Abstract
The Fungitell assay (Associates of Cape Cod, Inc.) is a commercial test that detects (1-3)-β-d-glucan (BG) and is intended for diagnosis of invasive fungal infections. To evaluate the Fungitell assay, we tested serum and plasma samples from healthy blood donors and from patients with blood cultures positive for yeast or bacteria. All 36 blood donors were BG negative, and 13 of 15 candidemic patients were BG positive. Of 25 bacteremic patients, 14 (10 with gram-positive bacteremia) were BG positive. One of the latter patients with Staphylococcus aureus bacteremia also had invasive candidiasis, based on histological findings in a tissue biopsy; therefore, the BG result was a true positive. The sensitivity, specificity, and positive and negative predictive values of the Fungitell assay, by patient, for these three groups were 93.3%, 77.2%, 51.9%, and 97.8%, respectively. We also performed the Fungitell assay on sera that had been tested for Aspergillus galactomannan or Histoplasma antigen. All six Histoplasma antigen-positive patients and 31 of 32 Aspergillus galactomannan-positive patients were also BG positive. BG results for the 10 Histoplasma antigen-negative and the 32 Aspergillus galactomannan-negative patients varied, but we were unable to confirm many of the results. Between-run coefficients of variance (CVs) for the assay ranged from 3.2% to 16.8%; within-run CVs were ≤4.8%. The correlation coefficient for an interlaboratory reproducibility study was 0.9892. Concentrations of hemoglobulin, bilirubin, and triglycerides that caused 20% interference were 588, 72, and 466 mg/dl, respectively. Our results suggest that the Fungitel assay may be most useful for excluding invasive fungal infection.
The rise in the incidence of invasive fungal infections (IFI) has increased dramatically since the early 1980s and parallels the increase in the population of persons who are immunocompromised (4, 27, 34, 38). Among this population are patients undergoing solid-organ or hematopoietic stem cell transplantation and patients infected with human immunodeficiency virus (1, 4, 6, 35). Other factors contributing to the increase in invasive fungal disease are more-effective immunosuppressive regimens, the widespread use of broad-spectrum antibiotics, and increased use of indwelling catheters and other implantable devices (6, 18, 38).
IFI in immunocompromised patients are difficult to diagnose, but successful treatment requires early intervention with antifungal drugs (11). Delay in instituting appropriate antifungal therapy contributes to the high death rate, which may approach 50% and 80% for invasive Candida and Aspergillus infections, respectively (6, 19, 27, 38). Traditional methods of culture and histopathologic examination of tissue biopsies require an invasive procedure (2, 27). Culture requires several days and may be negative in up to 50% of patients who have systemic Candida or Aspergillus infections (6, 27). Also, invasive procedures necessary to obtain biopsies may be precluded in critically ill patients (27, 29). Without reliable diagnostic information, antifungal drugs are often administered empirically (17).
Several nonculture methods for diagnosing systemic fungal infections have been described. These methods involve detection of fungal antigens or metabolites and molecular methods using PCR (11, 29). A double-sandwich enzyme immunoassay (EIA) that detects Aspergillus galactomannan is commercially available (27, 29). Assays for the Candida metabolites mannan and enolase have been reported (20); however, these methods are not commercially available, may require specialized equipment, and are not generally performed in most clinical laboratories.
(1→3)-β-d-glucan (BG) is a component of the cell wall of fungi except Cryptococcus neoformans and zygomycetes (20, 21, 26). Its presence in the bloodstream correlates with IFI (22, 23). Assays for BG are based on the activation of the horseshoe crab coagulation cascade (31). Liposaccharide and BG initiate the coagulation cascade by activating different serine protease zymogens, factors C and G. Liposaccharide specifically activates factor C, while BG activates factor G. Limulus polyphemus amebocyte lysates are made specific for BG by removal of factor C.
The goal of this study was to assess the utility of the Fungitell BG assay (Associates of Cape Cod, Inc. [ACCI], East Falmouth, MA), which was cleared by the U.S. Food and Drug Administration, as an aid in the diagnosis of IFI. The Institutional Review Board of the University of Utah approved this study.
MATERIALS AND METHODS
Specimens.
