a higher stringency condition of 65°C without appreciable loss of signal, further confirming the expression of Golfalpha.
3.2. Reverse transcription polymerase chain reaction
Figure 2 shows that RT-PCR amplification of total RNA obtained from the vestibular end organs (cristae and maculae) and vestibular ganglia yielded a single band of 445 bp, as expected based on our Golfalpha primer design. The authenticity of this product was confirmed by restriction mapping using BamHI restriction endonuclease (Fig. 3). The experiments omitting the reverse transcriptase step yielded no amplification product thus demonstrating the absence of genomic DNA. The correct amplicon size produced by the RT-PCR also demonstrates the absence of genomic DNA since the primer design spanned introns.
Fig. 2.
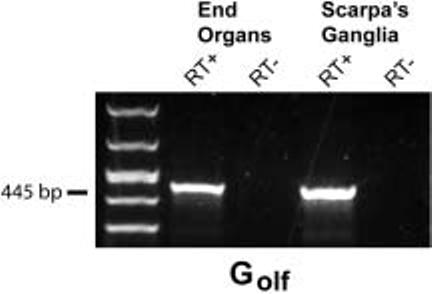
RT-PCR demonstrating expression of rat Golfalpha (Golf) in end organs (cristae and maculae) (lanes 2 and 3) and Scarpa's ganglia (lanes 4 and 5). Negative control reactions (RT-) are shown in lanes 3 and 5. A 50-1,000 bp DNA ladder is shown in lane 1 (Cambrex, East Rutherford, NJ). The observed amplicon size in lanes 2 and 4 is 445 base pairs and was authenticated to be Golfalpha by restriction endonuclease mapping using BamHI (see Fig. 3).
Fig. 3.
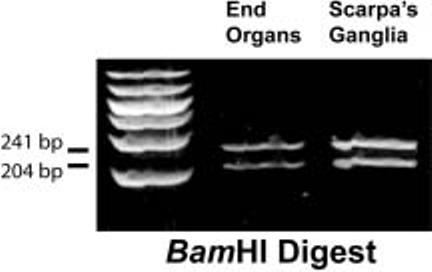
Confirmation of rat Golfalpha PCR product using BamHI restriction endonuclease digestion. Since there was a single BamHI site within the 445 base pair (bp) amplicon, two fragments were expected to result after digestion, one 241 bp and the other 204 bp. These can be seen in lanes 2 and 3 for the vestibular end organs and Scarpa's ganglia, respectively. A 50-1,000 bp DNA ladder is shown in lane 1 (Cambrex, East Rutherford, NJ).
4. Discussion
The RT-PCR experiments reported herein demonstrate that Golfalpha mRNA expression occurs within both the vestibular primary afferent neurons in Scarpa's ganglia as well as within the vestibular end organs. However, it can be inferred that there are higher levels of Golfalpha mRNA expression in the Scarpa's ganglia somata compared to the crista ampullaris. This is suggested by the demonstration of in situ hybridization to this mRNA within the ganglia but not the cristae or maculae. To demonstrate this relative difference of expression it would be necessary to complete real-time PCR experiments.
Regarding the apparent absence of Golfalpha in the vestibular end organs based on the in situ hybridization data, sensitivity of in situ hybridization vs. RT-PCR is an issue to consider. RT-PCR is an extraordinarily sensitive technique for detecting specific mRNAs, which is why control experiments are so crucial. Being certain that genomic DNA is not the template for the specific PCR amplification rather than the target cDNA is imperative. There are two control experiments that can be performed to accomplish this goal. First, if the expected amplicon size is seen after PCR amplification with primers designed to span introns, contamination with genomic DNA has not occurred. This is the case because the expected amplicon size would be much greater when amplified from genomic DNA since portions of the two exons plus the intron would be amplified. The second control experiment involves omitting the reverse transcription (RT) step of the protocol. This eliminates the conversion of mRNA to the template cDNA used for the PCR and therefore only genomic DNA (if present) would be available as a template for the amplification. We performed both of these control experiments. Unfortunately, with the RT-PCR technique cell specific context is lost, which is why in situ hybridization is a valuable technique. However, in situ hybridization in contrast to RT-PCR is not as sensitive. There are a variety of reasons why this is the case, including the need for the probe to penetrate the tissue, efficiency of hybridization, specificity of hybridization, and due to the additional limitations inherent in the techniques used for visualization of the hybridized complexes.
