Abstract
To assess the prevalence and clinical significance of hepatitis B virus (HBV) genotypes and precore and core promoter mutations in Taiwan, a cohort of 200 Taiwanese chronic hepatitis B patients was analyzed. The HBV genotypes and sequences of the precore and the core promoter regions were determined in 66 asymptomatic carriers and 134 patients who had liver biopsy-verified chronic hepatitis and liver cirrhosis. The HBV e-antigen (HBeAg)-negative patients had a higher frequency of mutations at core promoter nucleotides 1753 and 1773 and precore nucleotides 1846, 1896, and 1899 than HBeAg-positive patients. Among the 200 patients, the frequencies of genotype C, T1762 and A1764, C1753, T1766 and A1768, and A1896 mutations increased and the frequencies of T or G1752, T1773, G1799, and C1858 mutations decreased with advancing liver diseases. These factors were different between those with HBeAg-positive status and those with HBeAg-negative status. Based on multiple logistic regression analysis, the risk factors of liver cirrhosis for 200 patients were the presence of T1762 and A1764 mutations (odds ratio [OR] = 11.11; 95% confidence interval [CI] = 3.91 to 31.25; P < 0.001), age ≥35 years (OR = 3.42; 95% CI = 1.33 to 8.77; P = 0.011), and genotype C (OR = 2.87; 95% CI = 1.21 to 6.81; P = 0.017). Further categorical analysis found that 62.1% of patients with genotype C, T1762 and A1764 mutations and age ≥35 years had liver cirrhosis. None of the 55 patients infected with the genotype B, A1762 and G1764 wild type and age <35 years showed liver cirrhosis. In conclusion, our data suggest that pathogenic differences between HBeAg-positive and -negative patients may exist. In Taiwan, HBV genotype C and the T1762 and A1764 mutations may play a role in HBV-related liver cirrhosis, and these could serve as molecular markers for prediction of the clinical outcomes of chronic HBV patients.
Worldwide, hepatitis B virus (HBV) infection is a major health problem. Chronic HBV infection is associated with a wide range of clinical manifestations, from an asymptomatic carrier status with a normal liver histology to severe and chronic liver disease, including cirrhosis and hepatocellular carcinoma (HCC) (11, 13, 25). The natural history of chronic HBV infection can be divided into three phases: immune tolerance, immune clearance, and residual or integrated phases (11, 13). In addition, a fourth reactivation phase has also been proposed. During the early phase of chronic hepatitis B (CHB) patients are positive for hepatitis B e antigen (HBeAg) and have frequent acute flares characterized by substantial increases in serum alanine aminotransferase (ALT) levels. Spontaneous HBeAg seroconversion during the course of chronic HBV infection usually correlates with the disappearance of biochemical markers of hepatitis and a drastic decrease in viremia. However, hepatitis recurred in 15 to 33.2% of patients, which may develop cirrhosis and HCC (12, 18).
HBeAg-negative CHB patients suffer from active liver disease. They have increased ALT levels and detectable serum HBV DNA by classical hybridization techniques (17). HBeAg-negative CHB is frequently associated with precore and core promoter mutants. The predominant precore variation is a G-to-A change at nucleotide (nt) 1896 (A1896), which creates a premature stop codon and which abolishes the synthesis of HBeAg (1, 4, 8). The most common core promoter mutations involve a two-nucleotide substitution: A-T at nt 1762 and G-A at nt 1764 (T1762 and A1764) (23, 34). Several transfection studies show that the 1762 and A1764 mutations decrease the level of pre-C mRNA by 50 to 70% and lead to reduced HBeAg synthesis (7, 16). Several studies have shown that HBeAg may be a target antigen on HBV-infected hepatocytes (39). Failure to produce a target antigen may be a means of evading immune clearance. Nevertheless, the significance of additional mutations in the precore and core promoter regions other than nucleotides 1762 and 1764 and nucleotide 1896 for HBeAg production is unknown.
Although A1896 has previously been reported to be associated with severe forms of chronic liver disease (6, 38), the real significance of A1896 is still unclear because this mutation is frequently detected among patients with mild liver disease (2, 36). In addition, mutations in T1762 and A1764 have frequently been reported to be associated with advanced liver disease and an increased risk for HCC (19, 28, 41), and the mutations have also been found in patients with nonadvanced liver disease (23). Thus, the real significance of the T1762 and A1764 mutations is still unclear. Furthermore, another two basal core promoter (BCP) mutations at nt 1766 (C to T) and nt 1768 (T to A) have previously been reported in patients with fulminant hepatitis (3), and the C1753 mutation was reported to be associated with the progression of liver disease (42). However, it is unclear whether other mutations in the precore and core promoter regions were associated with the progression of liver disease.
HBV can be classified into eight genotypic groups (genotypes A to H) (33, 35, 40). Genotypes B and C are the most prevalent variants in Taiwan, and it has been reported that the genotype C variant is associated with liver diseases more severe than those with the genotype B variant is associated (20, 24). Several previous studies have indicated that genotype C has a higher frequency of T1762 and A1764 mutations than genotype B (26, 29). Thus, it is unclear whether different clinical outcomes between genotype B and genotype C infections correlate with the T1762 and A1764 mutations.
