Abstract
A rare case of a thyroid abscess due to mixed anaerobic flora containing Fusobacterium mortiferum in an immunocompetent patient is described. The patient was successfully treated with immediate surgical intervention and appropriate antimicrobial agents.
CASE REPORT
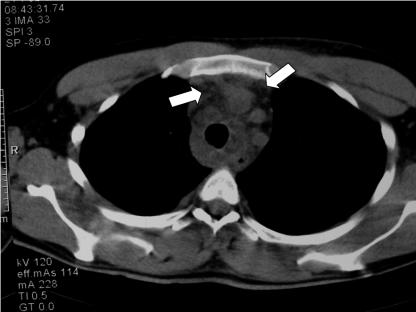
A 27-year-old white male patient was admitted with an enlarging, painful neck mass accompanied by high fever. He reported a viral upper respiratory tract illness 3 weeks prior to admission, which 10 days later was complicated by pain in the area of the thyroid. After extensive investigation that included ultrasound, a computed tomography scan, and nuclear scanning, he was diagnosed with subacute thyroiditis. He was initially treated with prednisone, and his symptoms improved. However, 5 days into treatment, he developed high fever and a painful neck mass, resulting in odynophagia and dysphagia. This did not respond to a course of antibiotics and the patient was admitted. He was acutely ill with a temperature of 38.4°C and tachycardia. Examination of the neck revealed a large tender, warm, and fluctuant mass occupying the region of the left lobe of the thyroid gland and no further extension. The trachea was shifted to the right. Laboratory investigations revealed a leukocyte count of 17,600 with 84% polymorphonuclear cells and a hemoglobin value of 9.5 g/dl. Plain radiographs of the neck and chest in frontal and lateral views showed a homogenous soft-tissue density anterior to the trachea, displacing it to the right. The retropharyngeal space had normal dimensions on radiographs. Computed tomography revealed cystic areas in both lobes of the thyroid gland. The larger was nonhomogeneous and extended into the superior mediastinum without any distortion of the mediastinal structures (Fig. 1). Needle aspiration of this lesion disclosed thick yellow pus. Free triiodothyronine and free thyroxine levels were mildly elevated, and thyroid-stimulating hormone levels were within normal range. The ear, nose, and throat (ENT) service intervened with incision and drainage of the affected area. The patient was treated with intravenous meropenem at a dose of 2 g three times a day and clindamycin at a dose of 900 mg three times a day. In the first few days of his admission, he developed pneumonia of the left lung and pericardial effusion. Cultures of the drained pus yielded Fusobacterium mortiferum, Streptococcus constellatus, and Prevotella melaninogenica. An API 20A kit (bioMérieux, Marcy-l'Etoile, France) was used for the identification of the anaerobic bacteria. F. mortiferum was identified with 99.9% certainty with the unique number 44204200 that corresponded to negative testing for indole, urease, catalase, gelatin liquefaction, and esculin hydrolysis; positive testing for fermentation of glucose, sucrose, salicin, mannose and raffinose; and no fermentation of mannitol, lactose, maltose, xylose, arabinose, glycerol, cellobiose, melezitose, sorbitol, rhamnose, or trehalose. No further molecular or susceptibility testing was used since the patient was clinically improving.
FIG. 1.
Computed tomography image revealing cystic areas in both lobes of the thyroid gland extending in the anterior mediastinum (white arrows).
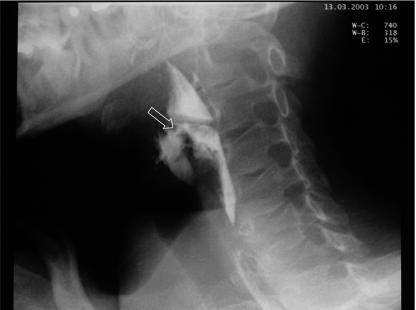
Further work-up did not reveal septic thrombosis of the jugular vein. The patient completed a 4-week course of intravenous antibiotics; he symptomatically improved and was discharged home 30 days after the initial episode. A barium esophagogram disclosed an anterior fistulous tract originating from the left pyriform sinus (Fig. 2), for which further ENT follow-up and surgery were arranged. He did not suffer any relapses and continues to do well.
