Abstract
Bacillus species causing food-borne disease produce multiple toxins eliciting gastroenteritis. Toxin assays with mammalian cell cultures are reliable but may take 24 to 72 h to complete and also lack sensitivity. Here, a sensitive and rapid assay was developed using a murine hybridoma Ped-2E9 cell model. Bacillus culture supernatants containing toxins were added to a Ped-2E9 cell line and analyzed for cytotoxicity with an alkaline phosphatase release assay. Most Bacillus cereus strains produced positive cytotoxicity results within 1 h, and data were comparable to those obtained with the standard Chinese hamster ovary (CHO)-based cytotoxicity assay, which took about 72 h to complete. Moreover, the Ped-2E9 cell assay had 25- to 58-fold-higher sensitivity than the CHO assay. Enterotoxin-producing Bacillus thuringiensis also gave positive results with Ped-2E9 cells, while several other Bacillus species were negative. Eight isolates from food suspected of Bacillus contamination were also tested, and only one strain, which was later confirmed as B. cereus, gave a positive result. In comparison with two commercial diarrheal toxin assay kits (BDE-VIA and BCET-RPLA), the Ped-2E9 assay performed more reliably. Toxin fractions of >30 kDa showed the highest degree of cytotoxicity effects, and heat treatment significantly reduced the toxin activity, indicating the involvement of a heat-labile high-molecular-weight component in Ped-2E9 cytotoxicity. PCR results, in most cases, were in agreement with the cytotoxic potential of each strain. Ribotyping was used to identify cultures and indicated differences for several previously reported isolates. This Ped-2E9 cell assay could be used as a rapid (∼1-h) alternative to current methods for sensitive detection of enterotoxins from Bacillus species.
Bacillus cereus is one of the major food-borne pathogens and is a spore-forming aerobic bacterium sometimes causing severe vomiting in 1 to 5 h and/or diarrhea within 8 to 16 h following ingestion of contaminated food. The difference in onset is due to the type of toxin the bacteria produce. In the former case, ingestion of the preformed emetic toxin manifests immediate symptoms, while for diarrhea, toxicoinfection results in enterotoxin production, thus requiring longer to induce gastrointestinal disorder (19, 50). While the emetic syndrome is generally associated with cereal foods including rice and pasta, diarrheal toxins are found in many foods, including milk, vegetables, and meat products (19, 31). B. cereus can grow over wide temperature and pH ranges, at high salt concentrations (31), and under aerobic or anaerobic conditions (19) and can also survive harsh food-processing treatments through spore formation; therefore, B. cereus is a serious concern in foods (36). Several other Bacillus species, such as B. thuringiensis, B. subtilis, B. licheniformis, B. pumilus, B. mojavensis, B. fusiformis, and B. sphaericus, are known to produce enterotoxin and could be food safety concerns (5, 16, 17, 30).
In addition to emetic or diarrheal enterotoxins, B. cereus also produces hemolysins, phospholipases, and other enterotoxins (30) (summarized in Table 1). The hemolysin HBL complex, composed of a B component and two L components (L1 and L2), is thought to be the primary virulence factor for B. cereus because it is hemolytic, cytotoxic, dermonecrotic, and increases vascular permeability (6, 7, 14, 33). While all three subunits are required for maximal activity, the L1 subunit alone is cytotoxic (7). Another three-component enterotoxin produced by B. cereus is the nonhemolytic enterotoxin (NHE), composed of the NHE-A, NHE-B, and NHE-C proteins (20, 33). As with the HBL complex, all three components must be present for full activity (34). Other enterotoxins produced by B. cereus include enterotoxin T (BcET), enterotoxin FM (EntFM), and cytotoxin K (CytK). BcET has either an unknown type of enterotoxic action or none at all (1, 12) but is generally found in more than half of outbreak-associated strains. EntFM, like BcET, has not been implicated as the enterotoxin responsible for a food poisoning outbreak, but EntFM is present in most outbreak-associated strains and is actually the most prevalent enterotoxin gene for all B. cereus strains (25, 52). Unlike EntFM and BcET, CytK has been implicated in B. cereus-related deaths due to necrotic enteritis (32).
TABLE 1.
Toxins produced by Bacillus cereus
| Toxins | Mol mass (kDa) | Activity | Reference(s) |
|---|---|---|---|
| Emetic toxin | ∼1.2 | Emesis (vomiting) | 30, 51 |
| Diarrheal toxin | 38-43 | Diarrhea | 30, 50 |
| Hemolysin (HBL) | Hemolytic, enterotoxic, | 6, 7, 14, 33 | |
| B component | 37.8 | dermonecrotic | |
| L component (L1) | 38.5 | ||
| L component (L2) | 43.5 | ||
| NHE | Enterotoxic | 20, 33, 34, 40 | |
| NHE-A | 41 | ||
| NHE-B | 39.8 | ||
| NHE-C | 36.5 | ||
| BcET | 41 | Unknown | 1, 12 |
| EntFM | 45 | Enterotoxic, induces vascular permeability | 25, 52 |
| CytK | 34 | Necrotic enteritis | 32 |
Traditional plating and biochemical assays are time-consuming and do not indicate Bacillus toxin production capabilities (31). In order to assess biological activity, a number of eukaryotic-cell-based assays have been employed (5, 11, 27). The emetic toxin is traditionally assessed using a HEp-2 tissue culture assay by observing vacuole formation (3, 26, 51) or colorimetrically, by using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) metabolic staining assay (15, 47). Another bioassay, based on the loss of motility of boar spermatozoa, has also been developed (4). Recently, an assay has been developed for emetic toxin based on its mitochondrial respiratory uncoupling activity on rat liver mitochondria (28). A number of cell lines have been employed to detect diarrheal enterotoxin activity, including the McCoy cell line (16, 27), where cytotoxicity was determined by microscopic observation. In the Vero cell line, enterotoxin activity was evaluated by measuring protein synthesis inhibition and decreased cell proliferation (14, 17, 35, 42, 56). Similar analysis was also done with the Caco-2 cell line (24, 47). A CHO-based assay was developed for detection of both the emetic and the diarrheal toxin through cytotoxic staining and measurement of metabolic activity (5, 25, 40). The drawback of these cell-based assays is the length of time required to complete the assays, 24 h to 72 h (5, 38, 54).
The PCR is generally used as a tool for toxin and strain characterization (18, 22, 25, 29, 41-43). PCR-based detection gives a high level of accuracy but does not reveal pathogenic potentials of strains, since phenotypic expression of genes is not always warranted. Two commercially available kits, the Bacillus Diarrheal Enterotoxin Visual Immunoassay (BDE-VIA;Tecra) and Bacillus cereus Enterotoxin-Reversed Passive Latex Agglutination (BCET-RPLA; Oxoid) kits, are used by the industry or clinicians (48). The drawback of these tests is that the BDE-VIA kit detects only the 41-kDa subunit of NHE, while the BCET-RPLA kit detects only the L2 subunit of HBL. Since it is known that different strains of B. cereus may produce both, only one, or perhaps neither of these subunits, the tests could easily produce false negatives (16) in a complex medium such asfood.