To assess the prevalence and clinical significance of HBV genotypes and mutations in the precore and core promoter regions, we compared the frequencies of HBV genotypes and these mutations between three groups of patients with CHB: asymptomatic carriers, patients with chronic hepatitis, and liver cirrhosis patients. We also analyzed whether these factors are different between HBeAg-positive and -negative CHB patients. In addition, we investigated the relationship between precore and core promoter mutations and HBeAg.
MATERIALS AND METHODS
Patients.
Between 1998 and 2001, 200 HBsAg-positive patients who had undergone long-term follow-up at Kaohsiung Chang Gung Memorial Hospital were analyzed. They included 66 patients (28 HBeAg-positive and 38 HBeAg-negative CHB carriers) who had persistently normal serum ALT levels, as detected by routine testing and confirmed by repeated tests every 3 to 6 months; who had undergone periodic ultrasound examinations showing a normal liver every 6 months for at least the preceding 3 years; and who were inferred to be asymptomatic carriers. In addition, the study included 134 CHB patients who had had abnormal liver functions for at least 6 months and who had received consecutive liver biopsies for the diagnosis of inflammation or fibrosis or for evaluation before lamivudine or interferon treatment. Among the latter group of patients, 95 patients had chronic hepatitis and 39 had liver cirrhosis. The histological grading of chronic liver disease was based on a modified Knodell histology index in relation to the degree of hepatic inflammation and fibrosis (HAI scores) (15). The necroinflammatory score (HAI-INF; score range, 0 to 18) and the fibrosis score (HAI-F; score range, 0 to 4) were analyzed independently. Patients were excluded if they had any evidence of autoimmune hepatitis or markers of hepatitis C virus, hepatitis D virus (HDV), or human immunodeficiency virus. The HBV genotype and the sequences of the precore and the core promoter regions were determined from sera collected at the last visit for asymptomatic carriers and at the time of liver biopsy for patients with chronic hepatitis and cirrhosis. HBV DNA levels were evaluated in patients with chronic hepatitis and cirrhosis at the time of the liver biopsy. The sera were frozen at −70°C until use.
Serology.
The presence of HBsAg, HBeAg, anti-HCV antibodies, and anti-HDV antibodies were determined with commercial assay kits (HBsAg, enzyme immunoassay [EIA], Abbott, North Chicago, IL; HBeAg, EIA, Abbott; anti-HCV, EIA 3.0, Abbott; anti-HDV, radioimmunoassay, Abbott). HBV DNA was determined by use of a hybridization capture kit (HBV test, Hybrid Capture II; Digene Corp., Gaithersburg, MD) with a detection limit for the standard test of 0.5 pg/ml (141,500 copies/ml).
PCR amplification and direct sequencing of BCP and precore regions.
DNA was extracted from 100 μl serum with a QIAamp DNA Mini kit (QIAGEN Inc., Hilden, Germany), according to the manufacturer's recommendations. For sequence analysis, the precore and the core promoter regions were amplified by nested PCR, and the primers described previously (9) were used. First-round PCR was performed with 5 μl of DNA extract in a 50-μl reaction mixture containing 10× buffer (100 mM Tris-HCl, pH 9.0, 500 mM KCl, 15 mM MgCl2, 1% Triton X-100), 2.5 mM deoxynucleoside triphosphates, 1 U Taq polymerase, and 20 μM external primers. PCR was performed as follows: 96°C for 2 min; 94°C for 1 min, 54°C for 1 min, and 72°C for 2 min for 36 cycles; and finally, 72°C for 10 min. For the second-round PCR, 1 μl of the first-round PCR product was reamplified by use of the same reaction mixture composition and PCR conditions used in the first-round reaction, except that internal primers were used. The sensitivity of this method is 100 copies/ml. All necessary precautions to prevent cross-contamination were taken, and negative controls were included in each assay. The nucleotide sequences of the amplified products were directly determined by using fluorescent-labeled primers with an ABI PRISM 377 genetic analyzer (Applied Biosystems, Foster City, CA). All of the tests were performed in duplicate to confirm the results.
HBV genotyping.
The HBV genotypes were determined from serum samples by PCR-restriction fragment length polymorphism genotyping, based on analysis of the surface gene (between nucleotide positions 256 and 796), as described previously (27).
Data analysis.
HAI scores were compared by the Mann-Whitney rank sum test. The chi-square, Fisher exact, and Student t tests were used for statistical analysis. The differences in the prevalence of gender, HBV genotypes, and precore and core promoter mutations with different stages of liver disease were examined by the chi-square test for linear trend. Spearman correlation analyses were used to evaluate the relationship between age and different stages of liver disease. Stepwise multiple logistic regression analysis was performed to assess the influences of various factors on the risk of liver cirrhosis. A P value below 0.05 was considered statistically significant.
RESULTS
Characterization of HBV in CHB patients.
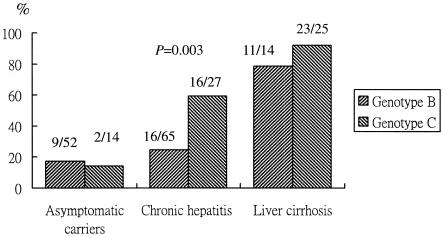
Among the 200 patients, there were 144 men and 56 women; the mean age was 36.7 ± 12 years. The clinical features, HBV DNA levels, and prevalence of HBV genotypes and mutants in the precore and core promoter regions with various stages of chronic liver disease are presented in Table 1. The mean age and male-to-female ratio were significantly increased with advancing clinical stages of liver disease. HBV DNA levels were significantly higher for chronic hepatitis patients than for liver cirrhosis patients. Overall, the frequencies of genotype C, C1753, T1766 and A1768, T1762 and A1764, and A1896 mutations had apparent increasing trends with advancing clinical stages; and the frequencies of T or G1752, T1773, G1799, and C1858 mutations had decreasing trends with advancing clinical stages. In addition, the frequency of the T1762 and A1764 mutations increased with advancing liver diseases in both genotype B- and genotype C-infected patients (P < 0.001 and P < 0.001, respectively) (Fig. 1).