FIG. 2.
A barium esophagogram disclosing an anterior fistulous tract originating from the left pyriform sinus.
Acute suppurative thyroiditis is a rare condition even in patients with impaired host defenses (5). Progression to thyroid abscess is also rare (12, 13) and carries the inherent dangers of advanced suppuration in the neck. Thyroid abscess and acute suppurative thyroiditis represent only 0.1 to 0.7% of surgically treated thyroid pathologies (14). Acute suppurative thyroiditis especially affects patients with preexisting thyroid gland pathology, and in childhood it is associated with local anatomic defects (13). The pyriform sinus fistula is the route of infection and is the most common underlying abnormality in acute suppurative thyroiditis cases (13). Fistulectomy may be necessary in order to prevent recurrent infections. Acute suppurative thyroiditis now rarely progresses to abscess formation due to the widespread use of antibiotics (11). The left side of the thyroid is more commonly involved than the right in cases with an abscess (16), and the usual treatment includes intervention of the ENT service with incision and drainage of the abscess or partial thyroidectomy, depending upon the presence or absence of underlying thyroid pathologies, together with intravenous antibiotics.
In the rare cases where a thyroid abscess is formed, the offending organisms include gram-positive pathogens such as Staphylococcus aureus and anaerobes of the oropharyngeal area (12, 13). S. constellatus and P. melaninogenica are classic mouth flora microorganisms and could have contributed to the presented infection. F. mortiferum, on the other hand, has rarely been isolated as a cause for an infection in mixed bacterial populations with other anaerobes (2, 10). The closely related Fusobacterium necroforum is implicated in the potentially life-threatening Lemmiere's syndrome (7). Our patient had no history or laboratory evidence of immunodeficiency and was only immunocompromised by virtue of the short antecedent steroid regimen. We were not able to find any other reports of F. mortiferum-associated acute suppurative thyroiditis and thyroid abscess in the literature.
Fusobacteria are obligately anaerobic non-spore-forming, nonmotile, pleomorphic rod-shaped bacilli. Morphological differences between various fusobacterial species exist. For example, F. necrophorum and F. mortiferum have extremely pleomorphic microscopic characteristics, with large cells frequently exhibiting bizarre shapes. On the other hand, Fusobacterium nucleatum is characterized by long, slender, and filamentous needle-shaped cells with tapered ends. Leptotrichia species can be confused with Fusobacterium spp. since they exhibit a similar needle-shaped cell appearance on Gram stain; but the cells are much larger, and colonies are large and gray, sometimes spreading and showing a “brain surface” texture. However, definitive identification of fusobacteria to species level requires additional biochemical testing. Fusobacterium spp. are resistant to vancomycin (5 μg) but susceptible to both colistin (10 μg) and kanamycin (1,000 μg) disk identification tests. F. mortiferum and Fusobacterium varium grow in the presence of bile, as do some strains of F. necrophorum. On the other hand F. nucleatum does not grow in 20% bile, and during biochemical testing, it will not show significant reactions except for positive-indole and negative-nitrate testing. The API 20A (bioMérieux, Marcy-l'Etoile, France) kit used in this case is an established system for identification of anaerobic bacteria (8, 15). Newer molecular techniques such as 16S RNA gene sequencing and 16S-23S rRNA gene spacer region sequencing help in further differentiating closely related fusobacterial species (9). Antimicrobial susceptibility testing was not performed in the present case. Such testing is not usually indicated unless a patient is not responding to therapy or is in critical condition. Fusobacterium spp. are usually susceptible to penicillin, clindamycin (except for F. varium), metronidazole, and chloramphenicol (3, 4). Resistance to macrolides is usual (6). The production of β-lactamase, especially from F. nucleatum and F. necrophorum (1), has led to recommendations to use β-lactamase-resistant antibiotics with anaerobic activity such as intravenous ampicillin-sulbactam, ticarcillin-clavulanate, metronidazole, or clindamycin for treatment of such infections.