To evaluate the Fungitell assay, specimens were tested from healthy blood donors, patients with blood cultures positive for yeast or bacteria, patients with suspected histoplasmosis (based on an order for the Histoplasma antigen EIA), and patients with suspected aspergillosis (based on an order for the Aspergillus galactomannan EIA). Blood was cultured for bacteria and yeast with the Bactec 9240 continuous monitoring system (BD Diagnostic Systems, Sparks, MD) at Associated Regional and University Pathologists, Inc. (ARUP). Serum samples were collected at ARUP Blood Services from healthy adults prior to their donating blood. Serum or plasma samples remaining from other laboratory tests that had been performed at the University of Utah Hospital or ARUP 5 days before or after the positive blood culture and then stored at −20°C were retrieved and tested for BG. Additionally, the medical records of patients with blood cultures that were positive for bacteria were reviewed to collect the following information: underlying disease; receipt of intravenous immunoglobulin, antimicrobial agents, and/or chemotherapeutic agents; treatment with hemodialysis; and diagnoses based on histological examination of tissue biopsies. Sera that remained after the requested tests for the Histoplasma antigen EIA, performed at MiraVista, Indianapolis, IN, and the Aspergillus galactomannan EIA, performed at ARUP, were retrieved from storage at −20°C and tested for BG. If there was a discrepancy between the Histoplasma or Aspergillus EIA and the BG results, an attempt was made to obtain pertinent clinical information. All samples were deidentified according to protocols approved by the University of Utah Institutional Review Board (IRB no. 13195).
Fungitell assay.
The Fungitell assay was performed according to the protocol supplied by the manufacturer (3). Briefly, 5 μl of serum or plasma was added to triplicate wells of a 96-well microtiter plate and incubated for 10 min at 37°C with 20 μl of a solution of 1 part 1.2 M KCl and 1 part 0.25 M NaOH. One hundred microliters of a Limulus amebocyte lysate with substrate was added to the sample. The plate was monitored at 405 nm for 40 min at 37°C in a Molecular Devices (Sunnyvale, CA) model 384 spectrophotometer equipped with Softmax software, version 4.7.1. The mean rate of optical density change was determined for each well, and the BG concentration was determined by comparison to a standard curve. Interpretation of BG values was as follows: <60 pg/ml, negative; 60 to 79 pg/ml, indeterminate; ≥80 pg/ml, positive.
Reproducibility.
Within-run, between-run, and interlaboratory variabilities of the Fungitell assay were assessed. To evaluate between-run variability, 15 different sera were tested by the Fungitell assay on three different days. Following the first assay, 15 sera with BG values ranging from 85 pg/ml to 2,197 pg/ml were tested again by the Fungitell assay on two separate days. Within-run variability of the Fungitell assay was determined by retesting four different serum samples (BG concentrations were 181, 953, 1,121, and 2,034 pg/ml) in triplicate in a single assay. For each replicate, three adjacent wells of a microtiter plate each contained 5 μl of serum sample, for a total of 15 μl. The three replicates of each sample were placed in different locations within the microtiter plate. The mean and coefficient of variation for the three replicates was determined for each of the four samples. To assess the interlaboratory variability of the Fungitell assay, two blinded panels, one with 25 samples and one with 24 samples, were prepared at ARUP and sent to ACCI for BG testing. Fungitell assay results determined at ARUP for the 49 samples were compared with those determined at ACCI for the same samples.
Interfering substances.
Interference in the Fungitell assay due to hemolysis, bilirubin, and lipemia was assessed by supplementation of a BG-positive serum sample with each of the individual interferents (25). To prepare sufficient quantities of BG-positive serum samples for this study, a unit of expired fresh frozen plasma that tested negative for BG was clotted with CaCl2. Serum was collected by centrifugation and sterilely filtered. A BG-positive serum sample control was produced by reconstituting lyophilized BG from Associates of Cape Cod, Inc., with the above-processed serum to a concentration of 161 pg/ml. The BG standard (pachyman) used in this study was the same as that supplied with the Fungitell kit. Bilirubin and triglycerides (Intralipid) were purchased from Sigma, St. Louis, Mo. A solution of hemoglobulin was prepared by lysis of washed red blood cells with pyrogen-free water (Associates of Cape Cod, Inc.). Stock solutions of bilirubin, triglycerides, and hemoglobulin, prepared in pyrogen-free water, were added individually to aliquots of the BG-positive serum prepared above to simulate a range of bilirubin, triglyceride, and hemoglobin concentrations of approximately 0 to 200 mg/dl, 0 to 1,000 mg/dl, and 0 to 2,500 mg/dl, respectively. Final concentrations of hemoglobin, bilirubin, and triglycerides were determined for each aliquot. Bilirubin and triglycerides were determined with a Modular Analytics P instrument (Roche Diagnostics Corporation, Indianapolis, Ind.) using reagents supplied by the manufacturer. Hemoglobin concentration was determined with a Beckman Coulter DU 800 spectrometer. Samples supplemented with interferents were tested by the Fungitell assay and compared to the BG-positive sample without an interferent. To estimate minimum interfering concentrations, interference plots for hemoglobin, bilirubin, and triglycerides were constructed by plotting the percentage of control serum as a function of the concentration of interferents.