TABLE 1.
Clinical features, HBV viremia, and prevalence of mutations in precore and core promoter regions in different clinical stages of chronic hepatitis B patients
| Characteristic | Asympatomatic carriers (n = 66) | Chronic hepatitis patients (n = 95) | Liver cirrhosis patients (n = 39) | P value |
|---|---|---|---|---|
| Age (yr) | 31.7 ± 10.2 | 36.5 ± 11.3 | 45.3 ± 11.9 | <0.001 |
| Sex (no. of males:no. of females) | 35:31 | 76:19 | 33:6 | <0.005 |
| ALT level (U/liter) | 23.8 ± 9.8 | 186.6 ± 187.1 | 122.8 ± 126.8 | NSa |
| HBV DNA concn (log pg/ml) | 2.4 ± 1.1 | 1.6 ± 1.1 | 0.001 | |
| HAI inflammation score | 6.1 ± 3.6 | 6.4 ± 3.4 | NS | |
| No. (%) of patients infected with HBV: | ||||
| Genotype B | 52 | 65 | 14 | <0.001 |
| Genotype C | 14 (21.1) | 27 (29.3) | 25 (64.1) | |
| T or G1752 | 28 (42.4) | 35 (36.8) | 8 (20.5) | <0.05 |
| C1753 | 0 | 3 (3.2) | 7 (17.9) | <0.001 |
| T1762/A1764 | 11 (16.7) | 32 (33.7) | 34 (87.2) | <0.001 |
| T1766/A1768 | 1 (1.5) | 7 (7.4) | 6 (15.4) | <0.01 |
| T1773 | 8 (12.1) | 4 (4.2) | 1 (2.6) | <0.05 |
| C1799 | 48 (72.7) | 67 (70.5) | 15 (38.5) | <0.005 |
| T1846 | 14 (21.2) | 17 (17.9) | 12 (30.8) | NS |
| C1858 | 7 (10.6) | 2 (2.1) | 1 (2.6) | <0.05 |
| A1896 | 13 (19.7) | 34 (35.8) | 19 (48.7) | <0.005 |
| A1899 | 5 (7.5) | 1 (1.1) | 4 (10.3) | NS |
NS, not significant.
FIG. 1.
Prevalence of T1762 and A1764 core promoter mutations in 200 genotype B and genotype C hepatitis B virus-infected patients with various liver diseases.
HBeAg status.
Our study population included 103 HBeAg-positive and 97 HBeAg-negative patients. Comparisons of the clinical features, HBV genotypes, HBV DNA levels, and levels of liver inflammation between HBeAg-positive and -negative patients are shown in Table 2. Compared with HBeAg-negative patients, the HBeAg-positive patients with chronic hepatitis and cirrhosis were significantly younger and had higher HBV DNA levels and lower fibrosis scores. Furthermore, these data were compared in relation to various stages of chronic liver diseases. The HBeAg-positive patients were younger (P = 0.005) and had higher HBV DNA levels (P < 0.001), if they were in the chronic hepatitis stage, than the HBeAg-negative patients.
TABLE 2.
Clinical features, HBV genotypes, HBV DNA levels, and liver inflammation in relation to HBeAg and clinical status
| HBeAg and clinical status | No. of patients | Age (yr) | Sex (no. of males:no. of females) | No. infected with genotype B:no. infected with genotype C | ALT level (U/liter) | HBV DNA concn (log pg/ml) | HAI inflammation score | HAI fibrosis score |
|---|---|---|---|---|---|---|---|---|
| HBeAg positive | ||||||||
| Asymptomatic carriers | 28 | 31.6 ± 8.9 | 13:15 | 20:8 | 25.3 ± 13.2 | |||
| Chronic hepatitis patients | 59 | 34 ± 10.0a | 45:14 | 37:19 | 201.9 ± 169.5 | 2.8 ± 1.0c | 6.4 ± 3.7 | 0.8 ± 1.1 |
| Liver cirrhosis patients | 16 | 44.4 ± 12 | 15:1 | 4:12 | 87.5 ± 58.6 | 2.0 ± 1.2d | 6.4 ± 4.5 | 4 |
| Subtotal | 103 | 35 ± 10.8b | 73:30 | 61:39 | 136.1 ± 152.1 | 2.6 ± 1.1e | 6.4 ± 3.8 | 1.5 ± 1.6f |
| HBeAg negative | ||||||||
| Asymptomatic carriers | 38 | 31.7 ± 11.3 | 22:16 | 32:6 | 22.8 ± 9.9 | |||
| Chronic hepatitis patients | 36 | 40.5 ± 12.1a | 31:5 | 28:8 | 161.5 ± 212.9 | 1.7 ± 1.0c | 5.6 ± 3.4 | 0.9 ± 1.2 |
| Liver cirrhosis patients | 23 | 45.8 ± 12 | 18:5 | 10:13 | 147.4 ± 154.6 | 1.3 ± 1.0d | 6.4 ± 2.5 | 4 |
| Subtotal | 97 | 38.4 ± 12.9b | 71:26 | 70:27 | 103.8 ± 162.3 | 1.5 ± 1.0e | 5.9 ± 3.1 | 2.1 ± 1.8f |
P = 0.0056.