Previously, a cytotoxicity assay based on a B-cell hybridoma, Ped-2E9, was developed that measures Listeria monocytogenes-induced cell damage in 1 to 2 h either by trypan blue staining or by an alkaline phosphatase (AP) release assay (8-10, 37, 53, 57). Recently a B-cell line-based biosensor assay was developed to sensitively detect Bacillus anthracis, Yersinia pestis, and Escherichia coli O157:H7 (45). In this study, we developed a Ped-2E9 cell-based assay as an alternative to the current B. cereus enterotoxin assays. Quantitative cytotoxic assessment was made by an alkaline phosphatase release assay, and results were compared to those of the traditional CHO assay and the commercial BDE-VIA and BCET-RPLA kits. Toxin gene profiles were determined by PCR, and culture identifications were done by ribotyping.
MATERIALS AND METHODS
Bacterial cultures and ribotyping.
The Bacillus cultures used in this study are listed in Table 2. Bacillus cultures were also isolated from nine ready-to-serve pasta and rice dishes procured from local restaurants (Table 2). Within 1 h of arrival, 11 g of each sample was added to 99 ml of 0.85% saline, dilutions were plated onto mannitol-egg yolk-polymyxin agar (Difco Laboratories, Sparks, MD) and incubated at 37°C for 24 h, and suspected Bacillus colonies were collected as natural isolates (44) for use with the cytotoxicity assay in a blind format.
TABLE 2.
Ped-2E9 cell-based cytotoxicity analysis and characterization of Bacillus species
| Culture (mo-day-yr) or food isolate | Riboprinter identification (Dupont ID) | Sourcea | Hemolysinb | Lecithinaseb | Cytotoxicity (%)c | Presence of the following gened by PCR analysis:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| bceT | cytK | hblA | nheA | nheB | nheC | ||||||
| Cultures | |||||||||||
| B. cereus PU1 | B. cereus DUP-6001 | Our collection | + | + | 20 ± 2.1 | − | − | + | + | − | + |
| B. cereus NRL 569 | B. cereus DUP-13212 | A. Aronson | + | + | 67 ± 5.3 | + | − | + | + | + | + |
| B. cereus T | B. thuringiensis DUP-6040 | A. Aronson | − | − | 13 ± 1.1 | + | − | + | + | + | + |
| B. cereus ATCC 3432 | B. cereus DUP-6078 | B. Applegate | + | + | 55 ± 3.3 | − | − | + | + | − | + |
| B. cereus CA1 | B. cereus DUP-6092 | B. Applegate | + | + | 0.2 ± 1.3 | + | − | − | + | − | − |
| B. cereus CA6 | NT | B. Applegate | + | + | 36 ± 1.3 | + | − | + | + | + | + |
| B. cereus ATCC 33018 | Bacillus sp., no matching | A. Wong | + | + | 43 ± 2.0 | − | − | − | + | + | + |
| B. cereus F837/76 | B. cereus DUP-6079 | A. Wong | + | + | 17 ± 1.3 | − | − | + | − | − | − |
| B. cereus A926 | B. cereus DUP-6082 | A. Wong | + | + | 88 ± 2.1 | + | + | + | + | + | + |
| B. cereus F4810 | B. cereus DUP-6012 | A. Wong | + | + | 60 ± 0.9 | − | − | − | + | + | + |
| B. cereus AS4-12 | B. cereus DUP-12561 | J. Handlesman | + | + | 78 ± 2.2 | − | − | + | + | + | + |
| B. cereus MS1-9 | B. cereus DUP-12561 | J. Handlesman | + | + | 80 ± 2.0 | − | − | + | + | + | + |
| B. cereus HS23-11 | B. cereus DUP-12561 | J. Handlesman | + | + | 72 ± 2.0 | − | − | + | + | + | + |
| B. cereus UW85 | B. cereus DUP-11561 | J. Handlesman | + | + | 69 ± 1.4 | − | − | + | + | + | + |
| B. cereus 1230-88 | B. cereus DUP-12561 | J. McKillip | + | + | 82 ± 3.0 | NT | NT | NT | NT | NT | NT |
| B. cereus ATCC 14579 | B. cereus DUP-6082 | J. McKillip | − | − | 64 ± 3.0 | + | + | + | + | + | + |
| B. subtilis | B. subtilis DUP-12546 | Our collection | − | + | 1 ± 1.2 | − | − | − | − | − | − |
| B. subtilis PY79 | B. subtilis DUP-12546 | G. Siragusa | − | − | − | NT | NT | NT | NT | NT | NT |
| B. subtilis ATCC 6633 | B. subtilis DUP-12551 | J. McKillip | − | NT | NT | NT | NT | NT | NT | ||
| B. megaterium MA | B. thuringiensis DUP-6044 | A. Aronson | + | + | 53 ± 1.2 | + | − | + | + | + | + |
| B. megaterium DSM 319 | B. subtilis DUP-9501 | J. McKillip | − | − | − | NT | NT | NT | NT | NT | NT |
| B. megaterium | B. megaterium | G. Siragusa | − | − | − | + | − | − | − | − | − |
| ATCC 9885 | DUP-16973 | ||||||||||
| B. thuringiensis H073 | B. thuringiensis DUP-6034 | A. Aronson | − | − | 37 ± 1.3 | + | − | + | + | + | + |
| B. thuringiensis subsp. | B. cereus DUP-13204 | J. McKillip | ± | + | 74 ± 1.1 | NT | NT | NT | NT | NT | NT |
| kurstaki #4 | |||||||||||
| B. thuringiensis subsp. | B. oleronius DUP-17021 | J. McKillip | − | − | − | NT | NT | NT | NT | NT | NT |
| kurstaki #7 | |||||||||||
| B. thuringiensis subsp. | B. cereus DUP-6082 | J. McKillip | + | + | 66 ± 2.8 | NT | NT | NT | NT | NT | NT |
| berliner | |||||||||||
| B. lentimorbis #11 | B. thuringiensis DUP-6040 | J. McKillip | + | + | 79 ± 3.7 | NT | NT | NT | NT | NT | NT |
| B. licheniformis #15 | B. pumilus DUP-6073 | J. McKillip | − | − | − | NT | NT | NT | NT | NT | NT |
| B. polymyxa B719 W | Paenibacillus polymyxa | K. Hayes | − | − | − | NT | NT | NT | NT | NT | NT |
| (3-18-76) | DUP-11063 | ||||||||||
| B. polymyxa B719 X | Paenibacillus polymyxa | K. Hayes | − | − | − | − | − | − | − | − | − |
| (1-20-65) | DUP-11063 | ||||||||||
| B. polymyxa B719 V | Paenibacillus polymyxa | K. Hayes | − | − | − | NT | NT | NT | NT | NT | NT |
| (8-20-69) | DUP-11063 | ||||||||||
| Food isolates | |||||||||||
| Baclo 2b | B. subtilis DUP-12544 | Lo mein (veg) | − | + | − | NT | NT | NT | NT | NT | NT |
| Baclo 2d | B. subtilis DUP-12551 | Lo mein (veg) | − | + | − | NT | NT | NT | NT | NT | NT |
| Baclo 2e | B. subtilis DUP-6098 | Lo mein (veg) | − | − | − | NT | NT | NT | NT | NT | NT |
| Baclo 3a | B. cereus DUP-6002 | Lo mein | + | − | 15 ± 6.5 | − | − | − | + | + | + |
| BacSR4a | B. subtilis DUP-6098 | Steamed rice | − | − | − | NT | NT | NT | NT | NT | NT |
| BacSpR8a | Bacillus sp., no matching | Spinach rice | − | − | − | NT | NT | NT | NT | NT | NT |
| BacSpR8b | B. subtilis DUP-12546 | Spinach rice | − | − | − | NT | NT | NT | NT | NT | NT |
| BacSpR8c | B. subtilis DUP-12544 | Spinach rice | − | − | − | NT | NT | NT | NT | NT | NT |
Sources of cultures are as follows: J. Handlesman, Department of Plant Pathology, University of Wisconsin; A. Wong, Food Research Institute, University of Wisconsin; B. Applegate, Department of Food Science, Purdue University; A. Aronson, Department of Biology, Purdue University; J. McKillip, Department of Biology, Ball State University; G. Siragusa, USDA-ARS, Russell Research Center, Athens, GA; K. Hayes, Department of Food Science, Purdue University. Veg, vegetable.