BG cross-contamination.
To assess the potential for BG cross-contamination, three 1-ml specimens that initially were negative for BG (24, 34, and 58 pg/ml) were transferred four times in succession into new transport tubes (catalogue no. 62.611.009; Sarstedt, Inc., Newton, NC). An aliquot consisting of the residual volume left in each tube was retained, and all five aliquots from each sample were tested simultaneously for BG by the Fungitell assay.
RESULTS
Clinical specimens.
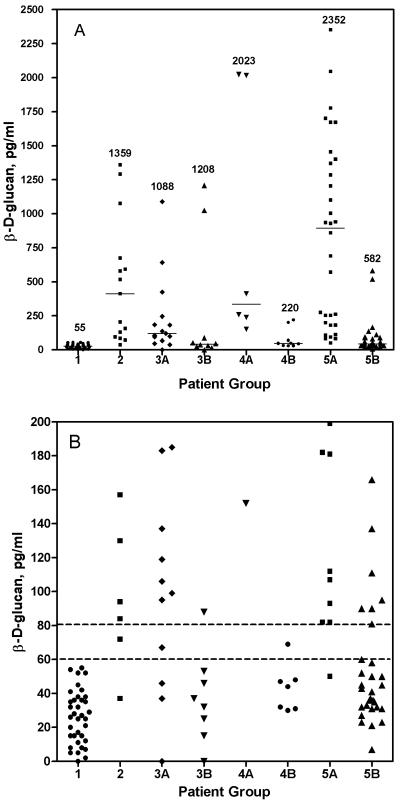
Patients with BG results were divided into eight groups: 1, healthy adult blood donors; 2, patients with blood cultures positive for yeast; 3A, patients with blood cultures positive for gram-positive cocci; 3B, patients with positive blood cultures for gram-negative bacilli; 4A, patients with positive EIA results for Histoplasma antigen; 4B, patients with negative EIA results for Histoplasma antigen; 5A, patients with positive EIA results for Aspergillus galactomannan antigen; and 5B, patients with negative EIA results for Aspergillus galactomannan antigen. Multiple samples were tested for most of the patients in groups 2, 3A, and 3B (105 samples for the 40 patients in these three groups). For groups 1, 4A, 4B, and 5A, only one sample for each patient was tested; for 3 of the 32 patients in group 5B, two samples were tested. BG results for the eight patient groups are shown by patient in Table 1 and in Fig. 1A and B. Table 1 shows the number of patients in each group with positive, negative, and indeterminate BG results. Figure 1A shows the range of BG results by patient for each patient group, and Fig. 1B shows the range of BG values for each patient group near the cutoff of ≥80 pg/ml. Organisms isolated from patients in groups 2, 3A, and 3B are listed in the legend to Fig. 1.
TABLE 1.
Fungitell BG assay results for various patient groups
| Group (no. of patients) | No. of patients with BG resulta of:
|
||
|---|---|---|---|
| Positive | Indeterminate | Negative | |
| Blood donors (36) | 0 | 0 | 36 |
| Blood culture positive for yeast (15) | 13 | 1 | 1 |
| Blood culture positive for gram-positive cocci (15) | 11b | 1 | 3 |
| Blood culture positive for gram-negative bacilli (10) | 3 | 2 | 5 |
| Histoplasma antigen positive (6) | 6 | 0 | 0 |
| Histoplasma antigen negative (10) | 2 | 2 | 6 |
| Aspergillus galactomannan positive (32) | 31 | 0 | 1 |
| Aspergillus galactomannan negative (32) | 9 | 1 | 22 |
Positive, ≥80 pg/ml; indeterminate, 60 to 79 pg/ml; negative, <60 pg/ml.
One patient had documented invasive candidiasis, based on histologic findings in a tissue biopsy.
FIG. 1.