P < 0.001.
P < 0.001.
P = 0.057.
P < 0.001.
P = 0.044.
The frequencies of age, male-to-female ratios, and infection with genotype C virus were significantly increased with advanced stages of liver disease in HBeAg-positive and -negative patients (for age, P = 0.001 and P < 0.001, respectively; for male-to-female ratios, P < 0.001 and P < 0.05, respectively; for genotype C, P < 0.01 and P < 0.005, respectively).
HBV mutations and HBeAg status.
The HBeAg-negative patients had a significantly higher frequency of mutations at core promoter nucleotides 1753 and 1773 and precore nucleotides 1846, 1896, and 1899 than HBeAg-positive patients (Table 3). The T1762 and A1764 mutations were detected in 33 of 102 (32%) of the HBeAg-positive patients and in 44 of 97 (45.4%) of the HBeAg-negative patients (borderline significance [P = 0.06]). In HBeAg-positive patients, the frequencies of the A1762 and A1764 mutations and the T1766 and A1768 mutations increased and the frequencies of the T or the G1752 and C1799 mutations decreased with advanced clinical stages of liver disease. In contrast, in HBeAg-negative patients, the frequencies of the T1762 and A1764, C1753 and A1896 mutations increased and the frequencies of the C1799 and C1858 mutations decreased with advanced clinical stages of liver disease.
TABLE 3.
HBV precore and core promoter mutations in relation to HBeAg and clinical status
| HBeAg and clinical status | No. (%) of patients
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | T or G1752 | C1753 | T1762/ A1764 | T1766/A1768 | T1773 | C1799 | T1846 | C1858 | A1896 | A1899 | |
| HBeAg positive | |||||||||||
| Asymptomatic carriers | 28 | 14 (50) | 0 | 2 (7.1) | 0 | 1 (3.6) | 20 (71.4) | 1 (3.6)g | 1 (3.6) | 1 (3.6) | 0 |
| Chronic hepatitis patients | 59 | 24 (40.7) | 1 (1.7) | 17 (28.8) | 2 (3.4) | 2 (3.4) | 41 (69.5) | 7 (11.9) | 2 (3.4) | 11 (18.6) | 0 |
| Liver cirrhosis patients | 16 | 2 (12.5) | 1 (6.2) | 14 (87.5) | 3 (18.7) | 0 | 5 (31.3) | 1 (6.2)h | 1 (6.3) | 3 (18.7) | 0 |
| Subtotal | 103 | 40 (38.8) | 2 (1.9)a | 33 (32)b | 5 (4.9) | 3 (2.9)c | 66 (64.1) | 9 (8.8)d | 4 (3.9) | 15 (14.6)e | 0f |
| HBeAg negative | |||||||||||
| Asymptomatic carriers | 38 | 14 (36.8) | 0 | 9 (23.7) | 1 (2.6) | 7 (18.4) | 28 (73.7) | 13 (34.2)g | 6 (15.8) | 12 (31.5) | 5 (13.2) |
| Chronic hepatitis patients | 36 | 11 (30.5) | 2 (5.5) | 15 (41.6) | 5 (13.9) | 2 (5.5) | 26 (72.2) | 10 (27.8) | 0 | 23 (63.8) | 1 (2.8) |
| Liver cirrhosis patients | 23 | 6 (26.1) | 6 (26.1) | 20 (86.9) | 3 (13) | 1 (4.3) | 10 (43.5) | 11 (47.8)h | 0 | 16 (69.6) | 4 (17.4) |
| Subtotal | 97 | 31 (31.9) | 8 (8.2)a | 44 (45.4)b | 9 (9.3) | 10 (10.3)c | 64 (66) | 34 (35.1)d | 6 (6.2) | 50 (51.5)e | 10 (10.3)f |
P = 0.05.
P = 0.06.
P = 0.044.
P < 0.001.
P < 0.001.
P = 0.001.
P = 0.004.
P = 0.012.
HBV mutations and genotypes and clinical features.
T1762 and A1764 mutants were found in 77 patients (38.5%), among whom 75 had T1762 and A1764 mutants, 7 had T1762 mutants alone, and 1 had T1764 mutant alone. Compared with wild-type virus, patients infected with the T1762 and A1764 mutants were older (41.8 ± 12.4 years versus 33.4 ± 10.5 years; P<0.001), had a higher rate of liver cirrhosis (34 of 77 patients [44.1%] versus 5 of 123 patients [4.1%]; P<0.001), were predominantly infected with HBV genotype C (41 of 77 patients [53.2%] versus 25 of 123 patients [20.3%]; P<0.001), and had lower HBV DNA levels (mean 1.9 ± 1.2 log pg/ml versus 2.3 ± 1.1 log pg/ml; P = 0.028) and higher inflammation scores (median [range], 7 [1 to 17] versus 5 [1 to 13]; P = 0.019) in the chronic hepatitis and the cirrhosis stages. There was no significant difference between patients infected with mutants and the wild type in terms of gender and ALT levels. In addition, the following mutations occurred more frequently in patients infected with HBV with the T1762 and the A1764 mutations than in those with wild-type HBV: C1753 (9 of 77 patients versus 1 of 123 patients; P = 0.001), T1766 and A1768 (13 of 77 patients versus 1 of 123 patients; P < 0.001), G1799 (34 of 77 patients versus 96 of 123 patients; P < 0.001), C1800 (10 of 77 patients versus 4 of 123 patients; P = 0.019), and A1899 (9 of 77 patients versus 1 of 123 patients; P = 0.001).