Hemolysin activity was determined on 5% sheep blood agar plates, and lecithinase activity was determined on plate count agar containing egg yolk. +, strongly positive; ±, weakly positive; −, negative.
Cytotoxicity values are averages from at least two experiments analyzed in triplicate. Values above 1% were considered positive based on the values produced by a negative-control strain, B. subtilis. −, no cytotoxicity.
+, presence of PCR product; −, absence of PCR product; NT, not tested.
All cultures were grown in Luria-Bertani (LB) broth for 18 h at 37°C and streaked onto LB agar (LB containing 1.5% agar) for isolation of single colonies. Isolated colonies were used for cytotoxicity and PCR assays and were ribotyped (21) using an automated Riboprinter (Qualicon, Inc.) with the EcoRI restriction enzyme. Ribopatterns were compared with the RiboPrinter database for culture identification.
Hemolysin and lecithinase activity.
Cultures were tested for hemolytic and lecithinase activity by spot inoculation onto 5% sheep blood agar plates (Difco) and 10% egg yolk agar plates (44), respectively.
Toxin preparation.
Crude toxins were prepared from bacterial-cell-free culture supernatants. Test strains were grown in LB for 18 h at 37°C in a shaker incubator (New Brunswick Scientific Co.) at 140 rpm and centrifuged (10,000 × g for 10 min at 4°C). The supernatants were passed through a 0.45-μM-pore-size filter (Nalgene), designated crude toxin preparation, and either used for cytotoxicity assays immediately or stored at refrigeration temperature (4°C) for no more than 48 h. The protein concentration of the crude toxin preparation was determined by using a Bio-Rad protein assay kit.
To determine the effect of heat, cell-free culture supernatants (toxin preparations) from B. cereus MS1-9, ATCC 14579, A926, CA-1, HS23-11, and F4810 were held at 58°C for 40 min (13) or at 70°C for 15 min, and cytotoxicity was determined on Ped-2E9 cells as described below.
Cell cytotoxicity assays.
The Ped-2E9-based cytotoxicity assay was modified slightly from the previous methods (8, 9). Ped-2E9 murine hybridoma lymphocytes were maintained in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% (vol/vol) fetal bovine serum (Atlanta Biologicals, Norcross, GA). Cells were cultured at 37°C under a humid atmosphere containing 7% CO2 and were subcultured every 2 to 4 days. Ped-2E9 cells were harvested, counted by trypan blue staining (0.4%), centrifuged (300 × g for 10 min), and resuspended to 1 × 106 to 2 × 106 cells/ml in serum-free medium (Sigma). The assay was performed in sterile 1.5-ml Eppendorf tubes with 1 ml Ped-2E9 cell suspension and 100 μl of toxin preparation. Negative controls of LB (100 μl) and positive controls of 1% Triton X (100 μl) in 20 mM phosphate-buffered saline, pH 7.0, were included. All tubes were incubated at 37°C for 1 h, and cytotoxicity for Ped-2E9 cells was determined by the AP release assay (9).
Cytotoxicity profiles of selected Bacillus cultures were also compared with the standard CHO cell-based MTT metabolic staining assay (5, 40) using an MTT assay kit (Roche Applied Science, Indianapolis, IN). Briefly, CHO (CHO-K1; ATCC, Manassas, VA) cell monolayers in T-25 flasks were trypsinized, and viable-cell concentrations were determined by trypan blue staining. About 4 × 104 viable cells were added to each well of a 96-well tissue culture plate. After an overnight incubation at 37°C in 7% CO2 under humidified conditions, the medium was replaced with 100 μl of the toxin preparation/well in triplicate, and the cells were incubated for an additional 24 h. Ten microliters of MTT labeling reagent (final concentration, 0.5 mg/ml) was added per well and incubated (37°C, 7% CO2) for an additional 4 h, followed by the addition of 100 μl/well of solubilization buffer. The plates were again incubated overnight or until the purple formazan salts were dissolved, and the absorbance was measured in a microplate reader (Bio-Rad) at 595/655 nm.
To compare the sensitivities of the Ped-2E9- and CHO-based assays, successive twofold dilutions of the crude toxin preparations from B. cereus MS1-9 and ATCC 14579 were tested with both cell lines by using AP release from Ped-2E9 cells (9) and an MTT assay with CHO cells (5). The protein concentration of each toxin preparation was determined by using a protein assay kit from Bio-Rad.
Determination of cytotoxic action of fractionated toxin preparations.
To identify the toxin components that are responsible for cell cytotoxicity, a crude toxin preparation from B. cereus MS1-9 was used. The crude toxin preparation was fractionated based on the molecular size by using molecular weight cutoff membranes in protein concentrators (Millipore-Amicon). Membranes with molecular cutoff values of 10, 30, and 50 kDa were used to yield fractions of 10 to 30 kDa (fraction a), 30 to 50 kDa (fraction b), and >50 kDa (fraction c). The protein concentration of each fraction was estimated by using the Bio-Rad protein assay kit and adjusted to a uniform concentration of about 40 μg/ml. All toxin fractions were tested for cytotoxicity on Ped-2E9 cells as described above.
PCR.
Individual cultures of Bacillus species were resuspended in nuclease-free water, and the genomic DNA was prepared by boiling for 10 min. Samples were centrifuged (930 × g, 10 min), and the supernatants were decanted into sterile Eppendorf tubes. One microliter of each supernatant was used as the template for PCR amplification (see Table 5). PCR products were amplified using Ready-To-Go PCR beads (Amersham Biosciences, Piscataway, NJ), and all reactions were carried out in a PTC-100 Programmable Thermal K-6 Controller (MJ Research, Inc., Watertown, MA). The hblA, bceT, and cytK genes were amplified individually, while the nheA, nheB, and nheC genes were multiplexed according to the PCR conditions given in Table 5. Amplified PCR products were analyzed by agarose (1 to 1.2%) gel electrophoresis and visualized by ethidium bromide staining.
TABLE 5.
Nucleotide sequences of specific primers and product sizes for toxin genes of B. cereus
| Toxin gene | Primer sequence (5′-3′) | PCR conditionsa | Product size (bp) | Reference |
|---|---|---|---|---|
| hblA | GCTAATCTAGTTTCACCTGTAGCAAC | 35 cycles of 94°C for 20 s, 58°C for 40 s, and 72°C for 40 s | 874 | 25 |
| AATCATGCCACTGCGTGGACATATAA | ||||
| bceT | TTACATTACCAGGACGTGCTT | 30 cycles of 94°C for 15 s, 55°C for 45 s, and 72°C for 2 min | 428 | 1 |
| TGTTTGTGATTGTAATTCAGG | ||||
| cytK | AACAGATATCGGTCAAAATGC | 30 cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min | 623 | 32 |
| CGTGCATCTGTTTCATGAGG | ||||
| nheA | TACGCTAAGGAGGGGCA | Multiplex reaction | 499 | 20 |
| GTTTTTATTGCTTCATCGGCT | ||||
| nheB | CTATCAGCACTTATGGCAG | 30 cycles of 94°C for 15 s, 55°C for 45 s, and 72°C for 2 min | 769 | |
| ACTCCTAGCGGTGTTCC | ||||
| nheC | CGGTAGTGATTGCTGGG | 581 | ||
| CAGCATTCGTACTTGCCAA |
Preceded by initial denaturation at 94°C for 5 min and followed by a final extension at 72°C for 7 min.