The membrane-bound adenylyl cyclases (ACs) are essential effectors of the Gαs family and act through cAMP production and protein kinase A (PKA) activation. In other tissues the cAMP pathway is also associated with several downstream signaling cascades, including cyclic nucleotide-gated ion channels and exchange protein directly activated by cAMP (Epacs) [6, 13]. While it is known that the olfactory-type cyclic nucleotide-gated ion channel is expressed in the cochlea of the rat [7], its expression in the vestibular periphery has not been studied to date.
The function of Golfalpha in the vestibular periphery is unknown; however, much has been learned about Golfalpha in the olfactory neuroepithelium (for review see Firestein [8], Ronnett and Moon [15]). Within the cilia of the olfactory neuroepithelium, Golfalpha activates the AC type III (AC3) isoform, increasing the level of cAMP, which opens a cyclic nucleotide-gated channel. This results in depolarization via the entry of Na+. Ca2+ also enters the cell and activates a Cl– channel, further increasing depolarization. In preliminary studies using RT-PCR we found that AC2, AC4, and AC5 were expressed in the vestibular periphery of the rat [17]. In addition to these three ACs, we have also been able to amplify AC3, AC6, AC8, and AC9 from a normalized cDNA library constructed from the vestibular end organs and Scarpa's ganglia harvested from 208 Rattus norvegicus temporal bones (unpublished data). Thus, the Golfalpha activation of AC3 within the vestibular periphery, as is the case in the olfactory epithelium is possible. The presence of these elements suggests that this transduction mechanism may play a role on the Na+, Ca2+, and Cl– metabolism within the vestibular periphery. However, more recently Régnauld et al. [14] showed that Golfalpha exerts no functional interaction with the AC3 isoform in transiently transfected HEK-293T cells, indicating that such a correlation may not have biologic significance. In that same body of work they demonstrated that the action of Golfalpha was to stimulate AC1 and AC8 isoforms preferentially. It is interesting to note that the Goto-Kakizaki (GK) rat, a widely accepted genetic rodent model of human type 2 diabetes mellitus, overexpresses AC1 and AC8 [11] suggesting that this may serve as a useful animal model to study the mechanisms of Golfalpha in the vestibular periphery.
While speculative in nature, it is intriguing to consider the potential role of mutated/dysfunctional Golfalpha proteins in two subsets of patients with vestibular dysfunction: those with psychiatric disorders and those who suffer with vestibular migraine. It is well known that there are neurological bases for balance-anxiety links [1,2], and an older less well known literature that suggests a relationship between peripheral vestibular function and schizophrenia [9,10]. More recently, there has been renewed interest in a genetic link between the genes responsible for schizophrenia and the genes for Usher syndrome type II [18]. It is particularly relevant that research focused on the basic mechanisms of the pathogenesis of Usher syndrome have implicated G-protein signaling [19]. Thus, it remains possible that a dysfunctional Golfalpha may be such a link. Berrettini et al. [3] identified two intronic polymorphisms in the human Golfalpha (GNAL) gene that may cause aberrant splicing of Golfalpha mRNA, thus it is possible that there exists a cohort of individuals with bilateral peripheral vestibular dysfunction due to this genetic basis. They subsequently determined the sequence and the genomic organization of GNAL which will allow further analysis of the GNAL locus at chromosome 18p11 which has been linked to bipolar disorder and schizophrenia [16].
Acknowledgments
This work was supported by NIH/NIDCD grant R01DC02971 and an intramural grant from the Toohill Research Fund of the Department of Otolaryngology and Communication Sciences.