The A1896 mutation was found in 66 patients (33%), among whom 18 had mixed infections. In comparison with patients infected with the wild-type virus, the patients infected with virus with the A1896 mutation were older (39.3 ± 12.5 versus 35.3 ± 11.6 years; P = 0.025), had a higher rate of liver cirrhosis (19 of 66 patients versus 20 of 134 patients; P = 0.024), and had lower HBV DNA levels in the chronic hepatitis and cirrhosis stages (mean, 1.8 ± 1.1 log pg/ml versus 2.3 ± 1.2 log pg/ml; P = 0.016). There was no significant difference between patients infected with mutants and the wild type in terms of gender, genotypes, ALT levels, and inflammation scores. For the precore regions, mutations from A to T at nucleotide 1846 and G to A at nucleotide 1899 were observed in 43 (21.5%) and 10 (5%) patients, respectively. Furthermore, compared with patients with the G1896 wild type, patients with the A1896 mutation had a higher frequency of the T1846 mutation (25 of 66 patients versus 18 of 134 patients; P < 0.001) and the A1899 mutation 10 of 66 patients versus 0 of 134 patients; P < 0.001).
Of the 200 patients, 131 were infected with genotype B and 66 were infected with genotype C. Compared with the genotype B-infected patients, the genotype C-infected patients had a higher incidence of liver cirrhosis (25 of 66 patients versus 14 of 131 patients; P < 0.001) and had lower log HBV DNA levels (mean, 1.9 ± 1.2 log pg/ml versus 2.3 ± 1.1 log pg/ml; P = 0.021); genotype C-infected patients with chronic hepatitis and cirrhosis had higher inflammation scores (median [range], 5 [1 to 14] versus 7 [1 to 17]; P = 0.034). There was no significant difference in terms of age, gender, ALT levels, and the rate of HBeAg positivity between genotype B- and genotype C-infected patients. In addition, patients with genotype B infection had a lower frequency of the T1762 and A1764 mutations (36 of 131 patients versus 41 of 66 patients; P < 0.001), the C1753 mutation (3 of 131 patients versus 7 of 66 patients; P = 0.018), and the T1766 and A1768 mutations (3 of 131 patients versus 11 of 66 patients; P < 0.001) and had higher rates of the T or G1752 mutation (65 of 131 patients versus 6 of 66 patients; P < 0.001), the G1799 mutation (119 of 131 patients versus 11 of 66 patients; P < 0.001), and the C1800 mutation (13 of 131 patients versus 1 of 66 patients; P = 0.037) than those with genotype C infection. However, the prevalence of T1762 and A1764 mutants was significantly higher in genotype C-infected patients than in genotype B-infected patients only in the chronic hepatitis stage (P = 0.003) (Fig. 1).
Risk factors for liver cirrhosis.
Stepwise multiple logistic regression was performed for the 200 patients by using the factors of age, gender, infecting HBV genotype, and precore and core promoter mutations (Table 4). It was found that the significant risk factors for liver cirrhosis were the presence of the T1762 and A1764 mutations (odds ratio [OR] = 11.11; 95% confidence interval [CI] = 3.91 to 31.25; P < 0.001), age ≥35 years (OR = 3.42; 95% CI = 1.33 to 8.77; P = 0.011), and infection with genotype C (OR = 2.87; 95% CI = 1.21 to 6.81; P = 0.017). However, the risk factors for liver cirrhosis for HBeAg-positive patients were the presence of the T1762 and A1764 mutations (OR = 16.95; 95% CI = 3.4 to 83.33; P = 0.001) and age ≥35 years (OR = 4.41; 95% CI = 1.03 to 19.0; P = 0.046); and the risk factors for HBeAg-negative patients were the presence of the T1762 and A1764 mutations (OR = 11.36; 95% CI = 2.99 to 43.48; P < 0.001) and infection with genotype C (OR = 4.01; 95% CI = 1.3 to 12.37; P = 0.016). When the patients were categorized according to these determinants, the rate of liver cirrhosis was the highest (18 of 29 [62.1%]) among patients who were infected with a genotype C, T1762 and A1764 mutant and who were ≥35 years of age. In contrast, none of the 55 patients who were infected with HBV genotype B, A1762 and G1764 wild type and who were <35 years of age had liver cirrhosis.
TABLE 4.