Commercial assay kits.
Two commercial immunological kits, BCET-RPLA (Oxoid) and BDE-VIA (Tecra), were used with cell-free culture supernatants to assess enterotoxin production according to the manufacturer's directions. The BCET-RPLA is based on agglutination, which detects antibody reaction to the L2 subunit of HBL. The BDE-VIA is a visual assay based on the enzyme-linked immunosorbent assay that detects the 41-kDa subunit of NHE.
Statistical analysis.
Data were analyzed using SAS software 8.02 (Cary, NC) for analysis of variance and residual plots. The differences in mean values were determined by Tukey's test; a P value of <0.05 was considered significant.
RESULTS
Characterization of Bacillus cultures.
Thirty-one previously isolated cultures obtained from various researchers were used in this study; these included 16 B. cereus, 4 B. thuringiensis, 3 isolates each of B. subtilis, B. megaterium, and B. polymyxa, and 1 isolate each of B. lentimorbis and B. licheniformis (Table 2). All B. cereus strains were identified as B. cereus by ribotyping except for B. cereus T, CA6, and ATCC 33018. T was identified as B. thuringiensis, CA6 was not typed, and ATCC 33018 did not give any matching with any known Bacillus ribopatterns in the RiboPrinter database. All B. cereus strains except ATCC 14579 were hemolytic and showed positive lecithinase activity. All B. subtilis and B. polymyxa strains were correctly identified. Of three B. megaterium strains tested, only one was identified as B. megaterium, while two others were identified as B. thuringiensis and B. subtilis, respectively. Of four B. thuringiensis strains tested, only one was identified as B. thuringiensis, while two were identified as B. cereus and one as B. oleronius. B. lentimorbis and B. licheniformis were identified as B. thuringiensis and B. pumilus, respectively. Besides B. cereus, only two of four B. thuringiensis strains were hemolytic and lecithinase positive. None of the other Bacillus isolates was hemolytic or lecithinase positive except for one strain of B. subtilis, which was lecithinase positive (Table 2).
A total of eight suspected Bacillus cultures were isolated from four of nine prepared, ready-to-serve rice and pasta dishes (Table 2). Six of those isolates were identified by ribotyping as B. subtilis, one (Baclo3a) was identified as B. cereus, and the remaining strain, BacSpR8a, was unknown. Although strain BacSpR8a was identified as a Bacillus sp. by ribotyping, no strain designation was assigned, because the fingerprinting similarity values were well below the acceptable range (<90%). Of the six B. subtilis strains, only two were positive for lecithinase activity and none were hemolytic. The B. cereus strain Baclo3a was hemolytic but lecithinase negative.
Cytotoxicity assay.
In all cytotoxicity assays, a strain was considered positive when its cytotoxicity value was above the value produced by a negative-control B. subtilis strain for that experiment. During initial screening (Table 3), cytotoxic effects of eight B. cereus isolates and one B. subtilis isolate were compared with Ped-2E9 and CHO cells. The B. subtilis strain in this set showed 16.4% ± 19.8% cytotoxicity with CHO cells and only 1% ± 1.2% with Ped-2E9 cells. Residual plots were generated with cytotoxicity values of all Bacillus isolates with CHO and Ped-2E9 cells (Table 3) separately to eliminate false negatives among test strains; this analysis allowed the cutoff to be set at 21% for the CHO assay and at 4% for the Ped-2E9 assay. All B. cereus strains from this set appeared to be cytotoxic to both cell lines, and the difference between the positive strains and the B. subtilis negative control in both the CHO and Ped-2E9 assays appeared to be significant at a P value of <0.05 (Table 3). These data indicated that the Ped-2E9 cell assay could potentially be used as a substitute for the CHO-based assay to test for B. cereus toxins.
TABLE 3.
Comparison of the Ped-2E9-based cytotoxicity assay with other cell culture-based and commercial assay kits
| Strain | Result by:
|
|||
|---|---|---|---|---|
| Commercial assaya
|
Tissue culture assay
|
|||
| BDE-VIA | BCET-RPLA | CHO diarrheagenic assayb,c | Ped-2E9 cytotoxicityb,d | |
| B. cereus | ||||
| F837/76 | +++ | +++ | 94.2 ± 5.57A | 17 ± 1.3F |
| ATCC 33018 | + | − | 28.5 ± 10.34E | 43 ± 2.0E |
| A926 | +/++ | +++ | 62.4 ± 4.27C | 88 ± 2.1A |
| F4810 | +++ | − | 24.9 ± 11.3E | 60 ± 0.9D |
| AS4-12 | + | +++ | 43.8 ± 12.69D | 78 ± 2.2B |
| MS1-9 | + | +++ | 41.4 ± 12.87D | 80 ± 1.9B |
| HS23-11 | + | +++ | 82.0 ± 8.18B | 72 ± 2.0C |
| UW85 | + | +++ | 107.6 ± 0.95A | 69 ± 1.4C |
| B. subtilis | − | − | 16.4 ± 19.8F | 1.0 ± 1.2G |
The BDE-VIA is NHE specific, while the BCET-RPLA is HBL specific. Results were scored visually as positive (+, ++, or +++) or negative (−) according to the manufacturers' directions.
Cytotoxicity is expressed as a percentage ± standard deviation. Data are averages from two experiments analyzed in duplicate. Values in the same column that are followed by different superscript letters (A through G) are significantly different (P < 0.05).
Cytotoxicity was determined using a 1:2 dilution of the toxin, and the assay needed at least 72 h to complete. Cytotoxicity values above 16.4%, the level produced by a negative-control strain (B. subtilis), were considered positive for diarrheagenic activity with the CHO cell-based assay.
Positive samples resulted in >1% cytotoxicity. Cytotoxicity values were obtained after a 1-h incubation.
Further testing of additional previously isolated Bacillus cultures with Ped-2E9 cells revealed that only B. cereus and B. thuringiensis strains were positive with this cell line, while other species were negative. Our ribotyping results differed in several cases from previous identifications (Table 2). Taking those into account, in our final estimate, of 17 B. cereus strains, only 1 (CA1) was negative while values for the remaining 16 ranged from 27 to 88%. All four B. thuringiensis strains were positive, with cytotoxicity values of 13 to 79% (Table 2). Heat treatment (58°C or 70°C) of the toxin preparations from several B. cereus strains resulted in significant loss (P < 0.05) of activity, suggesting that the Ped-2E9-specific component(s) is heat sensitive (Table 4).
TABLE 4.