Range of BG values for patient groups measured by the Fungitell assay. (A) The highest BG concentration for each patient is plotted for each patient group. Numbers indicating the highest BG value within each group are shown. Horizontal bars indicate the median value for each group. (B) Samples of ≤200 pg/ml are shown for each patient group. Horizontal lines indicate the cutoff for positive (>80 pg/ml) and negative (<60 pg/ml) samples. Patient groups are as follows: 1, healthy adult blood donors; 2, patients with blood cultures positive for yeast (8 patients with Candida albicans, 3 with Candida glabrata, 2 with Candida rugosa, 1 with Candida parapsilosis, and 1 with Candida lusitaniae); 3A, patients with blood cultures positive for gram-negative bacteria (1 with Klebsiella pneumoniae, 1 with Klebsiella oxytoca, 4 with Escherichia coli, 1 with Salmonella species, 2 with Pseudomonas aeruginosa, and 1 with Enterobacter cloacae); 3B, patients with blood cultures positive for gram-positive bacteria (9 with Staphylococcus aureus, (3 with coagulase-negative staphylococci, 1 with Streptococcus mitis, 1 with Streptococcus pneumoniae, and 1 with Enterococcus faecium); 4A, patients who were Histoplasma antigen positive (n = 6); 4B) patients who were Histoplasma antigen negative (n = 10); 5A, patients who were Aspergillus galactomannan positive (n = 32); and 5B, patients who were Aspergillus galactomannan negative (n = 32).
All 36 samples from healthy blood donors (group 1) were negative for BG (<60 pg/ml). Of the 39 samples from the 15 patients with blood cultures positive for yeast, 30 samples were positive for BG (range, 84 to 1,359 pg/ml), 2 were indeterminate, 6 were negative, and 1 was invalid due to excessive hemolysis. Thirteen of the 15 patients (86.7%) had at least one specimen that was positive. Blood cultures of both remaining patients grew Candida rugosa. One of these patients had one specimen that was indeterminate and three that were negative for BG; the other had two negative specimens and one that was invalid.
Twenty-two of 36 samples from 15 patients with blood cultures positive for gram-positive cocci (group 3A) were BG positive (range, 0 to 1,088 pg/ml). Of the 15 patients in group 3A, 11 (73.3%) (6 with Staphylococcus aureus, 3 with coagulase negative staphylococci, 1 with Streptococcus mitis, and 1 with Enterococcus faecium) had ≥1 specimen positive for BG, 1 patient (with S. aureus bacteremia) had only 1 specimen that was indeterminate, and all specimens from 3 patients (2 with S. aureus and 1 with Streptococcus pneumoniae) were negative. Of the 11 BG-positive patients in group 3A, 1 patient (with S. aureus bacteremia) also had a positive biopsy for Candida. The BG result for this patient, therefore, was a true positive. Another BG-positive patient in group 3A (with S. aureus bacteremia) received kidney dialysis and also received a β-lactam antibiotic (nafcillin). One patient with coagulase-negative Staphylococcus bacteremia and a positive BG result received intravenous immunoglobulin. Five other patients from group 3 who were BG positive also received β-lactam antibiotics (imipenem-cilastatin, cefazolin, amoxicillin, nafcillin, and piperacillin-tazobactam). However, another patient in group 3A with S. aureus bacteremia who received a β-lactam antibiotic (penicillin) was BG negative, and four other patients who did not receive a β-lactam antimicrobial were BG positive.
Of the 10 patients whose blood cultures grew gram-negative bacilli (group 3B), 3 (2 with Escherichia coli, 1 with Salmonella species) had ≥1 specimen positive for BG (range, 0 to 1,208 pg/ml), 2 (1 with Klebsiella pneumoniae and Enterobacter cloacae, 1 with E. coli) had at least 1 sample that was indeterminate, and all specimens from 5 patients (2 with Pseudomonas aeruginosa and 1 each with K. pneumoniae, Klebsiella oxytoca, and E. coli) were negative. Of the three patients in group 3B with positive BG results, one patient had received intravenous immunoglobulin and a β-lactam antibiotic (penicillin). Of the two other BG-positive patients in group 3B, one (with E. coli bacteremia) received no antibiotics, and the other received an antibiotic other than a β-lactam, doxycycline. Three other patients in group 3B received β-lactam antibiotics (nafcillin and piperacillin-tazobactam) but were BG negative. Counting the sample from the patient with biopsy confirmed IFI as a true positive, the sensitivity, specificity, and positive and negative predictive values of the Fungitell assay, by patient, for groups 1, 2, 3A, and 3B were 93.3%, 77.2%, 51.9%, and 97.8%, respectively.
All six patients with specimens that were positive for Histoplasma antigen (group 4A) were also BG positive. Of the 10 patients with specimens that were Histoplasma antigen negative (group 4B), 6 were negative for BG, 2 were indeterminate, and 2 were positive (202 and 220 pg/ml). Thirty-one of the 32 patients with specimens that were positive for Aspergillus galactomannan (1 sample/patient) were also positive for BG (range, 82 to 2,352 pg/ml). Of 32 patients with specimens that were negative for Aspergillus galactomannan (group 5B), 22 were negative for BG, 1 was indeterminate, and 9 were positive (range, 81 to 582 pg/ml). We were able to obtain clinical information for 8 of the patients with a negative Aspergillus antigen and positive BG. Two of the eight BG-positive patients had confirmed IFI: one patient had a Candida infection, and the other had Aspergillus. The remaining six patients had no laboratory evidence of IFI; therefore, the BG results were considered falsely positive.