Association of predictive factors with the risk of liver cirrhosis in patients with chronic hepatitis B virus infection
| Factor | OR (95% CI)
|
||
|---|---|---|---|
| Total (n = 200) | HBeAg positive (n = 103) | HBeAg negative (n = 97) | |
| Age ≥35 vs <35 yr | 3.42 (1.33-8.77) | 4.42 (1.03-19.0) | |
| Genotype C vs genotype B infection | 2.87 (1.21-6.81) | 4.01 (1.3-12.37) | |
| Presence vs absence of T1762 and A1764 mutant | 11.11 (3.91-31.25) | 16.95 (3.4-83.33) | 11.36 (2.99-43.48) |
DISCUSSION
Ever since the discovery of HBeAg by Magnius and Espmark in 1972 (32), its function has remained an enigma. There is still no solid evidence about the events that lead to seroconversion from HBeAg to anti-HBe status, but a strong contender has been the mutations in A1896 and in T1762 and A1764, which could prevent and reduce the production of HBeAg (1, 4, 8, 23, 34). In the present study, the HBeAg-negative patients had a significantly higher frequency of core promoter nucleotides C1753 and T1773 and precore nucleotides T1846, A1896, and A1899 than HBeAg-positive patients. Many studies have shown that the A1896 mutant can be detected in 0 to 80% of HBeAg-positive patients (5, 31). However, precore mutant ratios tended to correlate with anti-HBe seroconversion, but high precore mutant ratios were associated with persistent hepatitis after anti-HBe seroconversion (14). Those findings are compatible with those of the present study. In addition, our data showed that A1899 mutation was found only in HBeAg-negative patients (10.3%) and was associated with the A1896 mutation. This finding is compatible with that of a previous study (30). A previous study also found that the frequency of T1864 increased after HBeAg clearance during follow-up (30). However, it remains unclear whether T1864 could affect HBeAg secretion.
The T1762 and A1764 mutations have been reported to reduce HBeAg production by approximately 50 to 70% (7, 16). However, it is controversial whether the T1762 and A1764 mutations lead to the HBeAg-negative phenotype (28, 29). Our study found the frequency of the T1762 and A1764 mutations had a borderline difference between HBeAg-positive and -negative patients. This suggests that the T1762 and A1764 mutations appear to be insufficient to lead to the HBeAg-negative phenotype, as approximately one-third of the HBeAg-positive patients had this mutation. In addition, our study also found a higher frequency of C1753 and T1773 in HBeAg-negative patients. Parekh et al. (37) demonstrated that mutations at nt 1753, 1762, 1764, and 1766 conferred lower levels of HBeAg expression than mutations at nt 1762 and 1764 alone.
Several factors that lead an increased risk of advanced liver diseases for patients with CHB have been identified. These include age, male gender, repeated episodes of severe acute exacerbation, and HBV reactivation after HBeAg seroconversion (10, 12, 18, 24). In this study, our results showed that the frequency of mutation at nucleotides 1752, 1753, 1766 or 1768, 1799, 1858, and 1896 increased or decreased with the progression of chronic liver disease and that these risk factors were different between HBeAg-positive and -negative patients. Age, male gender, infection with genotype C, and the presence of the T1762 and A1764 mutations were the same risk factors in both HBeAg-positive and -negative patients. Takahashi et al. found that C1753 is a common mutation in genotype C patients (>85%) and is more closely associated with the progression of liver disease in HBeAg-positive patients (42). In our study, C1753 was a less common BCP mutation and was found in only 5.2% of all patients (10.7% of genotype C-infected patients), and it was associated with the progression of liver disease only in HBeAg-negative patients. Either or both of the T1766 and A1768 mutations were previously reported in a patient with fulminant hepatitis (3). These changes were detected in 7% of our patients and were associated with the progression of liver disease, especially among HBeAg-positive patients. A previous study demonstrated that the G1752 and the G1799 mutations were linked to genotype B but not to the progression of liver disease (19). Our study showed that G1752 and G1799 appeared together with genotype B infection and that the frequency was significantly decreased with advanced clinical stages of chronic liver diseases.
Previous studies have indicated that the T1762 and A1764 mutations were more predominant in HBV genotype C-infected patients (26, 29). However, it is unclear whether both the T1762 and A1764 mutations and genotype C infection were independent factors for advanced liver disease. In this study, the frequency of the T1762 and A1764 mutant increased with advanced liver disease in both genotype B- and genotype C-infected patients. These findings were compatible with those of a previous study (19). By using multiple logistic regression analysis, our results consistently showed that old age, genotype C infection, and the presence of the T1762 and 1764 mutation were independent risk factors for liver cirrhosis. The T1762 and A1764 mutations were found in the dominant viral species at the late HBeAg-positive phase and the anti-HBe stages of HBV infection. Thus, CHB patients infected with T1762 and A1764 mutant may have a longer duration of active replication. Previous studies found that HBV genotype C infection was associated with later HBeAg seroconversion and multiple episodes of acute exacerbation without HBeAg seroconversion than genotype B HBV infection (21, 22). The delayed HBeAg seroconversion may prolong the inflammation process and subsequently result in more severe liver damage. In addition, our study also showed that patients with genotype C infection or T1762 and A1764 mutant infection had more severe hepatic necroinflammation. Therefore, the detection of HBV genotype C and the T1762 and A1764 mutant may be useful as markers for the identification of immunoactivation.
In summary, the predictive factors for the progression of chronic liver disease were different between HBeAg-positive and -negative patients, and these findings suggest that pathogenic differences between the two groups may exist. Several nucleotide mutations in the precore and the core promoter regions might contribute to reduced HBeAg production and were associated with advanced liver disease. In Taiwan, genotype C and the T1762 and A1764 mutant may play a role in HBV-related liver cirrhosis and could serve as molecular markers for prediction of the clinical outcomes for patients with chronic HBV infection.
Acknowledgments
This study was supported by grant CMRPG 83057 from Chang Gung Memorial Hospital and grant NSC 93-2314-B-182A-146 from the National Council of Science, Taiwan.