Cytotoxicity analysis of heat-treated toxin preparations from Bacillus cereus
| Bacillus cereus toxin preparation (treatment) | % Cytotoxicity (avg ± SD)a |
|---|---|
| F 4810b (none) | 66.56 ± 0.11A |
| F 4810 (58°C, 40 min) | 0.75 ± 0.01D |
| HS23-11 (none) | 45.59 ± 0.15B |
| HS23-11 (58°C, 40 min) | 0.94 ± 0.02D |
| CA1c (none) | 4.13 ± 0.04D |
| CA1 (58°C, 40 min) | 2.46 ± 0.07D |
| A926 (none) | 51.56 ± 2.35B |
| A926 (58°C, 40 min) | 8.20 ± 1.02C |
| A926 (70°C, 15 min) | 2.48 ± 0.05D |
| ATCC 14579 (none) | 63.89 ± 3.01A |
| ATCC 14579 (58°C, 40 min) | 11.04 ± 5.58C |
| ATTC 14579 (70°C, 15 min) | 5.06 ± 0.04D |
Data are averages from two experiments analyzed in duplicate. Values followed by different superscript letters (A through D) are significantly different (P< 0.05). In this experiment, the cytotoxicity value for the negative-control strain (B. subtilis) was about 2.24%.
Emetic-toxin-producing strain.
Considered a noncytotoxic strain (see Table 2).
The ability of this Ped-2E9-based assay to detect cytotoxicity of nascent food isolates was investigated. Eight suspected Bacillus cultures obtained from four of nine food samples were tested with Ped-2E9 cells without any prior knowledge of their “species” identity. Only one strain (Baclo3a) gave positive cytotoxicity (15%); the remaining strains did not show any cytotoxicity (Table 2). Upon ribotyping, the cytotoxic strain (Baclo3a) was identified as B. cereus DUP-6002 and the remainder of the isolates were identified as B. subtilis or a Bacillus sp. Although a small number of natural isolates were tested, this Ped-2E9 assay accurately detected the cytotoxic potential of a single B. cereus strain.
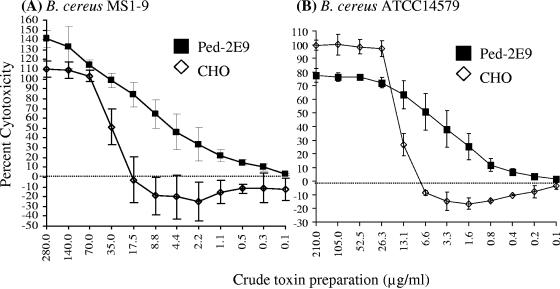
The sensitivities of Ped-2E9 cells to toxins of two diarrheagenic strains of B. cereus, MS1-9 and ATCC 14579, were compared with the MTT-based CHO assay (Fig. 1A and B). When B. cereus MS1-9 toxin was used with Ped-2E9 cells, 50% cytotoxicity was observed at a toxin concentration of 4.4 μg/ml, whereas 35 μg/ml was needed to show 50% cytotoxicity (P < 0.05) in the CHO cell assay. Cytotoxicity titration end points revealed that a minimum toxin concentration of 0.3 μg/ml caused positive cytotoxicity in Ped-2E9 cells while a concentration of >17.5 μg/ml was required to show positive cytotoxicity in CHO cells, indicating that the Ped-2E9 assay is about 58-fold more sensitive than the CHO assay (Fig. 1A). A similar pattern of cytotoxicity was observed for another B. cereus strain, ATCC 14579 (Fig. 1B), where 50% cytotoxicity in Ped-2E9 cells was observed at a protein concentration of about 6.6 μg/ml, whereas >13 μg/ml of toxin was needed to show 50% cytotoxicity in CHO cells. In this case, a minimum toxin concentration of 0.4 μg/ml caused positive cytotoxicity in Ped-2E9 cells, while a concentration of ∼10 μg/ml was required to show positive cytotoxicity in CHO cells, indicating that the Ped-2E9 assay is 25-fold more sensitive than the CHO assay (Fig. 1B). Cytotoxicity titration curves for Ped-2E9 cells with toxin preparations from both B. cereus strains revealed a gradual decline in cytotoxicity values with decreasing concentrations of toxins (0.3 to 0.4 μg/ml), whereas examination of the same curves for CHO cells revealed a sudden drop in cytotoxicity values, approaching zero percent, at toxin concentrations between 17.5 and 6.6 μg/ml. In this experiment, the cytotoxicity titration end point was established in comparison with a negative-control (B. subtilis) strain that gave a cytotoxicity value of ∼1%. Taken together, these data indicate that the Ped-2E9 assay is 25- to 58-fold more sensitive than the CHO assay based on the lowest concentrations of toxins that gave positive cytotoxicity values on both cell lines.
FIG. 1.
Determination of sensitivities of the Ped-2E9-based assay and the CHO-based assay with toxin preparations from Bacillus cereus MS1-9 and ATCC 14579. Protein concentrations of each toxin preparation were determined, and preparations were serially diluted (twofold dilutions) in LB broth and tested on the CHO and Ped-2E9 cells. All dilutions were tested with Ped-2E9 at 2 × 106 hybridoma cells/ml, and cytotoxicity values were determined by the AP release assay at 1 h. All dilutions were also tested on confluent CHO cell monolayers in a 96-well plate, and cytotoxicity values were determined by the MTT assay after 72 h. Data are averages for three experiments analyzed in duplicate. Negative values indicate higher CHO viability in the diluted toxin samples than in the negative control.
Identification of toxin fractions with cytotoxic effects on Ped-2E9 cells.
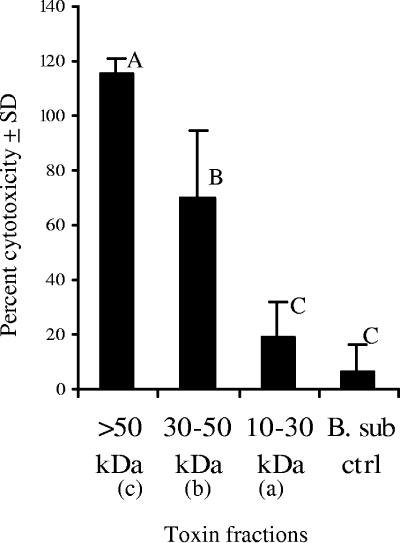
The total protein concentrations for B. cereus MS1-9 crude toxin fractions a, b, and c were adjusted to ∼40 μg/ml and were analyzed by the cytotoxicity assay with Ped-2E9 cells. Fraction c (>50 kDa) showed the maximum cytotoxicity (>100%) in the Ped2E9 assay, followed by fraction b (30 to 50 kDa) with 70% activity, levels that were significantly different (P < 0.05) from that of fraction a (10 to 30 kDa), with 19.2% activity, or the toxin preparation from the negative-control B. subtilis strain, with 6.5% activity (Fig. 2). This study demonstrated that the majority of cytotoxicity effects are associated with high-molecular-weight toxin components (>30 kDa).
FIG. 2.
Cytotoxicity analyses of toxin fractions of B. cereus MS1-9. Toxin fractions were obtained after separation by using specific-molecular-weight cutoff membranes (see the text for details), and the protein concentration for each fraction was adjusted to ∼40 μg/ml. Fractions were designated a (10 to 30 kDa), b (30 to 50 kDa), c (>50 kDa), and B. subtilis toxin preparation (B. sub ctrl) and were tested with 2 × 106 hybridoma cells/ml. Results are averages for three experiments. SD, standard deviation. Bars marked with different letters (A, B, C) are significantly different (P < 0.05).
PCR analysis of toxin genes.