Fungitell assay reproducibility.
The between-run coefficients of variance (CVs) for three runs ranged from 3.2% for a serum sample with a BG concentration of 1,643 pg/ml to 16.8% for the serum sample with a BG concentration of 85 pg/ml. The mean between-run CV for all 15 samples was 9.1%. The within-run CVs for the four samples tested ranged from 1.3% for a sample with a BG concentration of 2,034 pg/ml to 4.8% for a sample with a BG concentration of 181 pg/ml. Samples with BG values of 953 pg/ml and 1,121 pg/ml had replicate CVs of 2.0% and 2.8%, respectively. The correlation coefficient (R2) between ACCI and ARUP for the 49 samples tested at both sites was 0.9892. There were nine discrepant results between the two laboratories. Two samples were indeterminate at ACCI and negative at ARUP (73 and 61 pg/ml versus 53 and 52 pg/ml, respectively). Two samples were indeterminate at ACCI and positive at ARUP (77 and 60 pg/ml versus 81 and 98 pg/ml, respectively). Three samples were negative at ACCI and positive at ARUP (50, 58, and 59 pg/ml versus 122, 81, and 81 pg/ml, respectively). Finally, two samples were negative at ACCI and indeterminate at ARUP (45 and 50 pg/ml versus 63 and 77 pg/ml, respectively).
Interfering substances.
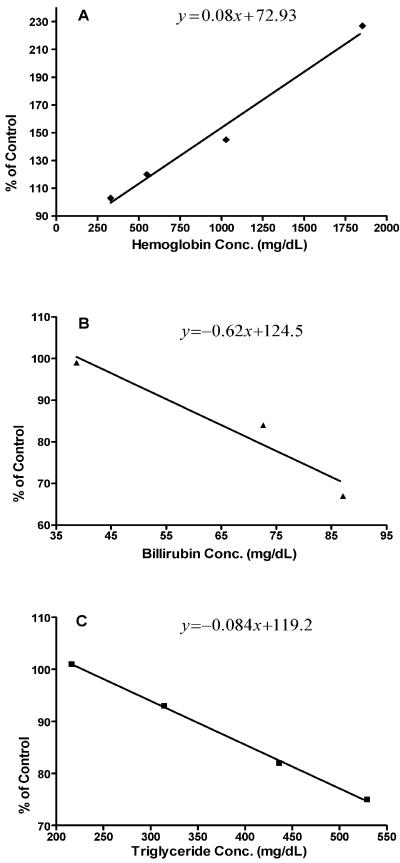
Figure 2 shows the effects of hemoglobulin, bilirubin, and triglycerides on the Fungitell assay. High concentrations of bilirubin and triglycerides were inhibitory and would cause false-negative results, while hemolysis would cause false-positive results. Using the equation for each graph and a 20% deviation in the assay as a clinically significant threshold for interference, the concentrations of each interferent at which a 20% interference would be expected to occur were 588 mg/dl for hemoglobin, 72 mg/dl for bilirubin (which would be unlikely to occur clinically), and 466 mg/dl for triglycerides.
FIG. 2.
Interference plots showing the effects of increasing concentrations of hemoglobulin, bilirubin, and triglycerides on the Fungitell assay. The plots show percent inhibition of a BG-positive serum sample as a function of increasing concentrations of hemoglobin (A), bilirubin (B), and triglycerides (C).
BG cross-contamination.
Two of three BG-negative specimens became positive after the third of four successive transfers of the samples into new transport tubes. For the sample with an initial BG concentration of 58 pg/ml, BG results for four aliquots from four successive transfers were 36, 38, 322, and 529 pg/ml. For the sample with an initial BG concentration of 24 pg/ml, BG values for the four aliquots were 21, 50, 83, and 102 pg/ml. BG values for the third sample were 34 (initial result) and 19, 16, 24, and 33 pg/ml, respectively, for the four transfers.