REFERENCES
- 1.Akahane, Y., T. Yamanaka, H. Suzuki, Y. Sugai, F. Tsuda, S. Yotsumoto, S. Omi, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1990. Chronic active hepatitis with hepatitis B virus DNA and antibody against e antigen in the serum. Disturbed synthesis and secretion of e antigen from hepatocytes due to a point mutation in the precore region. Gastroenterology 99:1113-1119. [DOI] [PubMed] [Google Scholar]
- 2.Akarca, U. S., S. Greene, and A. S. F. Lok. 1994. Detection of precore hepatitis B virus mutants in asymptomatic HBs-positive family members. Hepatology 19:1366-1370. [PubMed] [Google Scholar]
- 3.Baumert, T. F., S. A. Rogers, K. Hasegawa, and T. J. Liang. 1996. Two core promoter mutations identified in a hepatitis B virus strain associated with fulminant hepatitis result in enhanced viral replication. J. Clin. Investig. 98:2268-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetto, M. R., M. M. Giarin, F. Oliveri, E. Chiaberge, M. Baldi, A. Alfarano, A. Serra, G. Saracco, G. Verme, and H. Will. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl. Acad. Sci. USA 88:4186-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetto, M. R., M. Giarin, G. Saracco, F. Oliver, P. Calvo, G. Capra, A. Randone, M. L. Abate, et al. 1993. Hepatitis B virus unable to secret e antigen and response to interferon in chronic hepatitis B. Gastroenterology 105:846-850. [DOI] [PubMed] [Google Scholar]
- 6.Brunetto, M. R., M. Stemler, F. Bonino, F. Schodel, F. Oliveri, M. Rizzetto, G. Verme, and H. Will. 1990. A new hepatitis B virus strain in patients with severe anti-HBe positive chronic hepatitis B. J. Hepatol. 10:258-261. [DOI] [PubMed] [Google Scholar]
- 7.Buckwold, V. E., Z. Xu, M. Chen, T. S. Yen, and J. H. Ou. 1996. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J. Virol. 70:5845-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. J. McGarvey, A. Makris, and H. C. Thomas. 1989. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet ii:588-591. [DOI] [PubMed] [Google Scholar]
- 9.Chan, H. L., M. Hussain, and A. S. F. Lok. 1999. Different hepatitis B virus genotypes are associated with different mutations in the core promoter and precore regions during hepatitis B e antigen seroconversion. Hepatology 29:976-984. [DOI] [PubMed] [Google Scholar]
- 10.Chan, H. L., S. W. Tsang, C. T. Liew, C. H. Tse, M. L. Wong, J. Y. Ching, N. W. Leung, J. S. Tam, and J. J. Sung. 2002. Viral genotype and hepatitis B virus DNA levels are correlated with histological liver damage in HBeAg-negative chronic hepatitis B virus infection. Am. J. Gastroenterol. 97:406-412. [DOI] [PubMed] [Google Scholar]
- 11.Chen, D. S. 1993. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science 262:369-370. [DOI] [PubMed] [Google Scholar]
- 12.Chu, C. M., S. J. Hung, J. Lin, D. I. Tai, and Y. F. Liaw. 2004. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am. J. Med. 116:829-834. [DOI] [PubMed] [Google Scholar]
- 13.Chu, C. M., and Y. F. Liaw. 1997. Natural history of chronic hepatitis B virus infection: an immunopathological study. J. Gastroenterol. Hepatol. 12:S218-S222. [DOI] [PubMed] [Google Scholar]
- 14.Chu, C. M., C. T. Yeh, C. S. Lee, I. S. Sheen, and Y. F. Liaw. 2002. Precore stop mutant in HBeAg-positive patients with chronic hepatitis B: clinical characteristics and correlation with the course of HBeAg-to-anti-HBe seroconversion. J. Clin. Microbiol. 40:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmet, V. J., M. Gerber, J. H. Hoofnagle, M. Manns, and P. J. Scheuer. 1994. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 19:1513-1520. [PubMed] [Google Scholar]
- 16.Günther, S., N. Piwon, and H. Will. 1998. Wild-type levels of pregenomic RNA and replication but reduced pre-C RNA and e-antigen synthesis of hepatitis B virus with C(1653)→T, A(1762) →T and G (1764) → A mutations in the core promoter. J. Gen. Virol. 79:375-380. [DOI] [PubMed] [Google Scholar]
- 17.Hadziyannis, S. J., and D. Vassilopoulos. 2001. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 34:617-624. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, Y. S., R. N. Chien, C. T. Yeh, I. S. Sheen, H. Y. Chiou, C. M. Chu, and Y. F. Liaw. 2002. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 35:1522-1527. [DOI] [PubMed] [Google Scholar]
- 19.Kao, J. H. P., J. Chen, M. Y. Lai, and D. S. Chen. 2003. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 124:327-334. [DOI] [PubMed] [Google Scholar]
- 20.Kao, J. H. P., J. Chen, M. Y. Lai, and D. S. Chen. 2000. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 118:554-559. [DOI] [PubMed] [Google Scholar]
- 21.Kao, J. H. P., J. Chen, M. Y. Lai, and D. S. Chen. 2002. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J. Clin. Microbiol. 40:1207-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao, J. H. P., J. Chen, M. Y. Lai, and D. S. Chen. 2004. Hepatitis B virus genotype and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J. Med. Virol. 72:363-369. [DOI] [PubMed] [Google Scholar]