In conclusion, we describe a rare case of subacute thyroiditis complicated by the development of acute suppurative thyroiditis and a thyroid abscess due to mixed anaerobic flora containing F. mortiferum. This was successfully treated with immediate surgical intervention and appropriate antimicrobial agents. Physicians should be aware of this rare complication of subacute thyroiditis and its association with mixed anaerobic bacterial populations.
REFERENCES
- 1.Appelbaum, P. C., S. K. Spangler, and M. R. Jacobs. 1990. β-Lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers. Antimicrob. Agents Chemother. 34:1546-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook, I. 1994. Fusobacterial infections in children. J. Infect. 28:155-165. [DOI] [PubMed] [Google Scholar]
- 3.Finegold, S. M. 2000. Anaerobic bacteria: general concepts, p. 2519-2537. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 4.George, W. L., B. D. Kirby, V. L. Sutter, D. M. Citron, and S. M. Finegold. 1981. Gram-negative anaerobic bacilli: their role in infection and patterns of susceptibility to antimicrobial agents. II. Little-known Fusobacterium species and miscellaneous genera. Rev. Infect. Dis. 3:599-626. [DOI] [PubMed] [Google Scholar]
- 5.Imai, C., T. Kakihara, A. Watanabe, Y. Ikarashi, H. Hotta, A. Tanaka, and M. Uchiyama. 2002. Acute suppurative thyroiditis as a rare complication of aggressive chemotherapy in children with acute myelogenous leukaemia. Pediatr. Hematol. Oncol. 19:247-253. [DOI] [PubMed] [Google Scholar]
- 6.Jousimies-Somer, H. R., P. H. Summanen, and S. M. Finegold. 1999. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and other anaerobic gram-negative rods and cocci, p. 690-711. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 7.Kara, E., A. Sakarya, C. Keles, H. Borand, G. Pekindil, and C. Goktan. 2004. Case of Lemierre's syndrome presenting with thyroid abscess. Eur. J. Clin. Microbiol. Infect. Dis. 23:570-572. [DOI] [PubMed] [Google Scholar]
- 8.Karachewski N. O., E. L. Busch, and C. L. Wells. 1985. Comparison of PRAS II, RapID ANA, and API 20A systems for identification of anaerobic bacteria. J. Clin. Microbiol. 21:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitron, D. M. 2002. Update on the taxonomy and clinical aspects of the genus Fusobacterium. Clin. Inf. Dis. 35(Suppl. 1):S22-S27. [DOI] [PubMed] [Google Scholar]
- 10.Matsukawa, Y., N. Kitamura, M. Kaneko, et al. 2003. Multibacterial sepsis in an alcohol abuser with hepatic cirrhosis. Intern. Med. 42:208-210. [DOI] [PubMed] [Google Scholar]
- 11.Menegaux, F., G. Biro, C. Schatz, and J. P. Chigot. 1991. Thyroid abcess. Apropos of 5 cases. Ann. Med. Interne (Paris). 142:99-102. [PubMed] [Google Scholar]
- 12.Minhas, S. S., J. C. Watkinson, and J. Franklyn. 2001. Fourth branchial arch fistula and suppurative thyroiditis: a life-threatening infection. J. Laryngol. Otol. 115:1029-1031. [DOI] [PubMed] [Google Scholar]
- 13.Rohondia, O. S., R. S. Koti, P. P. Majumdar. T. Vijaykumar, and R. D. Bapat. 1995. Thyroid abscess. J. Postgrad. Med. 41:52-54. [PubMed] [Google Scholar]
- 14.Schneider, U., R. Birnbacher, S. Schick, W. Ponhold, and E. Schober. 1995. Recurrent suppurative thyroiditis due to pyriform sinus fistula: Eur. J. Pediatr. 154:640-642. [DOI] [PubMed] [Google Scholar]
- 15.Summanen, P., and H. Jousimies-Somer. 1988. Comparative evaluation of RapID ANA and API 20A for identification of anaerobic bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 7:771-775. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita, H., T. Noguchi, and M. Takahashi. 1995. Reccurent cervical abscess due to piriform sinus fistula. J. Larygol. Otol. 109:886-888. [DOI] [PubMed] [Google Scholar]