DISCUSSION
In this study, we assessed the performance of the Fungitell assay for the diagnosis of IFI in a diverse population of patients. In healthy adult blood donors (group 1), who theoretically all should be BG negative, and patients with candidemia (group 2), who by definition have proven IFI and should be BG positive, the sensitivity and specificity of the assay with ≥80 pg/ml as the positive cutoff (per the manufacturer's recommendations) were 92.9% and 100%, respectively. In a similar population (30 healthy adults and 30 nonneutropenic patients with candidemia), studied by Odabasi et al. to establish a cutoff for the Fungitell assay, the sensitivity and specificity of the assay with ≥60 pg/ml as the positive cutoff were 97% and 93%, respectively (24). When bacteremic patients (groups 3A and B) were included in our assessment of the performance of the Fungitell assay, the specificity and positive predictive value decreased considerably (to 77.2% and 51.9%, respectively), due to the high number of false-positive results, particularly in samples from those patients with gram-positive bacteremia. This specificity is considerably lower than that reported by Odabasi et al. (24) for neutropenic patients with acute myelogenous leukemia or myelodysplastic syndrome (90% in patients with proven or probable IFI and 96% in patients with proven, probable, or possible IFI) and by Pazos et al. (26) for adult patients with hematological cancer (89.6%). Digby et al., on the other hand, reported positive BG results in patients in an intensive care unit who had confirmed bacterial infections (5). However, because these investigators used >20 pg/ml as the cutoff for a positive BG value, rather than the manufacturer-recommended cutoff of ≥80 pg/ml, it is impossible to accurately interpret their results.
False-positive BG reactions are known to occur in patients with renal failure who are undergoing hemodialysis with cellulose membranes, patients treated with intravenous immunoglobulins, and specimens or patients exposed to gauze or other materials that contain glucans (10, 14, 15, 16, 21). Albumin, coagulation factors, and plasma protein fraction manufactured by certain vendors for intravenous injection also have been shown to contain high levels of BG (8, 9, 32). Certain streptococci are known to produce glucan or glucan-like polymers (5). In addition, we showed in our cross-contamination experiment that excess manipulation of a sample can result in its contamination with BG. In our study, two bacteremic patients with positive BG levels were receiving intravenous immunoglobulin and one was undergoing hemodialysis for renal failure, which would explain their false-positive BG results. Additionally, one patient with a false-positive BG result had streptococcal bacteremia (S. mitis). None of our patients had had recent surgery, but we could not reliably determine if there had been any exposure to gauze or other materials that contained glucans. All of our samples had been manipulated at least once; therefore, the possibility of laboratory contamination exists.
There are other potential, yet unproven, reasons for false-positive BG reactions. These include exposure to antitumor polysaccharides such as lentinan, polysaccharide K, and schizophyllan, which are derived from different species of mushrooms; mucosal damage from chemotherapy or radiotherapy, which could allow BG from dietary sources or from Candida colonizing the gastrointestinal tract to enter the bloodstream; and receipt of antibiotics derived from fungal sources (12, 28, 30, 33, 36). None of our patients received antitumor polysaccharides. Only one bacteremic patient received cytotoxic chemotherapy (cytarabine, mitoxantrone, and dexamethasone 10 days before the sample tested for BG was collected). Five bacteremic patients who were BG positive had been given β-lactam antibiotics, which are produced by large-scale fermentation of fungi, but so did three bacteremic patients who were BG negative. This suggests that these antibiotics are an unlikely cause of the false-positive BG results, but this should be confirmed in a larger, controlled study. In most of the bacteremic patients in our study, the reason for the false-positive BG result remains unknown.
For groups 4 and 5 (patients with suspected histoplasmosis and aspergillosis, respectively), despite a concerted effort, we were unable to obtain pertinent clinical information in several cases where there was a discrepancy between the Histoplasma antigen or Aspergillus galactomannan and BG results. For this reason, we did not include these groups in our assessment of the Fungitell performance. However, it is most likely that the BG result is accurate when the Histoplasma antigen or Aspergillus galactomannan result and the BG result are either both positive or both negative. When results are discordant, on the other hand, it is impossible to know which is correct without patient information. Results that were positive for BG but negative for galactomannan or Histoplasma antigen could reflect differences in the sensitivities of the assays. Sensitivities for the Platela assay have been reported to be as low as 30%, although other studies have reported sensitivities above 90% (7, 13, 26, 30). Kami et al. found the Platela assay to be less sensitive (58%) than a BG assay (67%) for diagnosis of invasive pulmonary aspergillosis (13). The Histoplasma EIA may not detect up to 20% of cases of disseminated histoplasmosis (37). Patients who were BG positive and either galactomannan or Histoplasma antigen negative could also have fungal infections other than Histoplasma or Aspergillus.