PCR was performed to screen various Bacillus isolates for the target genes listed in Table 5. The hblA gene, coding for hemolysin BL, was found in all strains except for B. cereus ATCC 33018, F4810, and Baclo3a. Phenotypically, these three strains showed zones of hemolysis on blood agar plates. This is probably because the PCR primers were targeted only toward the B component of the hemolysin gene, which is absent in these strains, but the two L components of HBL may be present in strains showing positive hemolysis (6, 7). Incidentally, these strains were also phospholipase (lecithinase) positive, which may aid in the hemolytic activity. In contrast, two B. thuringiensis strains and a B. cereus strain were hblA positive but did not show hemolysis on blood agar, indicating a possible lack of expression of HBL in these strains. Furthermore, these strains were also negative for lecithinase activity (Table 2).
Genes coding for nonhemolytic components A, B, and C (nheA, nheB, and nheC) were uniformly distributed in all B. cereus strains tested except for F837/76. The cytK gene was observed in only one B. cereus strain (A926), and bceT was found in six B. cereus strains and in B. megaterium and B. thuringiensis strains (Table 2).
Comparison with commercial assay kits.
Eight B. cereus strains used in this experiment gave positive enzyme-linked immunosorbent assay results with the BDE-VIA, which detects the 41-kDa subunit of NHE (Table 3). However, in the BCET-RPLA assay (HBL L2 subunit expression), all but two B. cereus strains (F4810 and ATCC 33018) were negative (Table 3). These data agree with the PCR data, where these two strains were found to be negative for the hblA gene (Table 2).
DISCUSSION
Sensitive and rapid detection of enterotoxin from B. cereus is highly desirable in order to protect consumers from food-associated illness and to promote food safety and food biosecurity. A Ped-2E9 cell-based assay to detect cytopathogenic Listeria species has been described (8, 9), and later the same cell line was found to be sensitive to B. cereus toxins (53). This cell line was used to measure cytotoxicity by assaying AP release spectrophotometrically. Cell-free culture supernatants from 31 previously isolated Bacillus cultures were tested, of which 17 were B. cereus and the remaining 14 belonged to other Bacillus species. All but one B. cereus strain gave positive cytotoxicity results, and a majority were positive for the hblA, nheA, nheB, and nheC genes. Furthermore, decreased cytotoxicity or no cytotoxicity was seen in strains in which all or some of the subunits of the NHE complex were absent. It has been shown that some subunits of the NHE toxin complex have some biological activity, but all are required for maximal activity (33). B. cereus CA1 is a food isolate and contained genes for bceT and one of the NHE subunits (nheA). This strain did not cause cytotoxicity (0.2%). This may be due to the lack of other subunits of NHE or the absence of a protein expression system. HBL is also an important cytotoxic factor; however, it may not be essential for Ped-2E9-mediated cytotoxicity, since hblA-negative B. cereus strains F4810 and ATCC 33018 exhibited positive cytotoxicity with Ped-2E9 cells (Table 3). Among the non-B. cereus cultures, only B. thuringiensis strains were positive with the Ped-2E9 assay, and those strains were also positive for the hbl or nhe gene (41). The cytotoxicity of these cultures was in agreement with published reports (5, 33, 46, 49). B. megaterium is also known to produce heat-stable emetic toxin (55). In this study, although we used three strains, only one was confirmed by ribotyping to be B. megaterium (ATCC 9885), and it showed no cytotoxicity effect on Ped-2E9 cells. This strain was also determined to be nonhemolytic and lecithinase negative, and PCR data indicated the absence of the hbl and nhe genes.
Among the eight nascent Bacillus isolates from food, only one strain was cytotoxic, and it was later confirmed by ribotyping to be B. cereus. This strain also contained nhe genes but lacked hblA, reiterating the significance of nhe gene sequences in Ped-2E9 cytotoxicity. Although a limited number of food samples or isolates were tested, this assay accurately detected the cytotoxic potential of a single B. cereus strain.
In order to elucidate the molecular weights of the toxins responsible for cytotoxic actions on Ped-2E9 cells, toxin preparations were fractionated and analyzed for activity. Fractions with Mrs of 30,000 and higher showed the most cytotoxic effects. As mentioned before, B. cereus produces multiple toxins, including hemolysins (HBL; 37.8, 38.5, and 43.5 kDa) (33) and NHE (39, 45, and 105 kDa) (40). Based on the protein analysis data, PCR results, and hemolysis on blood agar plates, we speculate that cytotoxic action on Ped-2E9 cells could be due to the NHE and/or HBL components, since these toxins were also found to be involved in cytotoxic activities by using other cell lines (39). As indicated above, HBL possibly enhances Ped-2E9 cytotoxicity induced by NHE but is not essential. Heat treatment significantly reduced the activity of the toxin on Ped-2E9 cells, suggesting the possible involvement of NHE (diarrheal enterotoxins) in cytotoxicity, since these toxins are heat labile (6, 46). The emetic toxin is thermostable and consists of low-molecular-weight cereulide (2, 55). The association of cytotoxic activity with a high-molecular-weight compound and its thermal inactivation strongly imply the involvement of NHE (diarrheal enterotoxins) in Ped-2E9 cytotoxicity. Furthermore, the heat-inactivated toxin preparation from a known emetic strain (B. cereus F4810) (23) did not show any cytotoxicity with the Ped-2E9 cells, providing circumstantial evidence that this cell line is not sensitive to emetic toxin.
The Ped-2E9-based assay was also compared with the standard CHO-based assay, which is sensitive to both diarrheagenic and emetic toxins (5, 40). In this study, CHO cells were sensitive to the enterotoxin-producing B. cereus strains and a B. subtilis strain. The latter strain is known to produce emetic toxin (25), and the CHO cell line is sensitive to emetic toxin. The Ped-2E9 assay did not show any cytotoxicity with B. subtilis, confirming the possible lack of response of Ped-2E9 cells to emetic toxin.
The sensitivity of the Ped-2E9-based assay was compared with that of the CHO cell assay by using crude toxin preparations from B. cereus MS1-9 and ATCC 14579. The Ped-2E9 assay was 58-fold more sensitive than the CHO assay for the toxin preparation from B. cereus MS1-9 and about 25-fold more sensitive for that from B. cereus ATCC 14579, based on the lowest concentrations of toxins showing positive cytotoxicity effects on both cell lines. Cytotoxicity titration curves for Ped-2E9 cells revealed a gradual decline in cytotoxicity values with decreasing concentrations of toxins (0.3 to 0.4 μg/ml), while the cytotoxicity titration curve for CHO cells revealed a sudden drop in cytotoxicity values, approaching zero percent at toxin concentrations of 17.5 μg/ml for B. cereus MS1-9 and >6.6 μg/ml for B. cereus ATCC 14579 (Fig. 1). These data indicate that CHO cells presumably require an optimum concentration of toxins, below which the lethal effects of toxins are attenuated, as indicated by a sudden drop in the curve. In contrast, Ped-2E9 cells are highly sensitive to a very low concentration of toxin, thus producing a linear cytotoxicity curve. Further, the Ped-2E9 assay is considerably faster (taking only 1 h to complete after addition of toxin) than the CHO assay, which required 72 h to complete (40).
The procedures of the commercially available toxin assay kits were relatively simple to perform; however, these assays are toxin subunit specific and have high likelihoods of false negatives, since strains do not always possess all subunits. Also, both kits must be used, since they each react with different toxin complexes, and some strains may possess only one or the other. The results of the BDE-VIA and BCET-RPLA did correlate with PCR results; however, they did not give any indication of the strain's pathogenic potential.