- 23.Kurosaki, M., N. Enomoto, Y. Asahina, I. Sakuma, T. Ikeda, S. Tozuka, N. Izumi, F. Marumo, and C. Sato. 1996. Mutations in the core promoter region of hepatitis B virus in patients with chronic hepatitis B. J. Med. Virol. 49:115-123. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. M., C. H. Chen, S. N. Lu, H. D. Tung, W. J. Chou, J. H. Wang, T. M. Chen, C. H. Hung, C. H. Huang, and W. J. Chen. 2003. Prevalence of clinical implications of hepatitis B virus genotypes in southern Taiwan. Scand. J. Gastroenterol. 1:95-101. [DOI] [PubMed] [Google Scholar]
- 25.Lee, C. M., S. N. Lu, C. S. Changchien, C. T. Yeh, T. T. Hsu, J. H. Tang, J. H. Wang, D. Y. Lin, C. L. Chen, and W. J. Chen. 1999. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer 86:1143-1150. [DOI] [PubMed] [Google Scholar]
- 26.Lin, C. L., L. Y. Liao, C. J. Liu, P. J. Chen, M. Y. Lai, J. H. Kao, and D. S. Chen. 2002. Hepatitis B genotypes and precore/basal core promoter mutants in HBeAg-negative chronic hepatitis B. J. Gastroenterol. 37:283-287. [DOI] [PubMed] [Google Scholar]
- 27.Lindh, M., A. S. Andersson, and A. Gusdal. 1997. Genotypes, nt 1858 variants, and geographic origin of hepatitis B virus—large-scale analysis using a new genotyping method. J. Infect. Dis. 175:1285-1293. [DOI] [PubMed] [Google Scholar]
- 28.Lindh, M., C. Gustavson, K. Mardberg, G. Norkrans, A. P. Dhillon, and P. Horal. 1998. Mutation of nucleotide 1762 in the core promoter region during hepatitis B e seroconversion and its relation to liver damage in hepatitis B e antigen carriers. J. Med. Virol. 55:185-190. [DOI] [PubMed] [Google Scholar]
- 29.Lindh, M., C. Hannoun, A. P. Dhillon, G. Norkrans, and P. Horal. 1999. Core promoter mutations and genotypes in relation to viral replication and liver damage in East Asian hepatitis B virus carriers. J. Infect. Dis. 179:775-782. [DOI] [PubMed] [Google Scholar]
- 30.Lok, A. S. F., U. S. Akarca, and S. Greene. 1994. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc. Natl. Acad. Sci. USA 91:4077-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lok, A. S. F., U. S. Akarca, and S. Greene. 1995. Predictive value of precore hepatitis B virus mutations in spontaneous and interferon-induced hepatitis B e antigen clearance. Hepatology 21:19-24. [PubMed] [Google Scholar]
- 32.Magnius, L. O., and J. A. Espmark. 1972. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J. Immunol. 109:1017-1021. [PubMed] [Google Scholar]
- 33.Norder, H., B. Hammas, S. Lofdahl, A. M. Courouce, and L. O. Magnius. 1992. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J. Gen. Virol. 73:1201-1208. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 68:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto, H., F. Tsuda, H. Sakugawa, R. I. Sastrosoewignjo, M. Imai, Y. Miyakawa, and M. Mayumi. 1988. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J. Gen. Virol. 69:2575-2583. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto, H., S. Yotsumoto, Y. Akahane, T. Yamanaka, Y. Miyazaki, Y. Sugai, F. Tsuda, T. Tanaka, Y. Miyakawa., and M. Mayumi. 1990. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J. Virol. 64:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parekh, S., F. Zoulim, S. H. Ahn, A. Tsai, J. Li, S. Kawai, S., N. Khan, C. Trépo, J. Wands, and S. Tong. 2003. Genome replication, virion secretion, and e antigen expression of naturally occurring hepatitis B virus core promoter mutants. J. Virol. 12:6601-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondo, G., R. Schneider, M. Stemler, V. Smedile, G. Rodino, and H. Will. 1990. A new hepatitis B virus variant in a chronic carrier with multiple episodes of viral reactivation and acute hepatitis. Virology 179:64-68. [DOI] [PubMed] [Google Scholar]
- 39.Schlicht, H. J., and H. Schaller. 1989. The secretory core protein of human hepatitis B virus is expressed on the cell surface J. Virol. 63:5399-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuyver, L., S. De Gendt, C. Van Geyt, F. Zoulim, M. Fried, R. F. Schinazi, and R. Rossau. 2000. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J. Gen. Virol. 81:67-74. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, K., K. Aoyama, N. Ohno, K. Iwata, Y. Akahane, K. Baba, H. Yoshizawa, and S. Mishiro. 1995. The precore/core promoter mutants (T1762A1764) of hepatitis B virus: clinical significance and an easy method for detection. J. Gen. Virol. 76:3159-3164. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, K., Y. Ohta, K. Kanai, Y. Akahane, Y. Iwasa, K. Hino, N. Ohno, H. Yoshizawa, and S. Mishiro. 1999. Clinical implications of mutations C-to-T1653 and T-to-C/A/G1753 of hepatitis B virus genotype C genome in chronic liver disease. Arch. Virol. 144:1299-1308. [DOI] [PubMed] [Google Scholar]