In summary, the Fungitell assay appears to be a useful test in the evaluation of patients at high risk for IFI. The assay is reproducible, except at concentrations very close to the cutoff. However, based on our cross-contamination experiment, we recommend minimal manipulation of specimens submitted for BG testing. Additionally, hemolytic and lipemic samples are likely to interfere with the assay and therefore should not be tested. Although the sensitivity of the test is good, several patients in our study had false-positive results, which limits the value of the test as a positive predictor of IFI. However, the negative predictive value in our study and others (5, 23, 26) was high (>95%). This suggests that the primary value of the Fungitell assay is to exclude the presence of IFI based on a negative result, with the caveat that some fungi (cryptococci and zygomycetes) produce low levels of BG. This could then eliminate the need to initiate toxic and expensive antifungal therapy. Controlled studies designed to further explore the cause of false-positive BG reactions will be valuable.
REFERENCES
- 1.Ampel, N. M. 1996. Emerging disease issues and fungal pathogens associated with HIV infection. Emerg. Infect. Dis. 2:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, f. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic IFI in immunocompromised patients with cancer and hematopoietic stem cell transplants; an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Associates of Cape Cod, Inc. 2004. Glucan assay for (1,3)-β-d-glucan in serum: Fungitell. [Online.] http://www.acciusa.com/pdfs/fungitell_insert.pdf.
- 4.Clark, T. A., and R. A. Hajjeh. 2002. Recent trends in the epidemiology of invasive mycoses. Curr. Opin. Infect. Dis. 15:569-574. [DOI] [PubMed] [Google Scholar]
- 5.Digby, J., J. Kalbfleisch, A. Glenn, A. Larsen, W. Browder, and D. Williams. 2003. Serum glucan levels are not specific for presence of fungal infections in intensive care units. Clin. Diagn. Lab. Immunol. 10:882-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellepola, A. N., and C. J. Morrison. 2005. Laboratory diagnosis of invasive candidiasis. J. Microbiol. 43:65-84. [PubMed] [Google Scholar]
- 7.Husain, S., E. J. Kwak, A. Obman, M. M. Wagener, S. Kusne, J. E. Stout, K. R. McCurry, and N. Singh. 2004. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am. J. Transplant. 4:796-802. [DOI] [PubMed] [Google Scholar]
- 8.Huszar, G., B. Jenei, G. Szabo, and G. A. Medgzesi. 2002. Detection of pyrogens in intravenous IgG preparations. Biologicals 30:77-83. [DOI] [PubMed] [Google Scholar]
- 9.Ikemura, K., K. Ikegami, T. Shimazu, T. Yoshioka, and T. Sugimoto. 1989. False-positive result in Limulus test caused by Limulus amebocyte lysate-reactive material in immunoglobulin products. J. Clin. Microbiol. 27:1965-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizuka, Y., H. Tsukada, and F. Gejyo. 2004. Interference of (1→3)-beta-d-glucan in the measurement of plasma (1→3)-beta-d-glucan. Intern. Med. 43:97-101. [DOI] [PubMed] [Google Scholar]
- 11.Jones, B. L., and L. A. McLintock. 2003. Impact of Diagnostic markers on early antifungal therapy. Curr. Opin. Infect. Dis. 16:521-526. [DOI] [PubMed] [Google Scholar]
- 12.Kakinuma, A., T. Asano, and H. Torii. 1981. Gelation of Limulus amebocyte lysate by an antitumor (1→3)-β-d-glucan. Biochem. Biophys. Res. Commun. 101:434-439. [DOI] [PubMed] [Google Scholar]
- 13.Kami, M., T. Fukui, S. Ogawa, Y. Kauyama, U. Machida, Y. Tanaka, Y. Kanda, T. Kashima, Y. Yamazaki, T. Hamaki, S. Mori, H. Akiyama, Y. Mutou, H. Sakamaki, K. Osumi, S. Kimura, and H. Hirai. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504-1512. [DOI] [PubMed] [Google Scholar]
- 14.Kanda, H., K. Kubo, K. Hamasaki, Y. Kanda, A. Nakao, T. Kitamura, T. Fujita, K. Yamamoto, and T. Mimura. 2001. Influence of various hemodialysis membranes on the plasma (1→3)-beta-d-glucan level. Kidney Int. 60:319-332. [DOI] [PubMed] [Google Scholar]
- 15.Kato, A., T. Takita, M. Furuhashi, T. Takahashi, Y. Maruyama, and A. Hishida. 2001. Elevation of blood (1→3)-beta-d-glucan concentrations in hemodialysis patients. Nephron 89:15-19. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, Y., A. Nakao, H. Tamura, S. Tanaka, and H. Takagi. 1995. Clinical and experimental studies of the limulus test after digestive surgery. Surg. Today 25:790-794. [DOI] [PubMed] [Google Scholar]