In summary, a sensitive cytotoxicity assay was developed for enterotoxin-positive Bacillus species using Ped-2E9 cells. This assay is considerably faster than other methods, showing a cytotoxic effect in just 1 h, saving at least a day compared to other cell-based assays. The Ped-2E9 assay is also relatively simple to perform and gives quantitative results through a simple enzyme-based colorimetric assay, as opposed to a metabolism-based MTT assay for other cell lines. Since the Ped-2E9 assay is sensitive to enterotoxin, it could be used for any Bacillus species capable of causing food-borne illnesses.
Acknowledgments
Sincere thanks to J. Handlesman (Department of Plant Pathology, University of Wisconsin), A. Wong (Food Research Institute, University of Wisconsin), B. Applegate and K. Hayes (Department of Food Science, Purdue University), A. Aronson (Department of Biology, Purdue University), J. McKillip (Department of Biology, Ball State University), and G. Siragusa (USDA-ARS, Russell Research Center, Athens, GA) for providing the cultures. Also our sincere thanks to Bruce Craig and Benjamin Tyner for assistance with statistical analyses and John McKillip and Jennifer Cutraro for critical reading of the manuscript.
This research was supported through a cooperative agreement with the Agricultural Research Service of the U.S. Department of Agriculture (project 1935-42000-035) and the Center for Food Safety and Engineering at Purdue University.
REFERENCES
- 1.Agata, N., M. Ohta, Y. Arakawa, and M. Mori. 1995. The bceT gene of Bacillus cereus encodes an enterotoxin protein. Microbiology 141:983-988. [DOI] [PubMed] [Google Scholar]
- 2.Agata, N., M. Ohta, M. Mori, and M. Isobe. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17-20. [DOI] [PubMed] [Google Scholar]
- 3.Agata, N., M. Ohta, and K. Yokoyama. 2002. Production of Bacillus cereus emetic toxin (cereulide) in various foods. Int. J. Food Microbiol. 73:23-27. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, M. A., R. Mikkola, J. Helin, M. C. Andersson, and M. Salkinoja-Salonen. 1998. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl. Environ. Microbiol. 64:1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie, S. H., and A. G. Williams. 1999. Detection of toxigenic strains of Bacillus cereus and other Bacillus spp. with an improved cytotoxicity assay. Lett. Appl. Microbiol. 28:221-225. [DOI] [PubMed] [Google Scholar]
- 6.Beecher, D. J., J. L. Schoeni, and A. C. L. Wong. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beecher, D. J., and A. C. L. Wong. 1997. Tripartite hemolysin BL from Bacillus cereus. J. Biol. Chem. 272:233-239. [DOI] [PubMed] [Google Scholar]
- 8.Bhunia, A., P. Steele, D. Westbrook, L. Bly, T. Maloney, and M. Johnson. 1994. A six-hour in vitro virulence assay for Listeria monocytogenes using myeloma and hybridoma cells from murine and human sources. Microb. Pathog. 16:99-110. [DOI] [PubMed] [Google Scholar]
- 9.Bhunia, A. K., and D. G. Westbrook. 1998. Alkaline phosphatase release assay to determine cytotoxicity for Listeria species. Lett. Appl. Microbiol. 26:305-310. [DOI] [PubMed] [Google Scholar]
- 10.Bhunia, A. K., D. G. Westbrook, R. Story, and M. G. Johnson. 1995. Frozen stored murine hybridoma cells can be used to determine the virulence of Listeria monocytogenes. J. Clin. Microbiol. 33:3349-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonventre, P. F. 1965. Differential cytotoxicity of Bacillus anthracis and Bacillus cereus culture filtrates. J. Bacteriol. 90:284-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choma, C., and P. E. Granum. 2002. The enterotoxin T (BcET) from Bacillus cereus can probably not contribute to food poisoning. FEMS Microbiol. Lett. 217:115-119. [DOI] [PubMed] [Google Scholar]
- 13.Choma, C., M. H. Guinebretiere, F. Carlin, P. Schmitt, P. Velge, P. E. Granum, and C. Nguyen-The. 2000. Prevalence, characterization and growth of Bacillus cereus in commercial cooked chilled foods containing vegetables. J. Appl. Microbiol. 88:617-625. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich, R., C. Fella, S. Strich, and E. Martlbauer. 1999. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 65:4470-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay, W. J. J., N. A. Logan, and A. D. Sutherland. 1999. Semiautomated metabolic staining assay for Bacillus cereus emetic toxin. Appl. Environ. Microbiol. 65:1811-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher, P., and N. A. Logan. 1999. Improved cytotoxicity assay for Bacillus cereus diarrhoeal enterotoxin. Lett. Appl. Microbiol. 28:394-400. [DOI] [PubMed] [Google Scholar]
- 17.From, C., R. Pukall, P. Schumann, V. Hormazabal, and P. E. Granum. 2005. Toxin-producing ability among Bacillus spp. outside the Bacillus cereus group. Appl. Environ. Microbiol. 71:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghelardi, E., F. Celandroni, S. Salvetti, C. Barsotti, A. Baggiani, and S. Senesi. 2002. Identification and characterization of toxigenic Bacillus cereus isolates responsible for two food-poisoning outbreaks. FEMS Microbiol. Lett. 208:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Granum, P. E., and T. Lund. 1997. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 157:223-228. [DOI] [PubMed] [Google Scholar]
- 20.Granum, P. E., K. O'Sullivan, and T. Lund. 1999. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 177:225-229. [DOI] [PubMed] [Google Scholar]
- 21.Guillaume-Gentil, O., P. Scheldeman, J. Marugg, L. Herman, H. Joosten, and M. Heyndrickx. 2002. Genetic heterogeneity in Bacillus sporothermodurans as demonstrated by ribotyping and repetitive extragenic palindromic-PCR fingerprinting. Appl. Environ. Microbiol. 68:4216-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinebretiere, M.-H., V. Broussolle, and C. Nguyen-The. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40:3053-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haggblom, M. M., C. Apetroaie, M. A. Andersson, and M. S. Salkinoja-Salonen. 2002. Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl. Environ. Microbiol. 68:2479-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy, S. P., T. Lund, and P. E. Granum. 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197:47-51. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh, Y. M., S. J. Sheu, Y. L. Chen, and H. Y. Tsen. 1999. Enterotoxigenic profiles and polymerase chain reaction detection of Bacillus cereus group cells and B. cereus strains from foods and food-borne outbreaks. J. Appl. Microbiol. 87:481-490. [DOI] [PubMed] [Google Scholar]
- 26.Hughes, S., B. Bartholomew, J. C. Hardy, and J. M. Kramer. 1988. Potential application of a HEp-2 cell assay in the investigation of Bacillus cereus emetic-syndrome food poisoning. FEMS Microbiol. Lett. 52:7-11. [Google Scholar]
- 27.Jackson, S. G. 1993. Rapid screening test for enterotoxin-producing Bacillus cereus. J. Clin. Microbiol. 31:972-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura-Sato, K., Y. Hirama, N. Agata, H. Ito, K. Torii, A. Takeno, T. Hasegawa, Y. Shimomura, and M. Ohta. 2005. Quantitative analysis of cereulide, an emetic toxin of Bacillus cereus, by using rat liver mitochondria. Microbiol. Immunol. 49:25-30. [DOI] [PubMed] [Google Scholar]
- 29.Kim, Y.-R., J. Czajka, and C. A. Batt. 2000. Development of a fluorogenic probe-based PCR assay for detection of Bacillus cereus in nonfat dry milk. Appl. Environ. Microbiol. 66:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microb. Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 31.Lattuda, C. P., and D. McClain. 1998. Examination of meat and poultry products for Bacillus cereus, p. 12-1-12-6. In B. P. Day (ed.), USDA/FSIS microbiology laboratory guidebook, 3rd ed. USDA/FSIS, Washington, D.C.