References
- 1.Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77(4–5):469–475. doi: 10.1016/s0031-9384(02)00935-6. [DOI] [PubMed] [Google Scholar]
- 2.Balaban CD, Thayer JF. Neurological bases for balance-anxiety links. J Anxiety Disord. 2001;15(1–2):53–79. doi: 10.1016/s0887-6185(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 3.Berrettini WH, Vuoristo J, Ferraro TN, Buono BJ, Wildenauer D, Ala-Kokko L. Human G(olf) gene polymorphisms and vulnerability to bipolar disorder. Psychiatr Genet. 1998;8(4):235–238. doi: 10.1097/00041444-199808040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cioffi JA, Erbe CB, Raphael R, Kwitek AE, Tiwari UK, Jacob HJ, Popper P, Wackym PA. Expression of G-protein alpha subunits in the rat vestibular periphery and their chromosomal mapping. Acta Otolaryngol (Stockh) 2003;123(9):1027–1034. doi: 10.1080/00016480310000773. [DOI] [PubMed] [Google Scholar]
- 5.Cioffi JA, Wackym PA, Erbe CB, Gaggl W, Popper P. Characterization of G-protein Gαi2 splice variants expressed in the vestibular periphery of Rattus norvegicus. Mol Brain Res. 2005;137(12):89–97. doi: 10.1016/j.molbrainres.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 6.De Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanisms of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 7.Drescher MJ, Barretto RL, Chaturvedi D, Beisel KW, Hatfield JS, Khan KM, Drescher DJ. Expression of subunits for the cAMP-sensitive, olfactory cyclic nucleotide-gated ion channel in the cochlea: implications for signal transduction. Mol Brain Res. 2002;98:1–14. doi: 10.1016/s0169-328x(01)00289-3. [DOI] [PubMed] [Google Scholar]
- 8.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 9.Fish B, Dixon WJ. Vestibular hyporeactivity in infants at risk for schizophrenia. Association with critical developmental disorders. Arch Gen Psychiatry. 1978;35(8):963–971. doi: 10.1001/archpsyc.1978.01770320057004. [DOI] [PubMed] [Google Scholar]
- 10.Gordon AG. Peripheral vestibular pathology in schizophrenic infants. Arch Gen Psychiatry. 1979;36(13):1462–1464. doi: 10.1001/archpsyc.1979.01780130080011. [DOI] [PubMed] [Google Scholar]
- 11.Guenifi A, Portela-Gomes GM, Grimelius L, Efendic S, Abdel-Halim SM. Adenylyl cyclase isoform expression in non-diabetic and diabetic Goto-Kakizaki (GK) rat pancreas. Evidence for distinct overexpression of type-8 adenylyl cyclase in diabetic GK rat islets. Histochem Cell Biol. 2000;113:81–89. doi: 10.1007/s004180050010. [DOI] [PubMed] [Google Scholar]
- 12.Iiri T, Bell SM, Baranski TJ, Fujita T, Bourne HR. A Gsa mutant designed to inhibit receptor signaling through Gs. Proc Natl Acad Sci USA. 1999;96:499–504. doi: 10.1073/pnas.96.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leech CA, Holz GG, Chepurny O, Habener JF. Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic β-cells. Biochem Biophys Res Commun. 2000;278:44–47. doi: 10.1006/bbrc.2000.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Régnauld KL, Leteurtre E, Gutkind SJ, Gespach CP, Emani S. Activation of adenylyl cyclases, regulation of insulin status, and cell survival by Gaolf in pancreatic β-cells. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R870–R880. doi: 10.1152/ajpregu.00374.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annu Rev Physiol. 2002;64:189–222. doi: 10.1146/annurev.physiol.64.082701.102219. [DOI] [PubMed] [Google Scholar]
- 16.Vuoristo JT, Berrettini WH, Overhauser J, Prockop DJ, Ferraro TN, Ala-Kokko L. Sequence and genomic organization of the human G-protein Golfalpha gene (GNAL) on chromosome 18p11, a susceptibility region for bipolar disorder and schizophrenia. Mol Psychiatry. 2000;5(5):495–501. doi: 10.1038/sj.mp.4000758. [DOI] [PubMed] [Google Scholar]
- 17.Wackym PA, Troyanovskaya M, Popper P. Differential amplification of adenylyl cyclase isoform cDNAs in the rat vestibular periphery. Brain Res. 2000;859(2):378–380. doi: 10.1016/s0006-8993(00)02007-2. [DOI] [PubMed] [Google Scholar]
- 18.Waldeck T, Wyszynski B, Medalia A. The relationship between Usher's syndrome and psychosis with Capgras syndrome. Psychiatry. 2001;64(3):248–255. doi: 10.1521/psyc.64.3.248.18467. [DOI] [PubMed] [Google Scholar]
- 19.Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74(2):357–366. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]