- 17.Klastersky, J. 2004. Empirical antifungal therapy. Int. J. Antimicrob. Agents 23:105-112. [DOI] [PubMed] [Google Scholar]
- 18.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillus case-fatality rate: systemic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 20.Mitsutake, K., T. Miyazaki, T. Tashiro, Y. Yamamoto, H. Kakeya, T. Otsubo, S. Kawamura, M. A. Hoosain, T. Noda, Y. Hirakta, and S. Kohno. 1996. Endolase antigen, mannan antigen, Cand-Tec antigen, and β-d-glucan in patients with candidemia. J. Clin. Microbiol. 34:1918-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki, T., S. Kohno, K. Mitsutake, S. Maesaki, K. Tanaka, N. Ishikawa, and K. Hara. 1995. Plasma (1→3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J. Clin. Microbiol. 33:3115-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obayashi, T., M. Yoshiba, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshima, S. Kohno, A. Horiuchi, A. Ito, H. Yamaguchi, K. Shimada, and T. Kawai. 1995. Plasma (1→3)-β-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 23.Obayashi, T., M. Yoshida, H. Tamura, J. Aketagawa, S. Tanaka, and T. Kawai. 1992. Determination of plasma (1→3)-beta-d-glucan: a new diagnostic aid to deep mycosis. J. Med. Vet. Mycol. 30:275-280. [DOI] [PubMed] [Google Scholar]
- 24.Odabasi, Z. G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. β-d-Glucan as a diagnostic adjunct for IFI: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 25.Owen, W. E., and W. L. Roberts. 2004. Performance characteristics of the IMMULITE 2000 erythropoietin assay. Clin. Chim. Acta 340:213-217. [DOI] [PubMed] [Google Scholar]
- 26.Pazos, C., J. Ponton, and A. D. Palacio. 2005. Contribution of (1→3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh, N., and D. L. Paterson. 2005. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 18:44-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, N., A. Obman, S. Husain, S. Aspinall, S. Mietzner, and J. E. Stout. 2004. Reactivity of Platelia Aspergillus galactomannan antigen with piperacillin-tazobactam: clinical implications based on achievable concentrations in serum. Antimicrob. Agents Chemother. 48:1989-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens, D. A. 2002. Diagnosis of fungal infections: current status. J. Antimicrob. Chemther. 49(Suppl. 1):11-19. [DOI] [PubMed] [Google Scholar]
- 30.Sulahian, A., F. Boutboul, P. Ribaud, T. Leblanc, C. Lacroix, F. Deouin. 2000. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer 91:311-318. [DOI] [PubMed] [Google Scholar]
- 31.Tamura, H., S. Tanaka, T. Oda, Y. Uemura, J. Aketagawa, and Y. Hashimoto. 1996. Purification and characterization of a (1→3)-beta-d-glucan-binding protein from horseshoe crab (Tachypleus tridentatus) amebocytes. Carbohydr. Res. 295:103-116. [DOI] [PubMed] [Google Scholar]
- 32.Usami, M., A. Ohata, T. Horiuchi, K. Nagasawa, T. Wakabayashi, and S. Tanaka. 2002. Positive (1→3)-β-d-glucan in blood components and release of (1→3)-β-d-glucan from depth-type membrane filters for blood processing. Transfusion 42:1189-1195. [DOI] [PubMed] [Google Scholar]
- 33.Viscoli, C., M. Machetti, P. Cappellano, B. Bucci, P. Bruzzi, M. T. Van Lint, and A. Bacigalupo. 2004. False positive galactomannan Platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 38:913-916. [DOI] [PubMed] [Google Scholar]
- 34.Walsh, T. J. A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissie. 2002. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10(Suppl. 1):48-66. [DOI] [PubMed] [Google Scholar]
- 35.Warnock, D. W. 1998. Fungal infections in neutropenia: current problems and chemotherapeutic control. J. Antimicrob. Chemother. 41(Suppl. D):95-105. [DOI] [PubMed] [Google Scholar]
- 36.Wasser, S. P. 2002. Medical mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60:258-274. [DOI] [PubMed] [Google Scholar]
- 37.Williams, B., M. Fojtasek, P. Connolly-Stringfield, and J. Wheat. 1994. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis, Ind. Arch. Pathol. Lab. Med. 118:1205-1208. [PubMed] [Google Scholar]
- 38.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]