- 32.Lund, T., M.-L. De Buyser, and P. E. Granum. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254-261. [DOI] [PubMed] [Google Scholar]
- 33.Lund, T., and P. Granum. 1997. Comparison of biological effect of the two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 143:3329-3336. [DOI] [PubMed] [Google Scholar]
- 34.Lund, T., and P. E. Granum. 1999. The 105-kDa protein component of Bacillus cereus non-haemolytic enterotoxin (Nhe) is a metalloprotease with gelatinolytic and collagenolytic activity. FEMS Microbiol. Lett. 178:355-361. [DOI] [PubMed] [Google Scholar]
- 35.Lund, T., and P. E. Granum. 1996. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 141:151-156. [DOI] [PubMed] [Google Scholar]
- 36.McKillip, J. L. 2000. Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review. Antonie Leeuwenhoek 77:393-399. [DOI] [PubMed] [Google Scholar]
- 37.Menon, A., M. L. Shroyer, J. L. Wampler, C. B. Chawan, and A. K. Bhunia. 2003. In vitro study of Listeria monocytogenes infection to murine primary and human transformed B cells. Comp. Immunol. Microbiol. Infect. Dis. 26:157-174. [DOI] [PubMed] [Google Scholar]
- 38.Mikami, T., T. Horikawa, T. Murakami, T. Matsumoto, A. Yamakawa, S. Murayama, S. Katagiri, K. Shinagawa, and M. Suzuki. 1994. An improved method for detecting cytostatic toxin (emetic toxin) of Bacillus cereus and its application to food samples. FEMS Microbiol. Lett. 119:53-57. [DOI] [PubMed] [Google Scholar]
- 39.Ostensvik, O., C. From, B. Heidenreich, K. O'Sullivan, and P. E. Granum. 2004. Cytotoxic Bacillus spp. belonging to the B. cereus and B. subtilis groups in Norwegian surface waters. J. Appl. Microbiol. 96:987-993. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen, P. B., M. E. Bjornvad, M. D. Rasmussen, and J. N. Petersen. 2002. Cytotoxic potential of industrial strains of Bacillus sp. Regul. Toxicol. Pharmacol. 36:155-161. [DOI] [PubMed] [Google Scholar]
- 41.Phelps, R. J., and J. L. McKillip. 2002. Enterotoxin production in natural isolates of Bacillaceae outside the Bacillus cereus group. Appl. Environ. Microbiol. 68:3147-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prub, B. M., R. Dietrich, B. Nibler, E. Martlbauer, and S. Scherer. 1999. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl. Environ. Microbiol. 65:5436-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radhika, B., B. P. Padmapriya, A. Chandrashekar, N. Keshava, and M. C. Varadaraj. 2002. Detection of Bacillus cereus in foods by colony hybridization using PCR-generated probe and characterization of isolates for toxins by PCR. Int. J. Food Microbiol. 74:131-138. [DOI] [PubMed] [Google Scholar]
- 44.Rhodehamel, E. J., and S. M. Harmon. 1998. Bacillus cereus, p. 14.01-14.08. In Food and Drug Administration bacteriological analytical manual, 8th ed. AOAC International, Gaithersburg, Md.
- 45.Rider, T. H., M. S. Petrovick, F. E. Nargi, J. D. Harper, E. D. Schwoebel, R. H. Mathews, D. J. Blanchard, L. T. Bortolin, A. M. Young, J. Chen, and M. A. Hollis. 2003. A B cell-based sensor for rapid identification of pathogens. Science 301:213-215. [DOI] [PubMed] [Google Scholar]
- 46.Rowan, N. J., G. Caldow, C. G. Gemmell, and I. S. Hunter. 2003. Production of diarrheal enterotoxins and other potential virulence factors by veterinary isolates of Bacillus species associated with nongastrointestinal infections. Appl. Environ. Microbiol. 69:2372-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowan, N. J., K. Deans, J. G. Anderson, C. G. Gemmell, I. S. Hunter, and T. Chaithong. 2001. Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl. Environ. Microbiol. 67:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rusul, G., and N. H. Yaacob. 1995. Prevalence of Bacillus cereus in selected foods and detection of enterotoxin using TECRA-VIA and BCET-RPLA. Int. J. Food Microbiol. 25:131-139. [DOI] [PubMed] [Google Scholar]
- 49.Salkinoja-Salonen, M. S., R. Vuorio, M. A. Andersson, P. Kampfer, M. C. Andersson, T. Honkanen-Buzalski, and A. C. Scoging. 1999. Toxigenic strains of Bacillus licheniformis related to food poisoning. Appl. Environ. Microbiol. 65:4637-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoeni, J. L., and A. C. L. Wong. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636-648. [DOI] [PubMed] [Google Scholar]
- 51.Shinagawa, K., H. Konuma, H. Sekita, and S. Sugii. 1995. Emesis of rhesus monkeys induced by intragastric administration with the HEp-2 vacuolation factor (cereulide) produced by Bacillus cereus. FEMS Microbiol. Lett. 130:87-90. [DOI] [PubMed] [Google Scholar]
- 52.Shinagawa, K., S. Ueno, H. Konuma, N. Matsusaka, and S. Sugii. 1991. Purification and characterization of the vascular permeability factor produced by Bacillus cereus. J. Vet. Med. Sci. 53:281-286. [DOI] [PubMed] [Google Scholar]
- 53.Shroyer, M., and A. Bhunia. 2003. Development of a rapid 1-h fluorescence-based cytotoxicity assay for Listeria species. J. Microbiol. Methods 55:35-40. [DOI] [PubMed] [Google Scholar]
- 54.Szabo, R. A., J. I. Speirs, and M. Akhtar. 1991. Cell culture detection and conditions for production of a Bacillus cereus heat-stable toxin. J. Food Prot. 54:272-276. [DOI] [PubMed] [Google Scholar]
- 55.Taylor, J. M. W., A. D. Sutherland, K. E. Aidoo, and N. A. Logan. 2005. Heat-stable toxin production by strains of Bacillus cereus, Bacillus firmus, Bacillus megaterium, Bacillus simplex and Bacillus licheniformis. FEMS Microbiol. Lett. 242:313-317. [DOI] [PubMed] [Google Scholar]
- 56.TeGiffel, M. C., R. R. Beumer, P. E. Granum, and F. M. Rombouts. 1997. Isolation and characterization of Bacillus cereus from pasteurized milk in household refrigerators in The Netherlands. Int. J. Food Microbiol. 34:307-318. [DOI] [PubMed] [Google Scholar]
- 57.Westbrook, D. G., and A. K. Bhunia. 2000. Dithiothreitol enhances Listeria monocytogenes mediated cell cytotoxicity. Microbiol. Immunol. 44:431-438. [DOI] [PubMed] [Google Scholar]